To the editor:
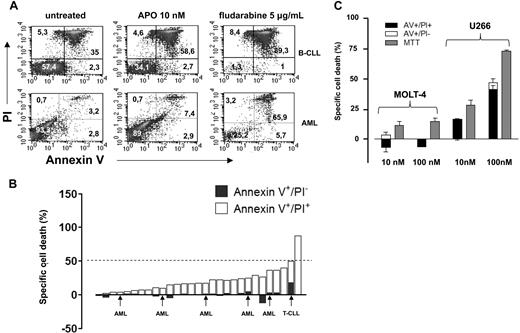
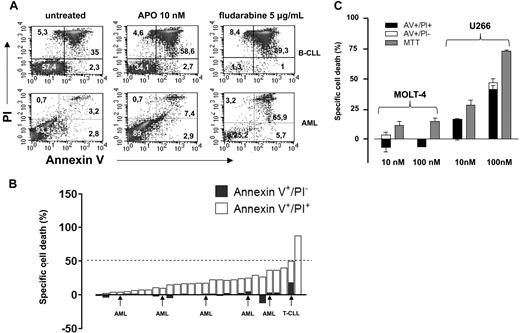
Nahimana and coworkers have recently reported that the nicotinamide phosphoribosyltransferase (NAMPT) inhibitor APO866 elicited massive cell death in primary leukemia cells and in numerous leukemia/lymphoma cell lines.1 In particular, in 32 primary leukemias (including 12 B-cell chronic lymphocytic leukemias [B-CLLs]) these authors found that a 96 hour-exposure to 10 nM APO866 resulted in a median “fraction of dead cells” (fdc, annexin-V [AV]+ cells) of 97%. Moreover, APO866 EC50, as measured by MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetratolium bromide] colorimetric assay in 45 established hematologic cancer cell lines ranged between 0.09 and 27.2 nM. Similar experiments were performed by our group on 29 primary leukemia cell samples (23 B-CLLs, 1 T-cell chronic lymphocytic leukemia [T-CLL], and 5 acute myeloid leukemias [AMLs]). We determined cell viability by AV–propidium iodide (PI) staining and flow cytometry. Specific cell death (scd) was calculated with the formula: (xt − xm/100 − xm) × 100, where xt was the number of AV+ cells in response to a given APO866 concentration and xm were the AV+ elements among the untreated cells. In our hands, susceptibility to APO866 among primary leukemia cells was heterogeneous (Figure 1A,B). Most cases exhibited a minor decrease in cell viability after a 96-hour exposure to APO866, while only in 1 B-CLL sample the scd was 89%. In almost all of our titration experiments (1 nM-1 μM), APO866 EC50 was not even reached. Finally, prolonging the exposure to APO866 (up to 10 days) did not substantially increase the efficacy of APO866 (data not shown). The observed failure to induce massive apoptosis by APO866 was not due to poor NAMPT inhibition by our compound batch as after a 48-hour treatment intracellular NAD+ levels were typically reduced by more than 90% compared with the control cells in primary leukemias and in leukemia cell lines as detected by high performance liquid chromatography (HPLC).2 We also evaluated the response of various established cancer cell lines to APO866. Here, our results were similar to those of Nahimana et al except for the MOLT4 (T-acute lymphoblastic leukemia) cells that we found to be resistant to APO866 for doses up to 100 nM (Figure 1C). Importantly, in agreement with Hasmann and Schemainda,3 we observed that the MTT method tends to anticipate and to overestimate APO866 efficacy compared with the AV/PI flow cytometric assay. We ascribe this discrepancy to the NAD+ dependent nature of tetrazolium-based assays, like MTT, XTT [2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] and WST-1 (4-[3-(4-ldophenyl)-2-(4-Nitrophenyl)-2H-5-Tetrazolio]-1,3-Benzene Disulfonate).3,4 It is therefore conceivable that, by not considering this methodologic drawback, Nahimana et al underestimated, at least in some cell lines, APO866 EC50. In conclusion, we wonder if Nahimana et al reported their data on primary cell viability as an absolute value or as scd (ie, normalized to the untreated cells), because in our experience frozen hematologic cancer cells may exhibit high basal death rates. Finally, we suggest that for viability experiments the authors use a more reliable test, independent of intracellular NAD+ levels such as sulforhodamine B.5 We believe APO866 is a promising agent in the treatment of hematologic malignancies. However, further studies are warranted to understand its mechanisms of action, as well as its potential in combination treatments.
Cytotoxic activity of APO866 in primary leukemia cells, MOLT4, and U266 cells. (A) Primary B-CLL (> 80% CD19+ cells, n = 23), T-CLL (> 90% CD3, CD2, CD5, CD7+, n = 1), and AML (> 70% blasts, n = 5) cells were isolated from peripheral blood (PB) samples by density gradient centrifugation. 106 cells/well were seeded in 24-well plates and cultured with or without 10 nM APO866 or 5 μg/mL fludarabine. Ninety-six hours later cells were harvested, washed, stained with fluorescein isothiocyanate (FITC)–conjugated annexin-V and PI, and analyzed by flow cytometry. (B) Quantification of early apoptotic (annexin-V+/PI−) and late apoptotic/necrotic (annexin-V+/PI+) cells on exposure to 10 nM APO866. The percentage of early apoptotic cells is shown as ▬ while late apoptotic cells are shown as ▭. (C) 3 × 104 MOLT4 and U266 cells/well were plated in 96-well plates and incubated with or without APO866 at the indicated concentrations for 96 hours. Thereafter, cell death was quantified by MTT colorimetric assay, and by AV/PI staining and flow cytometry. Results are presented as means of triplicate wells and SD.
Cytotoxic activity of APO866 in primary leukemia cells, MOLT4, and U266 cells. (A) Primary B-CLL (> 80% CD19+ cells, n = 23), T-CLL (> 90% CD3, CD2, CD5, CD7+, n = 1), and AML (> 70% blasts, n = 5) cells were isolated from peripheral blood (PB) samples by density gradient centrifugation. 106 cells/well were seeded in 24-well plates and cultured with or without 10 nM APO866 or 5 μg/mL fludarabine. Ninety-six hours later cells were harvested, washed, stained with fluorescein isothiocyanate (FITC)–conjugated annexin-V and PI, and analyzed by flow cytometry. (B) Quantification of early apoptotic (annexin-V+/PI−) and late apoptotic/necrotic (annexin-V+/PI+) cells on exposure to 10 nM APO866. The percentage of early apoptotic cells is shown as ▬ while late apoptotic cells are shown as ▭. (C) 3 × 104 MOLT4 and U266 cells/well were plated in 96-well plates and incubated with or without APO866 at the indicated concentrations for 96 hours. Thereafter, cell death was quantified by MTT colorimetric assay, and by AV/PI staining and flow cytometry. Results are presented as means of triplicate wells and SD.
Authorship
Acknowledgments: This work was supported by AIL Sezione Liguria, AIRC (Genova, Italy; A.N.), and the University of Genoa, Genoa, Italy.
Contribution: M.C., G.Z., and A.N. conceptualized and designed research, performed research, analyzed data, and wrote the paper; S.B. and F. Fruscione performed research and analyzed data. E.M., A.G., I.R., and G.C. analyzed data; and S.C., F.O., I.P., A.C., F. Ferrando, R.G., M.G., A.B., and F.P. provided data and samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Alessio Nencioni, Department of Internal Medicine, University of Genoa, V le Benedetto XV 6, 16132 Genoa, Italy; e-mail: A.Nencioni@gmx.net.