Epstein-Barr virus (EBV)–associated nasopharyngeal carcinoma (NPC) is the third most frequent virus-associated human malignancy. How this tumor escapes immune recognition despite the expression of several viral antigens has remained poorly understood. Our previous in vitro studies have shown that NPC cells release exosomes containing high amounts of galectin-9, a ligand of the membrane receptor Tim-3, which is able to induce apoptosis in mature Th1 lymphocytes. Here, we sought to determine whether galectin-9–carrying exosomes were produced in NPC patients and whether such exosomes might play a role in the immune evasion of NPC cells. We report that galectin-9–containing exosomes are selectively detected in plasma samples from NPC patients and mice xenografted with NPC tumors. The incorporation into exosomes protects galectin-9 against proteolytic cleavage but retains its Tim-3–binding capacity. Importantly, NPC exosomes induce massive apoptosis in EBV-specific CD4+ cells used as a model of target T cells. This effect is inhibited by both anti–Tim-3 and antigalectin-9 blocking antibodies. These results indicate that blocking galectin-9/Tim-3 interaction in vivo might alleviate the Th1-suppressive effect of NPC exosomes and sustain antitumoral T-cell responses and thereby improve clinical efficacy of immunotherapeutic approaches against NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)–associated malignancy and the third most frequent virus-associated human malignancy after hepatocarcinomas and cervix carcinomas. Each year, approximately 80 000 new cases are diagnosed worldwide. The geographic distribution of NPC is not uniform. It is relatively rare in European and North American countries. Very high incidence foci are found in South China, especially in Guandong and Guangxi provinces (25-40 per 100 000 per year).1 Areas of intermediate incidence (approximately 3-8 per 100 000 per year) include a large number of developing or emerging countries, especially in North and Central Africa (Tunisia, Algeria, Morocco, Somalia, and Kenya) and in Southeast Asia (Philippines, Vietnam, Indonesia).
NPC is an epithelial malignancy with a complex etiology involving viral, environmental, and hereditary factors. Except for some very rare cases of atypical highly differentiated NPC occurring in Western countries, the intact EBV genome is always contained in the nuclei of all malignant cells.2,3 Many of the approximately 80 EBV genes are silent, but several viral RNAs and proteins are consistently expressed in NPC and contribute to the malignant phenotype.4 NPC oncogenesis also requires a variable assortment of cellular genetic or epigenetic alterations.5 Another important biologic feature of NPC is the presence of a massive lymphoid infiltrate in the primary tumor. This infiltrate contains mostly T lymphocytes and a minority of B cells, monocytes, dendritic cells, and eosinophils. The abundant production by malignant NPC cells of inflammatory cytokines, including interleukin-1α (IL-1α), macrophage-inhibitory protein 1 (MIP1), and CXCL10, is likely to favor the leukocyte infiltrate.6,,–9
Several EBV proteins are consistently expressed in NPCs, including EBNA1, LMP1, LMP2, and BARF1.10,–12 In addition, there is indirect evidence that viral latency is occasionally disrupted in small foci of malignant cells, resulting in transient production of lytic cycle proteins.13,–15 Production of the early lytic transactivator BZLF1, which is highly immunogenic, has been formally demonstrated in NPC specimens.16 The emergence of a malignant process producing several immunogenic viral proteins in a context of local inflammation and heavy leukocytic infiltration is one major paradox of NPC pathogenesis. Previous reports have shown that NPC cells retain a functional antigen-presenting machinery, suggesting that immune escape by NPC is brought about by specific features of the tumor microenvironment.17,18 However, for a long time, the search for local immunosuppressive factors has been disappointing. For example production of Fas ligand by NPC cells is not frequently observed at early stages of the disease, and the transforming growth factor-β (TGF-β) gene is not expressed at a higher level in tumor tissue compared with normal adjacent mucosa.7,19
Our recent findings of a consistent galectin-9 production by NPC cells have brought new insights in this field. Galectin-9 is a β-galactoside binding lectin that contains 2 carbohydrate recognition domains (CRD) joined by a “linker” region. It is expressed in various mammalian tissues, especially by kidney cells, thymus epithelial cells, and M cells of mucosal lymphoid structures.20,21 Galectin-9 expression can be induced in various cell types by cytokines, especially interferon-γ and IL-1β.20 It is also inducible by EBV infection.22 We have shown that galectin-9 is extremely abundant in NPC cells, in xenografted tumors, as well as in clinical specimens.23 Moreover, when NPC cells are grown in vitro, galectin-9 is released into the cell culture medium in association with exosomes.24 Meanwhile, galectin-9 has been identified as a specific agonist of Tim-3, which is a death-inducing receptor expressed by mature Th1 cells.25 In this study, we intended to investigate the structural and physiologic characteristics of NPC exosomes as carriers of galectin-9 and agonists of Tim-3 on Th1 T cells. We report that galectin-9–containing exosomes are selectively detected in the plasma of NPC patients or mice xenografted with NPC tumor lines. In vitro they induce apoptosis in EBV-specific CD4+ lymphocytes through galectin-9/Tim-3 interactions. These findings support a model in which NPC exosomes exert Th1 suppressive functions at both the tumor and systemic levels.
Methods
Tumor and cell lines
C15 and C17 are xenografted EBV-positive NPC tumor lines permanently propagated by subcutaneous passage in nude mice.26 As previously reported, nonmalignant cells of human origin were eliminated from the xenografts beyond the first mouse passage. Therefore all human cells carried by mice grafted with C15 and C17 tumors are malignant NPC cells.26,27 Suspensions of NPC cells were obtained by dispersion of xenografted tumors as previously described.28 All animal procedures were performed by certified personnel according to French and European regulations in the animal facility of the Gustave Roussy Institute of Oncology (Villejuif, France). C666-1, an EBV-positive NPC tumor line propagated in vitro, was kindly provided by D. P. Huang and K. W. Lo (Chinese University of Hong Kong, Hong Kong).29 It was grown in RPMI medium supplemented with 25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) and 7.5% fetal calf serum (FCS). The HeLa cervix carcinoma cell line was cultured in Dulbecco modified Eagle medium (DMEM) with 5% FCS. All experiments with nude mice were performed in the Gustave Roussy Institute of Oncology with required permissions and approval of the governing board of our animal facility.
Generation of EBV-specific CD4+ T-cell clones
This procedure has been described previously.30,–32 Briefly, peripheral blood mononuclear cells (PBMCs) were obtained from healthy EBV carriers, and CD4+ cells were selected over magnetic cell sorting (MACS) columns (Miltenyi Biotec, Bergisch Gladbach, Germany). Initial stimulation was performed using autologous PBMCs pulsed with recombinant EBV proteins: EBNA1, BZLF1, EBNA3C, and BLLF1 (gp350 envelope glycoprotein). These proteins were produced as histidine (His)–tagged proteins by transient transfection of HEK 293T cells and purification over Nickel-NTA agarose beads (QIAGEN, Hilden, Germany). Autologous PBMCs (106/mL) cultured in AIM-V medium (Invitrogen, Karlsruhe, Germany) were pulsed with 500 ng/mL of the appropriate EBV recombinant protein for 24 hours, washed, irradiated (40 Gy), and mixed with an equal number of 106/mL autologous CD4+ PBMCs in T-cell medium (RPMI 1640 with 10 mM HEPES and 10% human serum). After 48 hours, 10 U/mL recombinant IL-2 (Chiron Behring, Marburg, Germany) were added to the cultures. T-cell clones were generated by limiting dilution cloning as described.30 They were maintained in culture by restimulation every 2 weeks using mini-lymphoblastoid cell lines (LCLs) pulsed with the appropriate EBV-recombinant protein. These mini-LCLs were established by infection of primary B cells with the genetically engineered mini-EBV strain and do not support lytic viral replication.31
Transient transfection of cell lines
Human galectin-9 was expressed in HeLa cells after transfection of the full-length cDNA of galectin-9 (long isoform) in the pBK-CMV plasmid (Stratagene, La Jolla, CA) using the calcium-phosphate method.
Protein extraction from cultured cells
Cell pellets were solubilized in prechilled radio immunoprecipitation assay (RIPA) buffer (150 mM NaCl, 25 mM Tris-HCl, pH 7.5, 5 mM ethylenediaminetetraacetic acid [EDTA], 0.5% sodium deoxycholate, 0.5% Nonidet P40 [NP40], 0.1% sodium dodecyl sulfate [SDS]) supplemented with complete protease inhibitor cocktail (Roche Applied Science, Neuilly-sur-Seine, France) and sonicated on ice. Extracts were clarified by centrifugation for 15 minutes at 16 000g at 4°C. Protein concentration was assayed by the Lowry method using a detergent-compatible microassay system (Bio-Rad Laboratories, Gif-sur-Yvette, France).
