The immune response in heparin-induced thrombocytopenia (HIT) is puzzling: heparin-naive patients can develop IgG antibodies and clinical HIT as early as day 5, and evidence for an anamnestic response on heparin reexposure is lacking. We assessed daily serum samples by anti-PF4/heparin enzyme-immunoassay (EIA) in patients receiving heparin thromboprophylaxis. Of 435 patients, 56.1% showed an increase in EIA optical density (OD) of more than or equal to 15%, with more than 90% starting between days 4 and 14. After reaching maximum reactivity by days 10 to 12, ODs declined despite heparin continuation, including in 2 patients with clinical HIT. Individual IgG/A/M classes showed identical time of onset (median, day 6). Most (58.7%) antibody-positive patients developed all 3 Ig classes; only 11.3% lacked IgG response. IgG/A/M increase usually occurred simultaneously (± 1 day) with no general tendency for IgM precedence. Consistent with the transient immune response, none of the IgG-EIA–positive (OD > 0.5) patients at discharge developed clinically evident thrombosis during extended low-molecular-weight heparin thromboprophylaxis. The rapid onset of the anti-PF4/heparin immune response, its transience, and the simultaneous appearance of antibodies of different classes with no IgM precedence suggest short-term activation of B cells that have previously undergone Ig-class switching even without previous pharmacologic heparin exposure.
Introduction
Heparin-induced thrombocytopenia (HIT) is one of the most important adverse drug reactions.1 It is usually caused by an immune response against platelet factor 4 (PF4)/heparin complexes.2,3 Typically, the anti-PF4/heparin antibodies observed in clinical HIT are of the immunoglobulin G (IgG) class.4 The central role of IgG class antibodies results from their ability to activate platelets through their Fc receptors.5,6
Many of the major issues of HIT have been elucidated. For example, the different clinical presentations of HIT are well described, there is increasing knowledge on how to perform and interpret laboratory assays for detecting HIT antibodies, and several treatment options for patients affected by HIT are available.7 Despite these advances, the immunobiology of HIT remains poorly understood. Indeed, there are certain well-known clinical features of HIT that differ from the patterns expected of a “classic” immune reaction. (1) Patients with clinical HIT often develop a platelet count decrease that begins as early as day 5 after commencing heparin treatment, even in the absence of any known previous heparin exposure8,9 ; these temporal features are atypical for a primary immune response, in which IgM antibodies should be initially generated and detectable after approximately 4 to 5 days, with IgG antibodies after several days later.10 (2) Features consistent with an “anamnestic” (secondary) immune response, that is, a stronger and more rapid immune response on heparin reexposure in a patient with a previous history of HIT, have not generally been observed in HIT8,11 ; rather, the situation of a rapid decline in platelet count among reexposed patients has been linked to the presence of already circulating HIT antibodies that resulted from a recent exposure to heparin.8 (3) Also unusual for a secondary immune response, antibody titers decline quickly in patients who are recovering from clinical HIT; antibodies typically become undetectable at a median of 50 to 80 days after the episode of HIT, depending on the test used to detect HIT antibodies.8 This differs from most other situations of blood cell alloimmunization or drug-dependent antibody formation, in which antibodies often persist for several months or years (eg, anti-D, quinine-dependent platelet-reactive antibodies)12,13 or immunization against viruses induced through vaccination.14
Understanding the temporal profile of the immune response in HIT may shed light on the nature of its underlying immunologic mechanisms. We used sera and clinical information systematically obtained in clinical studies to address the following questions:
Is the temporal profile of the day of onset of the immune response against PF4/heparin, as assessed by antibody reactivity in a PF4/heparin enzyme immunoassay (EIA), consistent with the features of a “primary” immune response (ie, relatively slow onset with a pronounced initial IgM component and a delayed and weaker IgG response) or a “secondary” immune response (a relatively more rapid onset with a more pronounced IgG component)?
