Pronounced thrombocytosis can result from decreased expression of the thrombopoietin (Tpo) receptor, Mpl, in late megakaryocytes and platelets. In this issue of Blood, Lannutti and colleagues1 and Tiedt and colleagues2 generate transgenic mice expressing Mpl and use these models to investigate the relationships between Mpl, expression and platelet production. In these mouse models, a 2 kb proximal Mpl promoter was used to express Mpl cDNA on an Mpl knock-out background. Mpl knock-out mice are viable but exhibit severe thrombocytopenia, as well as defects in hematopoietic stem cells (HSCs) and early myeloid progenitors,3-6 consistent with key roles of Mpl at multiple stages of hematopoiesis.7 Tiedt et al used the 2 kb upstream promoter sequence of Mpl to engineer a transgenic construct that drives expression of Mpl, as this promoter has been previously shown to target expression of exogenous proteins, such as placental alkaline phosphatase, to megakaryocytes and platelets.8 In transgenic mice obtained on the background of the Mpl knock-out mice, expression of Mpl mRNA appeared normal in early megakaryocytes, but drastically lower in late megakaryocytes and platelets. Strikingly, these mice exhibited thrombocytosis. A dominant effect of the transgene was ruled out since thrombocytosis disappeared when one endogenous copy of Mpl was expressed in addition to the transgene. It seemed possible that the thrombocytosis reflected the absence of the alternatively spliced version of Mpl (Mpl-truncated), which down-modulates Mpl function. However, this was not the case since crossing the transgenic mice with Mpl Δ60 knock-in mice, which cannot generate the alternatively spliced Mpl-truncated form,9 also prevented thrombocytosis.
What can be the mechanism that explains thrombocytosis in the Mpl transgenic mice? Based on the well-established role of platelet Mpl in clearing circulating Tpo,10,11 a model was suggested where lack of uptake and destruction of Tpo by the Mpl-deficient platelets led to high serum Tpo levels. As a result, early megakaryocyte progenitors expanded to a level where they themselves would suffice to clear excess serum Tpo. In this scenario, higher serum Tpo levels would be expected in transgenic animals. However, the Mpl transgenic mice of Tiedt et al had normal Tpo serum levels. These data could either point to a perfect compensation by the increased early megakaryocyte population, or suggest that some yet-to-be-understood negative signaling occurs via Mpl at the late megakaryocyte stage. To settle this question, ideally one would have to redo the experiment and express lower levels of Mpl in the same transgenic model, which should then lead to increased serum Tpo levels.
Lannutti et al performed exactly this experiment in a separate and very complementary study. The same upstream 2 kb promoter was used to drive the Mpl transgene, but a different 3′-UTR and vector sequences were employed for the transgene, resulting in a lower level of in vivo Mpl expression as compared with the Tiedt et al study. These mice also developed thrombocytosis, albeit milder, with decreased Mpl expression in megakaryocytes and platelets, and a clear increase in the number of megakaryocytes in the marrow. Importantly, mice exhibited increased serum Tpo levels, supporting the notion that an altered Tpo clearance mechanism might be a major contributor to thrombocytosis. Nevertheless, given that only the lower expressing Mpl transgenic mice exhibited high serum Tpo levels, and that Mpl knock-out mice are known to exhibit high Tpo levels,3 other mechanisms might still contribute to thrombocytosis in the transgenic mice. Interestingly, Lannutti et al showed that the 2 kb Mpl promoter does not work properly at the HSC level, as suggested by diminished reconstitution ability of transgenic bone marrow cells. Taken together, both studies establish that the 2 kb Mpl upstream sequences do not suffice to provide physiologic expression levels of Mpl in HSCs, late megakaryocytes, and platelets.