Antibodies
In Western blot analyses, galectin-9 was detected using an affinity-purified rabbit polyclonal serum (anti-G9–CT-L1) raised against a recombinant peptide derived from the C-terminal CRD2.23,24,33 For flow cytometric analysis, exosome capture, or blocking of ligand-receptor binding, we used a monoclonal antibody directed to residues 208-215 of galectin-9 (9M1-3). This epitope, FSTPAIPP, is located at the N-terminal border of the C-terminal CRD of galectin-9 (CRD2). Monoclonal antibodies against CD9 (TS9) and CD63 (TS63) have been previously published.34 The DA6.147 was used for Western blot detection of the DR-α chain of the human leukocyte antigen (HLA) class II antigens.35 The glucose-regulated protein 94 (Grp94 or gp96) was visualized with a rat monoclonal antibody (Stressgen, Ann Arbor, MI). For detection of Tim-3 by flow cytometric analysis and blocking of ligand-receptor binding, we used a monoclonal antibody (2E2) directed to its ligand-binding site.36,37 This reagent was kindly provided by David Anderson and Vijay Kuchroo (Center for Neurologic Diseases, Harvard Medical School, Boston, MA).
Recombinant proteins and galectin-9 detection by enzyme-linked immunosorbent assay
The m-isoform (medium-sized) of the wild-type galectin-9 lacks exon 5 and has a shorter linker region than the full-length l-isoform (long-sized).20,38 Galectin-9 NC(null) or NC is an artificial deletion mutant lacking the linker region, which increases its resistance to proteases (no linker peptide between the N- and C-CRD).39 Both the m-isoform and galectin-9 NC were produced in Escherichia coli using the pET11a vector and purified by affinity chromatography on a lactose-agarose column.39 Final preparations were checked for absence of endotoxin contamination. Detection of galectin-9 by enzyme-linked immunosorbent assay (ELISA) was performed according to the protocol used for synovial fluid from rheumatoid arthritis patients.40 Due to unidentified factors inhibitory for this ELISA system, human plasmas required substantial dilution (8×), resulting in poor sensitivity. Mouse plasmas did not contain such inhibitory factors.
Exosome production in vitro
C17 NPC cells obtained by dispersion of xenografts were incubated in 24-well plates at 106 cells/well in 1.5 mL RPMI medium supplemented with 1.5% FCS. The in vitro–propagated epithelial cell lines, HeLa and C666-1, were grown to 60% confluence in 75-cm2 flasks and incubated in 10 mL appropriate culture medium with 1.5% FCS. Conditioned media were collected after 48 hours of incubation at 37°C, centrifugated at 300g for 10 minutes to remove biggest cell remnants and debris, and frozen at −80°C.
Preparation of exosomes from plasma samples
Heparinized blood samples were collected from mice carrying various xenografted tumors by puncture of the nasal venous sinus. Plasmas were separated from red cells by centrifugation at 3000g at 4°C for 10 minutes and frozen at −80°C. On the other hand, human plasma samples were collected from 26 donors. This procedure was done with patient informed consent obtained in accordance with the Declaration of Helsinki and Institutional Review Board (IRB) approval from the Institut Gustave Roussy and the Salah Azaiz Institute. Clinical and pathologic data on donor patients are summarized in Tables S1 through S4 (available on the Blood website; see the Supplemental Materials link at the top of the online article). A first series of plasma samples were obtained from 12 NPC patients (A to L) diagnosed at the ENT Department of the Salah Azaiz Institute (Tunis, Tunisia). All these patients had nonkeratinizing carcinomas of the undifferentiated type and were previously untreated (Table S1). The nonkeratinizing type of NPC is known to be constantly associated to EBV, irrespective of the ethnic background.2,3 A second series of plasma samples were collected prospectively from 5 NPC patients (M to Q) referred to the Gustave Roussy Institute of Oncology (Villejuif, France) or to Paris hospitals working in collaboration with the Institute (Table S2). For each of these 5 patients, EBV association was confirmed by EBV-encoded RNA (EBER) in situ hybridization (Figure S1). Control plasma samples were obtained from 1 healthy volunteer and 8 patients referred for non-NPC malignant tumors to the Salah Azaiz (TA, TB, and TC) or the Gustave Roussy (TD to TH) Institute (Tables S3,S4). For exosome preparation, 1.5 mL thawed plasma samples were diluted 1:4 times in phosphate-buffered saline (PBS) and subjected to a first centrifugation in a SW41Ti Beckman rotor at 12 000g for 45 minutes. The 12-kg pellet was discarded, whereas the supernatant was subjected to a second centri-fugation at 110 000g for 2 hours using the same rotor. The 110-kg pellet, containing low-density vesicles, was washed by resuspension in PBS and centrifuged in a TL100 Beckman rotor for 1 hour at 110 000g. HLA class II–positive exosomes were isolated from this second pellet by immunomagnetic capture.
Purification of exosomes from culture media using a sucrose gradient
This procedure was adapted from the method described by Lamparski et al.41 All steps were performed at 4°C. Thawed culture supernatants (at least 75 mL) were clarified by 2 consecutive centrifugations, first at 1900g for 15 minutes and then at 12 000g for 30 minutes. Clarified supernatants were then subjected to ultracentrifugation at 66 000g for 2 hours using a Ti45 Beckman rotor, resulting in a pellet of low-density vesicles. Exosomes contained in this pellet were further purified by flotation on a cushion made of a sucrose solution in deuterium oxide (D2O; Sigma-Aldrich Chimie, Lyon, France). Practically, the low-density pellet was resuspended in filtered PBS (10 mL for an initial volume of a 7-mL supernatant), loaded on top of a 1-mL sucrose/D2O solution (20 mM Tris, 30% sucrose D2O, pH 7.4) in a SW 41 polycarbonate tube. This 2-phase discontinuous gradient was subjected to ultracentrifugation at 76 000g for 90 minutes on a SW41 Ti Beckman rotor. The cushion containing the exosomes was then collected without disturbing the pellet. The exosomes were diluted 1:5 and pelleted by ultracentrifugation at 110 000g in a SW41 rotor for 90 minutes. Two additional washing steps were performed in a smaller volume (ultracentrifugation at 110 000g using a TLA100 Beckman rotor). Washed exosomes were then resuspended in PBS, subjected to protein assay, and 35-μL aliquots with a protein concentration of 1 to 5 μg/μL were stored at −80°C. When prepared for immunomagnetic capture, low-density vesicles were directly subjected to the washing steps without prior purification on a sucrose cushion.
Immunomagnetic capture of exosomes from conditioned culture media or plasma samples
Materials subjected to capture were low-density vesicles (110-kg pellets) derived either from 50 mL conditioned culture medium or from 1.5-mL plasma samples. Beads used for capture were 4.5-μm-diameter magnetic beads. In most experiments, they were coated with an anti–HLA class II monoclonal antibody (Dynabeads–HLA class II; Dynal, Invitrogen, Cergy Pontoise, France). The same type of magnetic beads carrying an irrelevant monoclonal immunoglobulin (Ig) was used as control beads. In one set of experiments (see Figure 2), the 9M1-3 monoclonal antibody directed to the galectin-9 CRD2 was loaded on magnetic beads (10 μg for 5 × 107 beads) coated with a secondary anti–mouse Ig (Dynabeads Pan Mouse IgG; Dynal, Invitrogen). Control beads were coated with irrelevant purified monoclonal mouse IgG (Sigma-Aldrich Chimie). For the capture process, particles contained in 110-kg pellets were resuspended in 250 μL serum-free culture medium and incubated for 5 hours at 4°C, with 3.5 × 107 beads under mild agitation. After the capture step, magnetic beads were washed 4 times in 1 mL PBS. Exosome-loaded beads intended for electron microscopy examination were resuspended in the fixative solution. For Western blot analysis of captured material, the beads were boiled for 5 minutes in Laemmli buffer to release proteins before gel loading.
Electron microscopy
For visualization of exosomes after immunomagnetic purification, loaded beads were fixed in glutaraldehyde and embedded in Epon as previously described.24 Ultrathin sections were cut with a Reichert Ultramicrotome III (Reichert, Depew, NY) and counterstained with uranyl acetate and lead citrate before being observed with a Tecnai Spirit transmission electron microscope (FEI, Hillsboro, OR). Images were recorded with a SIS MegaviewIII charge-coupled device (CCD) camera driven by the AnalySIS 3.1 image processing software (Silicon Integrated System, Taiwan, Republic of China).