Are there differences in the temporal profile of antibody response between patients showing a strong (potentially secondary) immune response, as judged by greater reactivity in the EIA, compared with those showing a moderate or weak immune response? This is a relevant issue because there is evidence that patients who form higher-magnitude immune responses, as judged by optical density (OD) levels in the EIA, are at greater risk of HIT and its thrombotic complications15,,–18 :
What is the natural course of anti-PF4/heparin antibody patterns if heparin is continued?
We addressed the aforementioned questions by studying serial blood samples obtained in a prospective clinical trial from more than 400 patients during thromboprophylaxis with heparin (study I). We also were able to document serial changes in antibody levels (study II), including in 2 patients with clinical HIT in whom heparin had been continued. Finally, we enrolled patients after major orthopedic surgery in whom anti-PF4/heparin antibody status could be determined at the time that extended thromboprophylaxis with low-molecular-weight heparin (LMWH) was commenced after hospital discharge (study III). This last study allowed us to address whether extended thrombosis prophylaxis with LMWH is a risk factor for new thrombosis in otherwise asymptomatic patients who have developed anti-PF4/heparin antibodies at the time of hospital discharge as a result of earlier in-hospital thrombosis prophylaxis with heparin or LMWH.
Methods
Study of temporal seroconversion profile of the anti-PF4/heparin immune response
In a previously unpublished prospective observational study on the incidence of HIT during thromboprophylaxis in trauma and orthopedic surgery, we obtained daily blood samples in 435 patients who underwent thrombosis prophylaxis, of whom 190 patients received unfractionated heparin (UFH) alone, 227 patients received UFH for 2 to 5 days followed by LMWH, 11 patients received LMWH exclusively, and 7 patients underwent more than one switch between different types of heparin. All samples were tested (baseline through end of heparin treatment) by an “in-house” combined anti-PF4/heparin IgG/A/M EIA, as described.19 The first day of heparin administration was designated as day 0.
All individual patterns of changes in daily OD values of the IgG/A/M EIA were analyzed by a statistical algorithm for changes in OD over the baseline value, in which an increase of ODs (defined as a minimum threshold of 15%) was present in at least 2 consecutive days. For further control, we plotted all identified individual curves of the Ig OD reactivity for each sample. Only samples were included in which 3 independent investigators agreed that the OD values clearly increased to a stable plateau for several days. Simple variations in EIA results were not included. Specifically, we identified the day before a first change in OD by visual inspection of individual curves. We then checked numerically whether the change occurring after this day consistently exceeded 15% of the baseline value. We analyzed the seroconversions according to the absolute magnitude of increase in OD values (ΔOD), grouped as follows: less than 0.1, 0.1 to less than 0.5, 0.5 to less than 1.0, and more than 1.0. Onset of the immune response was defined as the day in which the first increase that met the threshold criterion in the OD was measurable, independent of the OD value that ultimately was attained.
We then tested those positive sera (with sufficient material for analysis) by anti-PF4/heparin EIA, testing for the individual Ig classes (IgG, IgM, and IgA), as described.19 For these 3 individual Ig classes, we analyzed the time of onset in relation to the magnitude of the change in OD. We also compared the time of onset for these 3 different Ig classes.