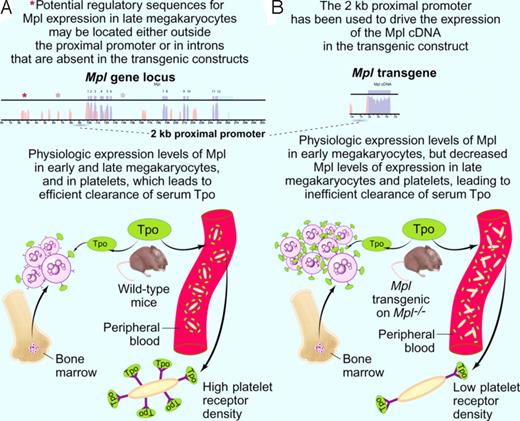
Model explaining the pronounced thrombocytosis detected in Mpl transgenic mice on an Mpl knock-out background (modified after Figure 7 of Tiedt et al2 ). (A top panel) A 2-kb proximal promoter (dashed line) was previously shown to drive expression of Tpo receptor (Mpl) in early megakaryocytes.7 Other elements (depicted as asterisks) regulating cell-type specific expression of the Mpl gene (exons in blue, introns in pink) might be located at distant sites from the proximal promoter or in the introns of the Mpl gene itself. (A botton panel) Clearance of the ligand Tpo (depicted in green) is physiologically accomplished by binding to Mpl receptor molecules (depicted as Y) on circulating platelets and on bone marrow megakaryocytes. Upon binding to Mpl, the ligand Tpo is internalized and degraded. (B top panel) The 2-kb Mpl proximal promoter (dashed line) was used to drive the expression of a transgene containing the cDNA (blue) coding for Mpl. (B bottom panel) Mpl transgenic mice on an Mpl knockout background show an increased number of bone marrow megakaryocytes and circulating platelets. While early megakaryocytes in the marrow exhibit Mpl receptor (depicted as Y) densities comparable to the wild-type mice, circulating platelets exhibit significantly lower surface Mpl densities (insert). Platelet clearance to Tpo is therefore diminished, and the higher Tpo levels are binding to the Mpl on early megakaryocytes in the marrows. This results in an expansion of this population, to the point where internalization and degradation to Tpo compensates for the decreased platelet clearance of Tpo. The expanded megakaryocyte population generates an increased number of circulating platelets. Professional illustration by Paulette Dennis.
Model explaining the pronounced thrombocytosis detected in Mpl transgenic mice on an Mpl knock-out background (modified after Figure 7 of Tiedt et al2 ). (A top panel) A 2-kb proximal promoter (dashed line) was previously shown to drive expression of Tpo receptor (Mpl) in early megakaryocytes.7 Other elements (depicted as asterisks) regulating cell-type specific expression of the Mpl gene (exons in blue, introns in pink) might be located at distant sites from the proximal promoter or in the introns of the Mpl gene itself. (A botton panel) Clearance of the ligand Tpo (depicted in green) is physiologically accomplished by binding to Mpl receptor molecules (depicted as Y) on circulating platelets and on bone marrow megakaryocytes. Upon binding to Mpl, the ligand Tpo is internalized and degraded. (B top panel) The 2-kb Mpl proximal promoter (dashed line) was used to drive the expression of a transgene containing the cDNA (blue) coding for Mpl. (B bottom panel) Mpl transgenic mice on an Mpl knockout background show an increased number of bone marrow megakaryocytes and circulating platelets. While early megakaryocytes in the marrow exhibit Mpl receptor (depicted as Y) densities comparable to the wild-type mice, circulating platelets exhibit significantly lower surface Mpl densities (insert). Platelet clearance to Tpo is therefore diminished, and the higher Tpo levels are binding to the Mpl on early megakaryocytes in the marrows. This results in an expansion of this population, to the point where internalization and degradation to Tpo compensates for the decreased platelet clearance of Tpo. The expanded megakaryocyte population generates an increased number of circulating platelets. Professional illustration by Paulette Dennis.
What are the implications of these results? On a fundamental level, a search can begin for those regulatory genomic sequences that are needed for physiologic Mpl expression, either away from the upstream promoter or within the Mpl gene itself. It would be interesting to test whether expression of the original placental alkaline phosphatase transgenic construct driven by the same 2 kb promoter7 is also low at late megakaryocyte and platelet stage. In patients, thrombocytosis might be induced by mutations or epigenetic changes that diminish Mpl expression in megakaryocytes and platelets. The down-modulation of megakaryocyte and platelet Mpl in myeloproliferative neoplasm (MPN) patients12 might in itself contribute to thrombocytosis. Careful examination of serum Tpo in MPN patients would be warranted, especially since “high Tpo” mice develop myelofibrosis.13 Finally, in congenital amegakaryocytic thrombocytopenia, gene therapy using an Mpl transgene driven by the 2 kb Mpl proximal promoter is unlikely to lead to a physiologic pattern of Mpl expression.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■