Western blot analysis
Cell or exosome protein extracts were separated on polyacrylamide gels in standard conditions except when planning detection of CD9 and CD63 tetraspanins, which require nonreducing conditions. Gels were blotted on polyvinylidene fluoride (PVDF) membranes (Immobilon P; Millipore, Molsheim, France) according to standard protocols. Specific protein bands were visualized using horseradish peroxidase–conjugated secondary antibodies and appropriate revelation systems, enhanced chemiluminescence (ECL) system (GE Healthcare, Buc, France) or Supersignal Westpico (Pierce, Perbio Europe, Belgium), depending on the required sensitivity. When required, densitometry of the chemiluminescence films was done using a GS-710 calibrated imaging densitometer with Quantity One software (Bio-Rad Laboratories).
Resistance to trypsin digestion assay
To solubilize lipid membrane components, purified C17 exosomes (35 μg proteins in 7 μL PBS) were subjected to lipid membrane lysis by 1% Triton X-100 for 30 minutes at 4°C. Mock-treated exosomes were incubated under the same conditions in the absence of Triton. Both treated and mock-treated exosome suspensions were then subjected to trypsin digestion at 37°C with 2 enzyme concentrations (15 and 0.01 μg/mL) and increasing times of incubation (5, 15, and 30 minutes; T4549; Sigma-Aldrich Chimie). Trypsin activity was only marginally affected by the presence of Triton (data not shown). After completion of trypsin incubation time, the samples were immediately mixed with an equal volume of Laemmli buffer and boiled for 3 minutes before gel loading.
Membrane immunostaining and flow cytometry
Single-cell suspensions of C17 and C666-1 NPC cells were incubated with 10 μg/mL of the anti–galectin-9 antibody 9M1-3 for 30 minutes, washed with PBS supplemented with 3% FCS, and then incubated with fluorescein isothiocyanate (FITC)–conjugated anti–mouse antibody for 30 minutes. Propidium iodide (PI; 5 μg/mL) was added before analysis. Similarly, EBV-specific CD4+ T cells were first incubated with 3 μg/mL anti–TIM-3 antibody 2E2 for 1 hour, washed, and then incubated with phycoerythrin (PE)–conjugated anti–mouse antibody for 30 minutes. After PI addition, stained cells were analyzed by flow cytometry using the CellQuest software (FACSCalibur; BD Biosciences, Erembodegem, Belgium).
Stimulation of the Tim-3 receptor on EBV-specific CD4+ T cells and assessment of apoptosis induction
CD4+ T cells from 4 clones specific for EBV proteins were chosen as targets of Tim-3–binding agents. These clones were specific for EBNA3C (GB3C), gp350 or BLLF1 (JM1H2), EBNA1, and BZLF1. These T cells (106 cells/well) were incubated for 5 hours with one of the following agents: (1) recombinant wild-type (WT) or modified (NC) galectin-9 at various concentrations, or (2) suspensions of gradient-purified exosomes, adjusted to a final protein concentration of 100 μg/mL. In some experiments, preincubation with blocking antibodies were performed, either on CD4+ target cells (2E2 anti–Tim-3 antibody, 10 μg/mL for 1 hour) or on exosomes (9M1-3 anti–galectin-9 antibody, 10 μg/mL for 1 hour). After Tim-3 stimulation, target cells were stained with annexin V–FITC and PI using the Annexin V–FITC Apoptosis Detection kit (Roche Applied Science). FITC and PI staining were analyzed by flow cytometry using the CellQuest software, FACSCalibur.
Results
Galectin-9–positive exosomes are produced by NPC cells in vivo
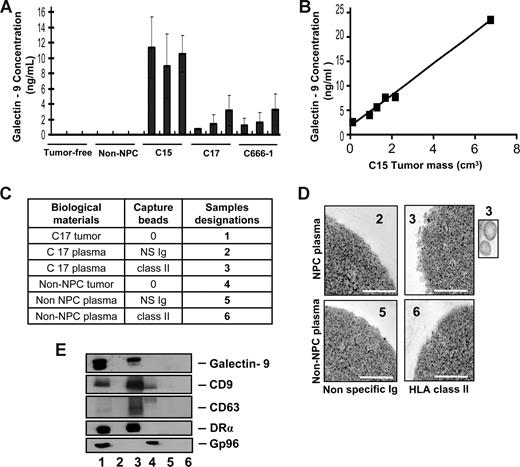
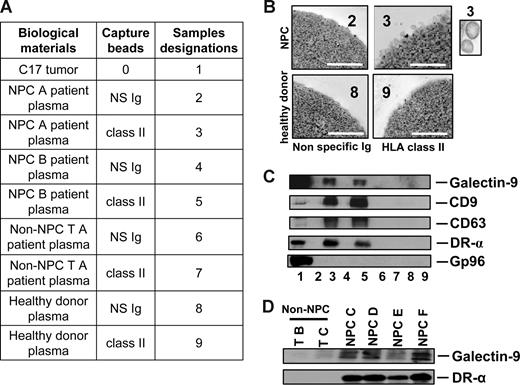
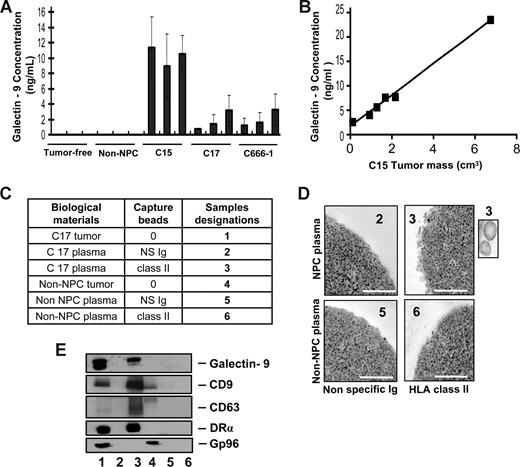
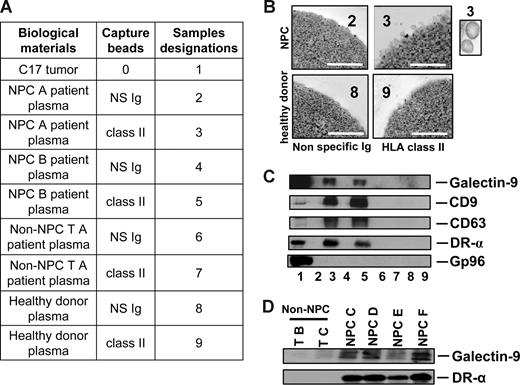
To investigate whether galectin-9–containing exosomes were produced by NPC cells in vivo, plasma samples were collected from nude mice xenografted with NPC tumors. Control samples were obtained from tumor-free nude mice and mice xenografted with a non-NPC human tumor. By ELISA, galectin-9 was specifically detected in the plasma samples from NPC-xenografted but not control mice (Figure 1A). In addition, galectin-9 concentration in mouse plasma samples was proportional to the tumor volume as shown for the C15 xenografts (Figure 1B). In the next step, low-density vesicles contained in plasma samples were collected as 110-kg pellets. These pellets were further treated by magnetic beads to isolate vesicles expressing surface HLA class II. As shown in Figure 1C-E, such vesicles were captured from xenografted NPC mouse plasma but not control samples. Upon electron microscopy examination, captured HLA class II–positive vesicles had size and morphology typical of exosomes. Western blot analysis of their proteins readily detected galectin-9 with a predominance of the l-isoform in contrast with the tumor extract containing equal amounts of the l- and m-isoforms.20,38 Identification of these vesicles as exosomes was substantiated by detection of the CD63 and CD9 tetraspanins, which are markers of the endosomal compartment, and the absence of the cytoplasmic marker gp96. A similar procedure was applied on plasma samples collected from a series of 17 NPC patients and 9 controls, including one healthy individual and 8 patients affected by various types of malignancies distinct from NPC. As shown in Figures 2 and S2, HLA class II–positive exosomes were captured, and exosome-derived galectin-9 was detected in 16 of 17 NPC plasma samples. In contrast, no galectin-9 was detected in 8 of 9 control samples processed according to the same procedure; only 1 of them was borderline or weakly positive (sample TF from a patient affected by an epithelioid sarcoma). This distribution of positive cases speaks strongly in favor of a specific relationship between NPC and detection of galectin-9–containing exosomes in patients' blood (2-tailored P = .001 by Fisher exact test). Because of its very poor sensitivity in the context of human plasma, our galectin-9 ELISA system could not be applied to human samples (see “Recombinant proteins and galectin-9 detection by enzyme-linked immunosorbent assay”). Nevertheless, data obtained from xenografted mice and NPC patients by exosome capture and Western blot analysis provide compelling evidence that galectin-9–containing exosomes are consistently produced by NPC cells in vivo and diffuse from the tumor interstitial fluids to the bloodstream.