Observations on antibody waning or disappearance despite continued heparin treatment
To assess the pattern of antibody reactivity in immunized patients despite maintaining heparin, we identified all patients enrolled in study I who received heparin for at least 12 days and compared the peak OD between day 7 and 12 with the OD at the last day of subsequent heparin administration (median, day 17) by paired t test. The decrease in OD values that we observed despite maintaining heparin in asymptomatic patients (presented subsequently in Figure 2 and “Anti-PF4/heparin EIAs determined separately for the Ig classes, IgG, IgM, and IgA”) raised the question as to whether this phenomenon of antibody level waning despite continued heparin administration might also occur in patients with clinically evident HIT. From the files at the McMaster University Platelet Immunology Laboratory, we identified 2 patients who had clinical and laboratory confirmed HIT, but in whom the diagnosis of HIT had been delayed (and thus heparin continued), and in whom by chance serial blood samples for HIT antibody testing were available for up to 8 and 13 days, respectively, after the onset of the HIT immune response. (These patients were not included in studies I, II, or III.) Patient heat-inactivated serum or plasma was tested in the serotonin release assay (SRA), as described.20 All samples regarded as testing positive exhibited significantly increased release compared with background (≥ 20%), as well as inhibition of platelet activation at high heparin concentrations (100 U/mL UFH) and also in the presence of Fc receptor-blocking monoclonal antibody, IV.3. Patient sera or plasma was additionally tested in 2 EIAs for detection of anti-PF4/H antibodies: EIA-GTI (a commercial EIA that detects IgG, IgA, and IgM class antibodies against PF4/polyvinylsulfonate) and an “in-house” EIA-IgG that detects antibodies of IgG class against PF4/H complexes.21 All samples were tested at the recommended 1/50 dilution.
Study on clinical relevance of asymptomatic seroconversion for PF4/heparin antibodies for long-term outpatient thrombosis prophylaxis with LMWH
In another prospective study, patients were tested routinely for HIT antibodies after hip or knee replacement surgery at day 16 (before discharge from hospital) by anti-PF4/heparin EIA19 and by the heparin-induced platelet activation (HIPA) test.22 These patients had been enrolled in a prospective before-after study, which has been reported previously.23 At the end of the in-patient phase of the study, all patients who had not developed clinical HIT or thrombosis were asked to participate in an additional (outpatient) observational study. Patients and investigators were blinded for the screening result of HIT antibodies (blood sample obtained at time of discharge from hospital). Patients who received extended outpatient thrombosis prophylaxis after day 16 by LMWH were followed by a questionnaire and telephone interview for new clinically manifest thrombosis or death within the next 3 months. The LMWH was prescribed by the treating family physician according to his or her standard practice and included any of 4 LMWH preparations approved in Germany for postoperative thrombosis prophylaxis (enoxaparin, dalteparin, certoparin, or nadroparin). Based on the observation that PF4/heparin antibodies wane despite continuing heparin (see “Observations on antibody waning or disappearance despite continued heparin treatment”), we hypothesized that the presence of antibodies at day 16 in patients without clinical evidence of HIT would not subsequently develop more thrombotic events or other features of HIT despite continuing outpatient LMWH thromboprophylaxis, in comparison with antibody-negative controls.
Statistical methods
Statistical results for categorical variables are presented as absolute numbers of cases and percentages. Mean values, medians, and SDs are reported as descriptive summary statistics for continuous data. Differences between means or medians were tested using nonparametric techniques (Mann-Whitney, Kruskal-Wallis, and Wilcoxon tests). The Fisher exact test was used for testing differences between proportions.
Ethics
All studies involving patients were approved by the respective ethics committee, and patient informed consent was obtained in accordance with the Declaration of Helsinki. For the 2 patients reported in study II, the report satisfied the ethical requirements of the Research Ethics Board of Hamilton Health Sciences/Faculty of Health Sciences, McMaster University (Hamilton, ON). All samples were tested retrospectively on archived serum samples.
Results
Study on temporal seroconversion profile of the anti-PF4/heparin immune response
Anti-PF4/heparin IgG/IgA/IgM combined EIA.
Of the 435 patients studied, 215 (49.4%) were female. The mean age of the patients was 53.8 years (range, 12-96 years). Patients were treated with heparin for a median of 11 days (range, 3-53 days). Daily blood samples were taken during the period of heparin application.