Specific detection of galectin-9–containing exosomes in the plasma of mice xenografted with NPC tumor lines. (A) A significant amount of galectin-9 is detected by ELISA in crude plasma samples from mice xenografted with NPC tumor lines (C15, C17, and C666-1), but not control mice either without xenografts (tumor-free) or xenografted with a non-NPC carcinoma tumor line (non-NPC). Assays were performed in triplicate on 3 plasma samples from 3 different mice. (B) A series of plasma samples were collected from 6 mice carrying C15 tumors of various sizes. Galectin-9 concentration in these samples is proportional to the tumor volume (y = 3.2× + 1.7, R2 = 0.994). (C) Captures with anti–HLA class II beads were performed on low-density vesicles (110-kg pellets) derived from pools of murine plasmas. Samples collected with anti–class II beads are designated nos. 3 and 6 (class II). Control samples collected with beads carrying nonspecific Ig are nos. 2 and 5 (NS Ig). In addition to plasma material, protein extracts from the C17 and the non-NPC xenografted tumors were used as controls for Western blot analyses (nos. 1 and 4). (D) Numerous vesicles approximately 70 nm in diameter are captured by HLA class II beads and visualized by electron microscopy when these procedures are applied to plasma vesicles from C17-xenografted but not control mice. Scale bar represents 500 nm. In the inset, 2 vesicles at original magnification (× 4). (E) Western blot analysis detects galectin-9, CD9, CD63, and the DR-α chain in C17 plasma vesicles. In contrast, none of these molecules are detected when the capture is performed on control mouse plasma. Note that CD63 is much more concentrated in C17 exosomes than in the tumor extract. Gp96, which is a cytoplasmic membrane protein, is detected in the C17 and non-NPC tumor extracts (lanes 1 and 4) but not in C17 exosomes (lane 3).
Specific detection of galectin-9–containing exosomes in the plasma of mice xenografted with NPC tumor lines. (A) A significant amount of galectin-9 is detected by ELISA in crude plasma samples from mice xenografted with NPC tumor lines (C15, C17, and C666-1), but not control mice either without xenografts (tumor-free) or xenografted with a non-NPC carcinoma tumor line (non-NPC). Assays were performed in triplicate on 3 plasma samples from 3 different mice. (B) A series of plasma samples were collected from 6 mice carrying C15 tumors of various sizes. Galectin-9 concentration in these samples is proportional to the tumor volume (y = 3.2× + 1.7, R2 = 0.994). (C) Captures with anti–HLA class II beads were performed on low-density vesicles (110-kg pellets) derived from pools of murine plasmas. Samples collected with anti–class II beads are designated nos. 3 and 6 (class II). Control samples collected with beads carrying nonspecific Ig are nos. 2 and 5 (NS Ig). In addition to plasma material, protein extracts from the C17 and the non-NPC xenografted tumors were used as controls for Western blot analyses (nos. 1 and 4). (D) Numerous vesicles approximately 70 nm in diameter are captured by HLA class II beads and visualized by electron microscopy when these procedures are applied to plasma vesicles from C17-xenografted but not control mice. Scale bar represents 500 nm. In the inset, 2 vesicles at original magnification (× 4). (E) Western blot analysis detects galectin-9, CD9, CD63, and the DR-α chain in C17 plasma vesicles. In contrast, none of these molecules are detected when the capture is performed on control mouse plasma. Note that CD63 is much more concentrated in C17 exosomes than in the tumor extract. Gp96, which is a cytoplasmic membrane protein, is detected in the C17 and non-NPC tumor extracts (lanes 1 and 4) but not in C17 exosomes (lane 3).
Specific detection of galectin-9–containing exosomes in the plasma of NPC patients. (A) Captures with anti–HLA class II beads were performed on low-density vesicles (110-kg pellets) derived from human plasma samples. Initially, plasmas were collected from 2 NPC patients (NPC A and B; samples 2, 3, 4, and 5), 1 patient with a non-NPC head and neck carcinoma (non-NPC TA; samples 6 and 7), and 1 healthy donor (samples 8 and 9). In each case, beads coated with nonspecific Ig (NS Ig) were used as negative controls. In addition to plasma material, a protein extract from the C17-xenografted tumor was used as a positive control for Western blot detection (no. 1). Clinical and pathologic data on donor patients are provided in Tables S1 and S3. (B) Numerous bilamellar vesicles approximately 70 nm in diameter are visualized by HLA class II capture and electron microscopy when these procedures are applied to plasma vesicles from NPC patients but not control subjects. Scale bar represents 500 nm. In the inset, 2 vesicles at original magnification (× 4). (C) In parallel experiments, Western blot analysis revealed expression of galectin-9, CD9, CD63, and the α chain of the DR molecule in NPC plasma vesicles. In contrast, none of these proteins is detected when the same procedure is applied to control plasmas. Gp96 is only detected in the tumor extract. (D) Galectin-9–carrying exosomes are captured by anti–HLA class II beads from 4 additional samples of NPC plasmas (NPC C, D, E, and F) but not from 2 control subjects with non-NPC tumors (non-NPC TB and TC). Clinical and pathologic data on donor patients are in Tables S1 and S3. Consistent results were obtained for 11 NPC and 5 non-NPC additional plasma samples (see Tables S1, S2, and S4 and Figures S1 and S2).
Specific detection of galectin-9–containing exosomes in the plasma of NPC patients. (A) Captures with anti–HLA class II beads were performed on low-density vesicles (110-kg pellets) derived from human plasma samples. Initially, plasmas were collected from 2 NPC patients (NPC A and B; samples 2, 3, 4, and 5), 1 patient with a non-NPC head and neck carcinoma (non-NPC TA; samples 6 and 7), and 1 healthy donor (samples 8 and 9). In each case, beads coated with nonspecific Ig (NS Ig) were used as negative controls. In addition to plasma material, a protein extract from the C17-xenografted tumor was used as a positive control for Western blot detection (no. 1). Clinical and pathologic data on donor patients are provided in Tables S1 and S3. (B) Numerous bilamellar vesicles approximately 70 nm in diameter are visualized by HLA class II capture and electron microscopy when these procedures are applied to plasma vesicles from NPC patients but not control subjects. Scale bar represents 500 nm. In the inset, 2 vesicles at original magnification (× 4). (C) In parallel experiments, Western blot analysis revealed expression of galectin-9, CD9, CD63, and the α chain of the DR molecule in NPC plasma vesicles. In contrast, none of these proteins is detected when the same procedure is applied to control plasmas. Gp96 is only detected in the tumor extract. (D) Galectin-9–carrying exosomes are captured by anti–HLA class II beads from 4 additional samples of NPC plasmas (NPC C, D, E, and F) but not from 2 control subjects with non-NPC tumors (non-NPC TB and TC). Clinical and pathologic data on donor patients are in Tables S1 and S3. Consistent results were obtained for 11 NPC and 5 non-NPC additional plasma samples (see Tables S1, S2, and S4 and Figures S1 and S2).
Relative resistance to proteolysis and surface presentation of galectin-9 carried by NPC exosomes
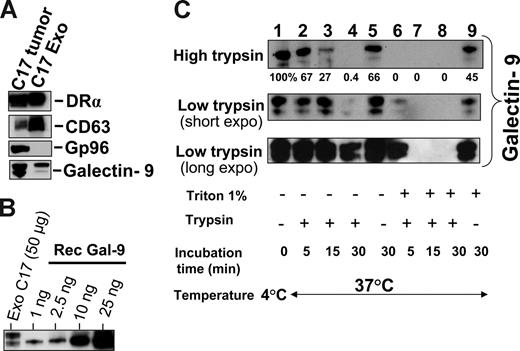
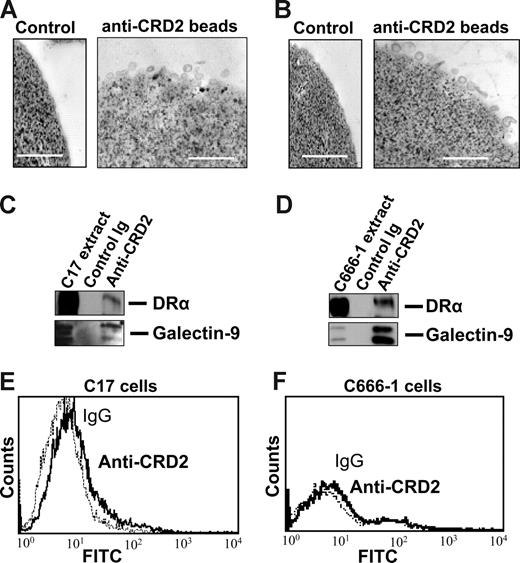
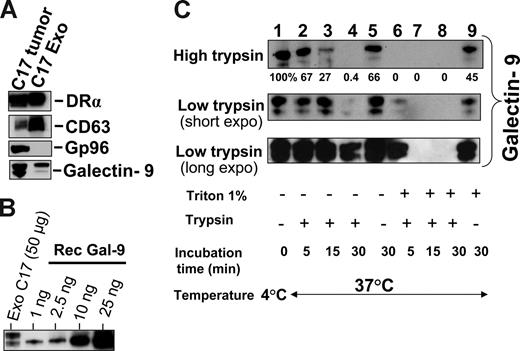
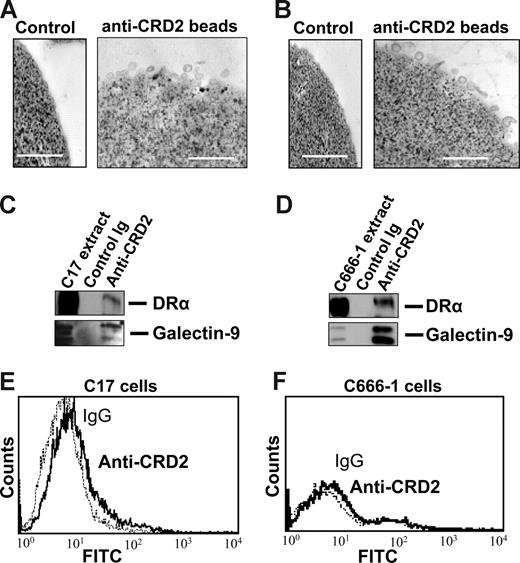
Subsequent experiments were performed on exosomes produced by NPC cells in vitro to investigate their characteristics as galectin-9 carriers. Exosomes produced by C17 NPC cells were purified on a sucrose gradient and assessed both qualitatively and quantitatively. As shown on Figure 3A, they contained HLA class II and CD63 molecules in the absence of detectable gp96. The amount of galectin-9 contained in the purified exosome fraction was assessed by Western blot analysis using recombinant galectin-9 as a reference (Figure 3B). Approximately 0.4 ng galectin-9 was secreted in exosomes by 1 million C17 cells within 48 hours. This amount is roughly equivalent to 1/10 000 of all exosomal proteins released by C17 cells. Like most proteins released in the extracellular space, galectin-9 is vulnerable to digestion by proteolytic enzymes.39,42 It was hypothesized that it could be protected from enzymatic degradation by its insertion into exosomes. Therefore, C17 exosomes were subjected to Triton lysis, to dissolve lipid membrane components, followed by trypsin treatment. Control exosomes were subjected to the same procedure, except with the absence of Triton. As shown in Figure 3C, trypsin degradation of galectin-9 was considerably enhanced by prior disruption of membrane exosomes, thus providing evidence of their protective role against proteolytic attack of galectin-9 (Figure 3C). Next we wanted to know whether galectin-9 carried by exosomes could behave as an agonist of the Tim-3 receptor. One important requirement was the presentation of galectin-9 CRDs at the surface of intact exosomes to allow its interaction with the Tim-3 receptor. To address this question, we used the 9M1-3 antibody directed to the FSTPAIPP epitope of the CRD2 domain of galectin-9. Magnetic beads coated either with the 9M1-3 antibody or with an antibody directed to the external part of HLA class II molecules were used in parallel to capture NPC exosomes. Capture efficiency was assessed by electron microscopy imaging and Western blot analysis of the DR-α chain eluted from beads. As shown in Figure 4, a significant amount of DR-α–positive exosomes were captured using the 9M1-3 antibody, providing evidence that the FSTPAIP epitope of the galectin-9 CRD2 is displayed at the surface of NPC exosomes. Interestingly, surface presentation of galectin-9 CRD2 was equivalent for the exosomes derived from the C666-1 and C17 NPC cells, although galectin-9 was absent on the plasma membrane of C666-1 cells and at a very low level on C17 cells.
Intact exosomes protect galectin-9 against trypsin digestion. (A) Western blot detection of DR-α, CD63, and galectin-9 in exosomes released by C17 cells and purified on a sucrose gradient. Gp96 is a cytoplasmic protein typically absent from exosomes. (Left side) C17 total extract. (Right side) Exosome proteins. (B) Galectin-9 contained in C17 exosomes is quantified in a Western blot analysis by comparison to a series of recombinant galectin-9 samples ranging from 1 to 25 ng (rec gal-9). On average, 2 to 5 ng galectin-9 are contained in 50 μg exosome proteins. (C) Trypsin digestion assay, the same amount of C17 exosomes was used for each condition (35 μg total exosome proteins). They were subjected either to Triton lysis or mock treatment before incubation with trypsin. Two different trypsin concentrations, 15 μg/mL (high trypsin) or 0.01 μg/mL (low trypsin), were used in distinct experiments. For samples treated with low amounts of trypsin, short and long exposures of the blotted membrane are presented. Controls are displayed in lane 1 (no Triton lysis, no trypsin addition, and incubation at 4°C), lane 5 (no Triton, no trypsin, 37°C), and lane 9 (Triton lysis without trypsin addition and incubation at 37°C). Triton lysis of exosomes greatly enhances trypsin digestion of exosome-bound galectin-9 as shown in lanes 6, 7, and 8 (Triton lysis followed by trypsin digestion) as compared with lanes 2, 3, and 4 (mock treatment followed by trypsin digestion). After Triton lysis, undigested galectin-9 remained detectable only for the shortest time of incubation (5 minutes) with the smaller concentration of trypsin (0.01 μg/mL; lane 6). In lane 9, a substantial decrease of galectin-9 is observed despite the absence of trypsin, suggesting an effect of endogenous proteolytic enzymes after Triton lysis.
Intact exosomes protect galectin-9 against trypsin digestion. (A) Western blot detection of DR-α, CD63, and galectin-9 in exosomes released by C17 cells and purified on a sucrose gradient. Gp96 is a cytoplasmic protein typically absent from exosomes. (Left side) C17 total extract. (Right side) Exosome proteins. (B) Galectin-9 contained in C17 exosomes is quantified in a Western blot analysis by comparison to a series of recombinant galectin-9 samples ranging from 1 to 25 ng (rec gal-9). On average, 2 to 5 ng galectin-9 are contained in 50 μg exosome proteins. (C) Trypsin digestion assay, the same amount of C17 exosomes was used for each condition (35 μg total exosome proteins). They were subjected either to Triton lysis or mock treatment before incubation with trypsin. Two different trypsin concentrations, 15 μg/mL (high trypsin) or 0.01 μg/mL (low trypsin), were used in distinct experiments. For samples treated with low amounts of trypsin, short and long exposures of the blotted membrane are presented. Controls are displayed in lane 1 (no Triton lysis, no trypsin addition, and incubation at 4°C), lane 5 (no Triton, no trypsin, 37°C), and lane 9 (Triton lysis without trypsin addition and incubation at 37°C). Triton lysis of exosomes greatly enhances trypsin digestion of exosome-bound galectin-9 as shown in lanes 6, 7, and 8 (Triton lysis followed by trypsin digestion) as compared with lanes 2, 3, and 4 (mock treatment followed by trypsin digestion). After Triton lysis, undigested galectin-9 remained detectable only for the shortest time of incubation (5 minutes) with the smaller concentration of trypsin (0.01 μg/mL; lane 6). In lane 9, a substantial decrease of galectin-9 is observed despite the absence of trypsin, suggesting an effect of endogenous proteolytic enzymes after Triton lysis.
Galectin-9 CRD2 is presented at the surface of NPC exosomes. Low-density vesicles (110-kg pellets) released by C17 or C666-1 NPC cells were incubated with magnetic beads coated with the 9M1-3 monoclonal antibody directed to the CRD2 of galectin-9. Beads coated with purified isotype-matched nonspecific Ig were used for control reactions. (Top panel) Numerous exosomes released by C17 (A) or C666-1 (B) are captured by anti-CRD2, but not control beads, and visualized by electron microscopy. (Middle panel) Western blot analysis detects the DR-α and galectin-9 proteins in C17 (C) and C666-1 (D) exosomes, whereas no similar proteins are recovered from control beads coated with irrelevant IgG. Positive controls are provided by total C17 and C666-1 cell extracts in the left lanes of panels C and D, respectively. (Bottom panel) A small amount of the galectin-9 CRD2 is detected at the surface of live C17 cells by flow cytometry (E), whereas it is absent at the surface of C666-1 (F) cells.
Galectin-9 CRD2 is presented at the surface of NPC exosomes. Low-density vesicles (110-kg pellets) released by C17 or C666-1 NPC cells were incubated with magnetic beads coated with the 9M1-3 monoclonal antibody directed to the CRD2 of galectin-9. Beads coated with purified isotype-matched nonspecific Ig were used for control reactions. (Top panel) Numerous exosomes released by C17 (A) or C666-1 (B) are captured by anti-CRD2, but not control beads, and visualized by electron microscopy. (Middle panel) Western blot analysis detects the DR-α and galectin-9 proteins in C17 (C) and C666-1 (D) exosomes, whereas no similar proteins are recovered from control beads coated with irrelevant IgG. Positive controls are provided by total C17 and C666-1 cell extracts in the left lanes of panels C and D, respectively. (Bottom panel) A small amount of the galectin-9 CRD2 is detected at the surface of live C17 cells by flow cytometry (E), whereas it is absent at the surface of C666-1 (F) cells.
Recombinant galectin-9 induces apoptosis in EBV-specific CD4+ T cells
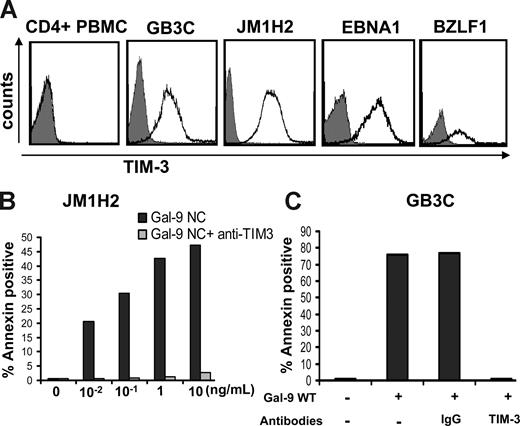
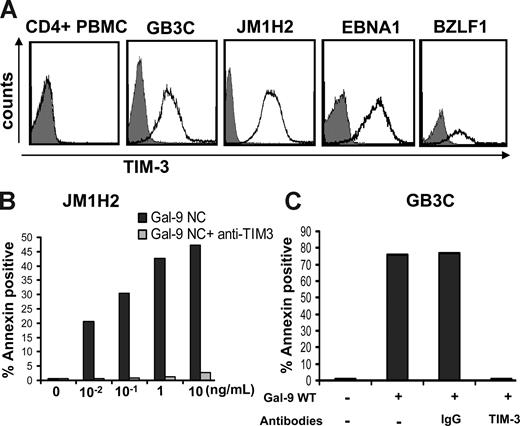
To directly assess the immunosuppressive functions of NPC exosomes, we used EBV-specific cytotoxic CD4+ T-cell clones as a model system. These clones were obtained from healthy EBV-carriers by in vitro stimulation of CD4+ cells with recombinant latent or lytic EBV proteins.31 Some of them have direct cytotoxic activity.31 The majority preferentially secrete Th1 cytokines.32 In preliminary experiments, most of the T-cell clones tested demonstrated intense expression of Tim-3 including GB3C specific for EBNA3C, JM1H2 specific for gp350, one clone specific for EBNA1, and one specific for BZLF1 (Figure 5A). Kinetics studies demonstrated a maximal expression of Tim-3 at day 6 after restimulation, precisely when they are at their highest level of anti-EBV activity (data not shown). This was in contrast with the absence of Tim-3 detection on CD4+ T cells sorted directly from PBMCs given by a healthy donor (Figure 5A). These observations are consistent with the notion that, among CD4+ T cells, Tim-3 is expressed exclusively on terminally differentiated Th1 cells.43 To find out whether the Tim-3 expression on the EBV-specific CD4+ T cells is of physiologic consequence, they were treated with recombinant galectin-9. Both GB3C and JM1H2 clones were found to be exquisitely sensitive to apoptosis induced by 2 distinct forms of recombinant galectin-9. Similar results were obtained with galectin-9 NC, which is a modified artificial species resistant to proteases, and the m-isoform of wild-type galectin-9 (Figure 5B,C).38,39 In both cases, the median-inhibitory dose (ID50) was close to 100 pg/mL, which is much lower than the 350 ng/mL described for PBMCs or human T-cell lymphotropic virus-1 (HTLV1)-infected cells in a previous publication.44
Recombinant galectin-9 induces apoptosis in EBV-specific CD4+ T cells. (A) Intense membrane expression of Tim-3 is detected on 4 EBV-specific CD4+ T cell clones of Th1 subtype but not on polyclonal CD4+ T cells sorted directly from PBMCs (CD4+ PBMC; gray curve, control staining). EBV-specific clones are directed against the EBV-proteins EBNA3C (GB3C), gp350 or BLLF1 (JM1H2), EBNA1, and BZLF1. (B) A recombinant modified form of galectin-9 with increased stability (Gal-9 NC) induces apoptosis in JM1H2 CD4+ T cells with an ID50 of approximately 100 pg/mL (apoptosis was assessed according to the percentage of annexin V–positive cells). (C) Recombinant wild-type galectin-9 (gal-9 WT) at a concentration of 100 pg/mL induces apoptosis in GB3C T cells. Induction of apoptosis is suppressed by preincubation of the T cells with 10 μg/mL blocking anti–Tim-3 monoclonal antibody, but not a control IgG.
Recombinant galectin-9 induces apoptosis in EBV-specific CD4+ T cells. (A) Intense membrane expression of Tim-3 is detected on 4 EBV-specific CD4+ T cell clones of Th1 subtype but not on polyclonal CD4+ T cells sorted directly from PBMCs (CD4+ PBMC; gray curve, control staining). EBV-specific clones are directed against the EBV-proteins EBNA3C (GB3C), gp350 or BLLF1 (JM1H2), EBNA1, and BZLF1. (B) A recombinant modified form of galectin-9 with increased stability (Gal-9 NC) induces apoptosis in JM1H2 CD4+ T cells with an ID50 of approximately 100 pg/mL (apoptosis was assessed according to the percentage of annexin V–positive cells). (C) Recombinant wild-type galectin-9 (gal-9 WT) at a concentration of 100 pg/mL induces apoptosis in GB3C T cells. Induction of apoptosis is suppressed by preincubation of the T cells with 10 μg/mL blocking anti–Tim-3 monoclonal antibody, but not a control IgG.
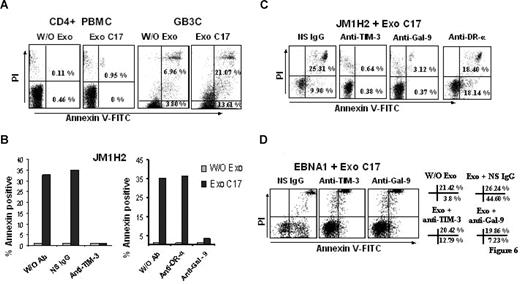
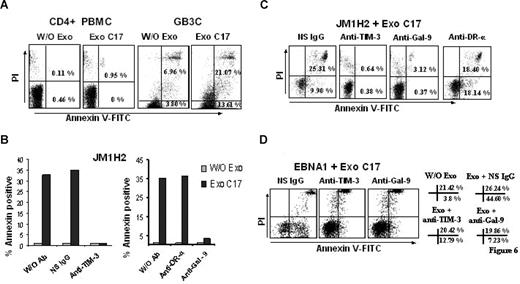
NPC exosomes induce apoptosis in EBV-specific CD4+ T cells through galectin-9/Tim-3 interaction
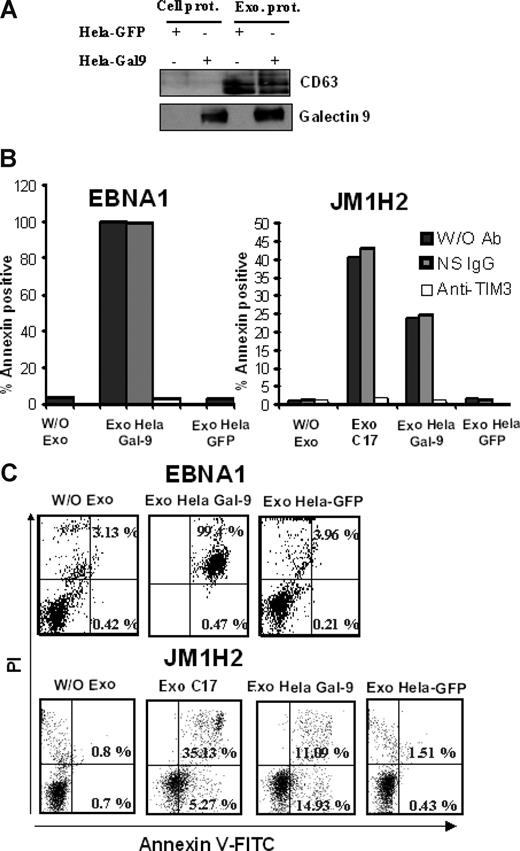
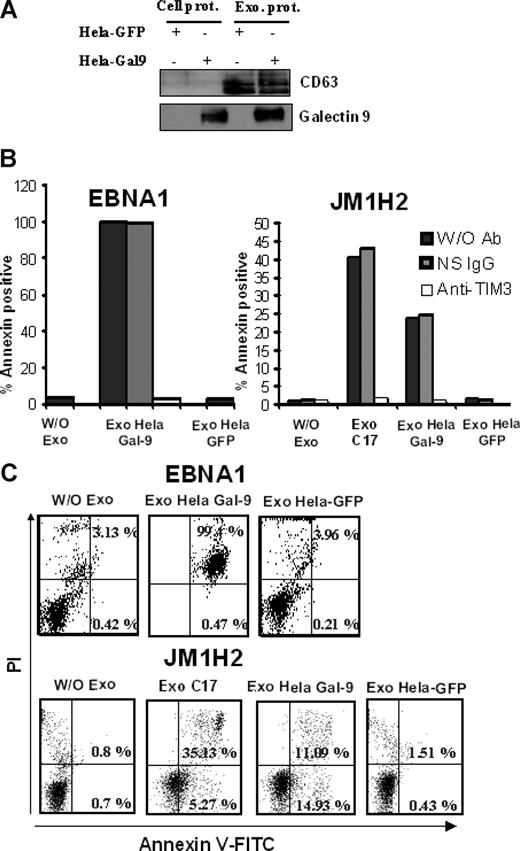
We then set out to address whether galectin-9 in exosomes would have a similar effect. As shown in Figure 6, purified exosomes from C17 NPC cells induced apoptosis in less than 5 hours in approximately 35% of the GB3C and JM1H2 CD4+ T cells (Figure 6A-C). When CD4+ cells from the EBNA1-specific clone were treated with C17 exosomes, apoptosis induction was even more massive (Figure 6D). A similar effect was obtained with the BZLF1-specific T cells (data not shown). All these responses were obtained with a concentration of exosome proteins of 100 μg/mL, equivalent to a galectin-9 concentration of 10 ng/mL (Figure 3B). In contrast, polyclonal CD4+ T cells sorted directly from PBMCs, which are negative for Tim-3, were almost insensitive to the same treatment (Figure 6A). Because exosomes are rich in membrane molecules, not all of which have been well characterized, it was important to confirm that the induction of apoptosis in the EBV-specific CD4+ T cells was the result of direct interaction between galectin-9 on the part of the exosomes and Tim-3 on the side of the T cells. Apoptosis induction by C17 exosomes was almost entirely inhibited by preincubation of target T cells with a blocking anti–Tim-3 antibody as well as by preincubation of NPC exosomes with the 9M1-3 antibody directed to galectin-9 CRD2 (Figure 6B-D). In control experiments, preincubation of NPC exosomes with an anti–DR-α antibody had no inhibitory effect. To further confirm the specific role of galectin-9 in apoptosis induction by NPC exosomes, CD4+ cells, specific for either gp350 (JM1H2) or EBNA1, were treated with exosomes from HeLa cells transfected with either a galectin-9 or a green fluorescent protein (GFP) expression construct. As expected, galectin-9 was only detectable in exosomes released by galectin-9–transfected Hela cells (Figure 7A). Apoptosis of EBNA1 and JM1H2 CD4+ cells was induced by galectin-9–positive exosomes, but not by exosomes from control Hela cells. It was inhibited by the anti–Tim-3 blocking antibody (Figure 7B,C).
NPC exosomes induce apoptosis in EBV-specific CD4+ T cells through galectin-9/Tim-3 interaction. (A) NPC exosomes (C17) containing galectin-9 induce apoptosis in EBV-specific CD4+ T cells (GB3C clone, anti-EBNA3C) without significant effects on polyclonal CD4+ T cells sorted directly from PBMCs (CD4+ PBMC). Tested cells were incubated for 5 hours with purified C17 exosomes at a final concentration of 100 μg/mL exosomal proteins (corresponding approximately to 10 ng/mL galectin-9; Exo C17). Control cells were incubated without exosomes (W/O Exo). Cell clusters visible at the top of the upper right portions of the graphs are related to nonspecific necrosis. (B,C) NPC exosomes (C17) induce apoptosis in EBV-specific CD4+ cells from the JM1H2 clone (anti-gp350). Apoptosis of CD4+ T cells is almost entirely suppressed by preincubating the T cells with a blocking anti–Tim-3 antibody or by preincubating the exosomes with the 9M1-3 antibody directed to the galectin-9 CRD2. In contrast, apoptosis induced by NPC exosomes is not prevented by a nonspecific Ig (NS IgG) or a monoclonal antibody directed to the extracellular portion of the DR-α chain (anti–DR-α, DA6.147). Flow cytometry graphs representative of some experiments summarized in panel B are displayed in panel C. PI-positive/annexin V–negative cells were consistently detected in the presence of anti–Tim-3 and anti–galectin-9 antibodies even in the absence of NPC exosomes, without satisfactory explanation. (D) NPC exosomes (C17) induce apoptosis in EBNA1-specific CD4+ cells. Percentages of annexin V–positive and annexin V/PI-positive cells are presented on the right side of the panel. Spontaneous cell death is more prevalent in this clone compared with GB3C and JM1H2: 3.8% annexin V and 21.42% annexin V/PI-positive cells in the absence of exosomes (W/O Exo). Nevertheless, the percentage of annexin V–positive cells is dramatically increased by the incubation with C17 exosomes, in the absence of blocking antibodies (Exo+ NS IgG). This effect is almost entirely reversed by anti–Tim-3 or anti–galectin-9 antibodies (Exo+ anti–Tim-3, Exo+ anti–Gal-9).
NPC exosomes induce apoptosis in EBV-specific CD4+ T cells through galectin-9/Tim-3 interaction. (A) NPC exosomes (C17) containing galectin-9 induce apoptosis in EBV-specific CD4+ T cells (GB3C clone, anti-EBNA3C) without significant effects on polyclonal CD4+ T cells sorted directly from PBMCs (CD4+ PBMC). Tested cells were incubated for 5 hours with purified C17 exosomes at a final concentration of 100 μg/mL exosomal proteins (corresponding approximately to 10 ng/mL galectin-9; Exo C17). Control cells were incubated without exosomes (W/O Exo). Cell clusters visible at the top of the upper right portions of the graphs are related to nonspecific necrosis. (B,C) NPC exosomes (C17) induce apoptosis in EBV-specific CD4+ cells from the JM1H2 clone (anti-gp350). Apoptosis of CD4+ T cells is almost entirely suppressed by preincubating the T cells with a blocking anti–Tim-3 antibody or by preincubating the exosomes with the 9M1-3 antibody directed to the galectin-9 CRD2. In contrast, apoptosis induced by NPC exosomes is not prevented by a nonspecific Ig (NS IgG) or a monoclonal antibody directed to the extracellular portion of the DR-α chain (anti–DR-α, DA6.147). Flow cytometry graphs representative of some experiments summarized in panel B are displayed in panel C. PI-positive/annexin V–negative cells were consistently detected in the presence of anti–Tim-3 and anti–galectin-9 antibodies even in the absence of NPC exosomes, without satisfactory explanation. (D) NPC exosomes (C17) induce apoptosis in EBNA1-specific CD4+ cells. Percentages of annexin V–positive and annexin V/PI-positive cells are presented on the right side of the panel. Spontaneous cell death is more prevalent in this clone compared with GB3C and JM1H2: 3.8% annexin V and 21.42% annexin V/PI-positive cells in the absence of exosomes (W/O Exo). Nevertheless, the percentage of annexin V–positive cells is dramatically increased by the incubation with C17 exosomes, in the absence of blocking antibodies (Exo+ NS IgG). This effect is almost entirely reversed by anti–Tim-3 or anti–galectin-9 antibodies (Exo+ anti–Tim-3, Exo+ anti–Gal-9).
Exosomes from HeLa cells induce apoptosis in EBV-specific CD4+ T cells only when they contain galectin-9. (A) Purified exosomes released by transfected HeLa cells expressing either GFP or galectin-9 (l-isoform) were analyzed by Western blot. The first 2 lanes on the left contain total extracts from cells transfected with GFP and galectin-9 (Cell prot). Exosome proteins are analyzed in the next 2 lanes (Exo prot). CD63, which is barely detectable in total cell extracts, is much more abundant in purified exosomes. In contrast to CD63, galectin-9 is detected in exosomes only when these are derived from cells transfected with the galectin-9 gene. (B,C) There is no induction of apoptosis in CD4+ T cells (EBNA1 and JM1H2 clones) treated with control HeLa exosomes (Exo Hela-GFP). In contrast, treatment with galectin-9–positive exosomes induces apoptosis in a large majority of the EBNA1-specific CD4+ cells (Exo Hela Gal-9). For JM1H2 cells (specific for gp350), the rate of apoptosis induced by Hela galectin-9 exosomes amount to 60% of the rate observed with C17 exosomes at the same concentration. It is entirely prevented by preincubation of target cells with the anti–Tim-3 antibody. Flow cytometry graphs representative of some experiments summarized in panel B are displayed in panel C.
Exosomes from HeLa cells induce apoptosis in EBV-specific CD4+ T cells only when they contain galectin-9. (A) Purified exosomes released by transfected HeLa cells expressing either GFP or galectin-9 (l-isoform) were analyzed by Western blot. The first 2 lanes on the left contain total extracts from cells transfected with GFP and galectin-9 (Cell prot). Exosome proteins are analyzed in the next 2 lanes (Exo prot). CD63, which is barely detectable in total cell extracts, is much more abundant in purified exosomes. In contrast to CD63, galectin-9 is detected in exosomes only when these are derived from cells transfected with the galectin-9 gene. (B,C) There is no induction of apoptosis in CD4+ T cells (EBNA1 and JM1H2 clones) treated with control HeLa exosomes (Exo Hela-GFP). In contrast, treatment with galectin-9–positive exosomes induces apoptosis in a large majority of the EBNA1-specific CD4+ cells (Exo Hela Gal-9). For JM1H2 cells (specific for gp350), the rate of apoptosis induced by Hela galectin-9 exosomes amount to 60% of the rate observed with C17 exosomes at the same concentration. It is entirely prevented by preincubation of target cells with the anti–Tim-3 antibody. Flow cytometry graphs representative of some experiments summarized in panel B are displayed in panel C.
Discussion
Galectin-9 plays a key role in the control of immune effector cells during various phases of immune responses in mammals. On one hand, it is a proinflammatory factor acting by stimulation of effector cells of innate immunity.36,45 For example, it induces maturation of monocyte-derived dendritic cells, and through this process, promotes Th1 immune responses.45 On the other hand, galectin-9 has a major role in the self-limitation of the immune response.43 While γ-interferon released by Th1 cells induces galectin-9 production by various cell types, galectin-9 creates a negative feedback by triggering apoptosis of mature Th1 cells through stimulation of their Tim-3 receptor.25 However, galectin-9, like other galectins, is not a cytokine stricto sensu. Although it is known to be secreted by various cell types, it has no signal sequence, and it cannot be exported into the extracellular space through the classical biosynthetic pathway.20 We have previously made one important step toward the solution of this paradox by demonstrating that NPC cells cultured in vitro release galectin-9 in the extracellular medium in association with exosomes.24 This suggested that galectin-9–carrying exosomes could be the missing link between galectin-9–producing cells and T cells expressing Tim-3. We have shown here that the same type of galectin-9–carrying exosomes is consistently and specifically detected in the blood of NPC patients. These observations confirm that galectin-9–carrying exosomes are produced by NPC cells in vivo and have enough stability for long-range diffusion from tumor interstitial fluids into the bloodstream. Some in vitro experiments confirm that exosomes can behave as efficient carriers of galectin-9: intact exosome structure ensures its protection against proteolytic enzymes without precluding interactions of its CRDs with external ligands (Figures 3,4).
This study was focused on NPC exosomes as carriers of galectin-9. Additional studies will be required to provide more details about other components of NPC exosomes with potentially important roles in host-tumor relationships. We have previously reported that cells from one NPC xenograft release exosomes in vitro containing both galectin-9 and the viral oncoprotein LMP1.24 While this work was in progress, Houali et al reported detection of LMP1-carrying exosomes in plasma samples obtained from NPC patients.46 Using our own approach based on immunomagnetic capture of HLA class II–positive exosomes, we have not been able to detect LMP1 in circulating exosomes from several NPC patients (data not shown). These results suggests that galectin-9 is more consistently and more abundantly associated with NPC exosomes than LMP1. Confirmation of this point will require further investigations especially on plasma samples collected at early stages of the disease. In this perspective, additional tools like galectin-9 ELISA systems with high sensitivity in human plasma samples will also be important. In another earlier report, Chan et al have published observations suggesting that apoptotic malignant cells release apoptotic bodies containing short viral DNA fragments that are detected in the peripheral blood of NPC patients.47 There is strong evidence that galectin-9–containing exosomes are distinct from these bodies. First, the production of NPC exosomes containing HLA class II molecules and galectin-9 is not dependent on apoptosis (Figure S3). Moreover NPC plasma vesicles captured on class II beads contain the CD9 protein that was reported to be excluded from apoptotic bodies48 (Figure 2C).
EBV-specific CD4+ T-cell clones have proven to be remarkable tools to investigate the role of galectin-9–positive exosomes as agonists of the Tim-3 receptor. These clones exhibited intense expression of Tim-3 and were exquisitely sensitive to apoptosis induction by recombinant galectin-9, with an ID50 as low as 100 pg/mL. The same apoptotic response was induced by NPC exosomes. Using blocking antibodies, we could formally demonstrate that apoptosis induction was mediated by the contact of galectin-9 CRDs with the Tim-3 receptor presented at the surface of exosomes and target T cells, respectively. A similar apoptotic response was induced by exosomes derived from HeLa cells transfected with the galectin-9 but not the GFP gene, confirming the key role of galectin-9 in this process. Other CD4+ T cells specific for non-EBV antigens are probably sensitive to galectin-9–positive exosomes, especially in conditions inducing expression of the Tim-3 receptor.
Although relatively neglected for a long time, EBV-specific CD4+ T cells increasingly appear to play a key role in the immune response against EBV-infected cells in healthy carriers as well as in patients affected by posttransplant lymphomas.32,49,50 This probably also applies to NPC and speaks strongly for a role of galectin-9–carrying exosomes in NPC immune evasion. Several studies have shown that the repertoire of the EBV-specific CD4+ and CD8+ T-cell memory is only mildly altered in NPC patients by comparison with healthy subjects.18,51,–53 Moreover, there are indications that precursors of EBV-specific cytotoxic T cells are abundant in the tumor tissue.52 Although they are functionally inactive in situ, they can be reactivated ex vivo.18 These observations suggest that inhibitory factors contained in the tumor microenvironment either block cytotoxic T-cell maturation or prevent their activation/proliferation, potentially by eliminating a subgroup of Th1 cells that are required to provide T-cell help. Moreover, Lau et al have reported the presence of large numbers of regulatory T cells (CD4+CD25+FoxP3+) in tumors and peripheral blood of NPC patients (in addition to a decrease in the absolute count of peripheral CD4+ T cells).54 Because pathways involving galectin-9 and or Tim-3 participate in the generation of CD4+CD25+ regulatory T cells, one can speculate that galectin-9 exosomes could contribute to their expansion in tumors and peripheral blood of NPC patients.55,56 Some data recently obtained in our laboratory support this hypothesis (data not shown). The possible influence of NPC exosomes on Th1 and regulatory T cells is likely to become an important topic in adoptive immunotherapy. This therapeutic modality has given promising results in early trials.57 As with other EBV-associated diseases, CD4+ T cells are expected to play a key role in the antiviral immune response.49 Monoclonal antibodies neutralizing the Tim-3–binding domain of galectin-9, as was done with the 9M1-3 antibody in this study, might be useful to enhance long-term survival of CD4+ EBV-specific T cells infused into NPC patients.
Beyond the problem of host-tumor relationships in NPC patients, our findings have important implications for the understanding of general aspects of immune regulations in health and disease. There are several cell types producing large amounts of galectin-9 in various physiologic or pathologic contexts, including follicle-associated epithelial cells, postnatal thymus in healthy subjects, and astrocytes of brain white matter in plaques of multiple sclerosis.20,21,36 In the future, it will be important to know whether these various cell types can produce exosomes carrying galectin-9 and whether these exosomes can behave as Tim-3 agonists on CD4+ T cells as well as cells of innate immunity, for example, dendritic and natural killer (NK) cells.36
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank David Anderson and Vijay Kuchroo (Harvard Medical School, Boston, MA) for providing the 2E2 antibody, Laurence Zitvogel and Sophie Viaud (Institut Gustave Roussy, Villejuif, France) for helpful advice and technical assistance in exosome purification, and Xiaohui Wang and Tadamasa Ooka (Faculté de Médecine Laennec, Lyon, France) for helpful discussions.
This study was supported by grants from the Ligue Nationale Contre le Cancer (Comité du Val de Marne), the Agence Nationale de la Recherche (EBV-inter), and the Herpesvirus and Cancer Network.
Authorship
Contribution: J.K. carried out xenografts, cell cultures, and exosome purification and was involved in most other studies; T.N. performed ELISA detection of galectin-9 in mouse plasma samples; C.P.-D. was involved in exosome purification and galectin-9 detection by Western blot analysis and flow cytometry. A.R., D.A., and J.M. produced CD4+ T-cell clones and were involved in assessment of apoptosis by flow cytometry; S.S. made electron microscopy observations. E.R. contributed important reagents related to tetraspanins; M.H. contributed essential reagents related to galectin-9; F.G., J.G., and S.L.M. collected plasma samples from NPC and non-NPC patients; J.M. participated in the design of the study and edited the manuscript; and P.B. conceived the study and its design and drafted the manuscript. All authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Busson, UMR 8126, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94805 Villejuif, France; e-mail: pbusson@igr.fr.