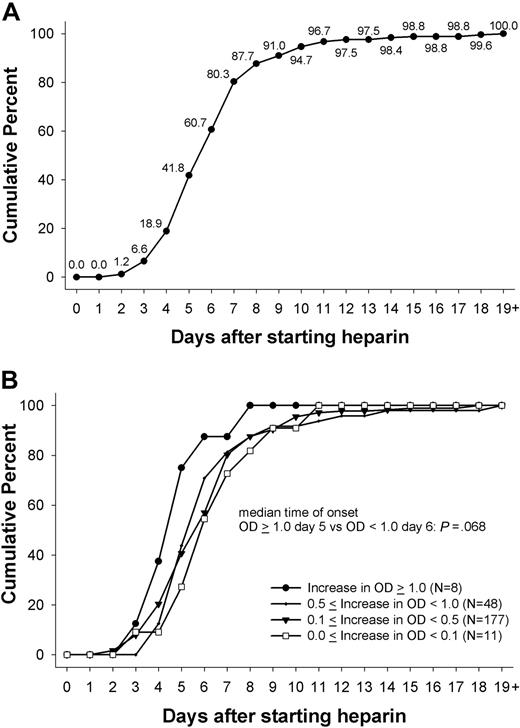
Of the 435 patients, 244 (56.1%) showed a change in OD of at least 15% (in OD units) in the PF4/heparin EIA that persisted for 2 or more days. The first day the immune response was measurable ranged between day 2 and day 24 (inclusive), with a median of day 6 (Figure 1A). More than 90% of immune responses, as measured by changes in OD, started between day 4 and 14 (inclusive), with more than two-thirds of patients responding between days 4 and 7 (inclusive): day 4 (12.3%), day 5 (23.0%), day 6 (18.9%), and day 7 (19.7%). Only 6.6% of immune responses began before day 4, and even fewer (1.6%) began after day 14. Mean duration of thromboprophylaxis was 12.2 plus or minus 7.0 days (range, 3-53 days). All patients received heparin thrombosis prophylaxis until the last day of the in-hospital stay. There was a statistically significant difference in the duration of heparin exposure between those showing a change in OD and those who did not (mean, 15.5 days ± 6.9; range, 5-53 days vs mean, 8.0 days ± 4.3; range, 3-34 days; P < .001).
Day of first onset of immune response against PF4/heparin. (A) The day at which the immune response against PF4/heparin complexes was first detected (first day of heparin use = day 0). There was a narrow time period between day 4 and day 12 during which the immune response occurred for combined IgG/IgA/IgM EIA; 100% represents the 244 patients who showed an increase in OD. (B) This pattern was the same whether the immune response was very weak (< 0.1 OD), weak (0.1- < 0.5 OD), or intermediate (0.5- < 1.0 OD), as indicated by change in the OD in the PF4/heparin EIA. There was a trend toward a slightly earlier onset in case of a strong (> 0.1 OD) immune response (median day 5 vs median day 6; P = .068).
Day of first onset of immune response against PF4/heparin. (A) The day at which the immune response against PF4/heparin complexes was first detected (first day of heparin use = day 0). There was a narrow time period between day 4 and day 12 during which the immune response occurred for combined IgG/IgA/IgM EIA; 100% represents the 244 patients who showed an increase in OD. (B) This pattern was the same whether the immune response was very weak (< 0.1 OD), weak (0.1- < 0.5 OD), or intermediate (0.5- < 1.0 OD), as indicated by change in the OD in the PF4/heparin EIA. There was a trend toward a slightly earlier onset in case of a strong (> 0.1 OD) immune response (median day 5 vs median day 6; P = .068).
The majority of immune responses were very weak (ΔOD, < 0.1) or weak (ΔOD, < 0.5) occurring in 188 patients (77.0%). An intermediate strength of immune response (ΔOD, 0.5- < 1.0) occurred in 48 (19.7%) of patients, whereas only 8 (3.3%) patients showed a strong immune reaction, with an absolute increase in OD more than 1.0. There was a trend for an earlier onset of the immune response in patients with a change in OD more than 1.0 (n = 8) compared with the onset in patients with an increase of OD less than 1.0 (5 days vs 6 days; P = .068; Figure 1B).
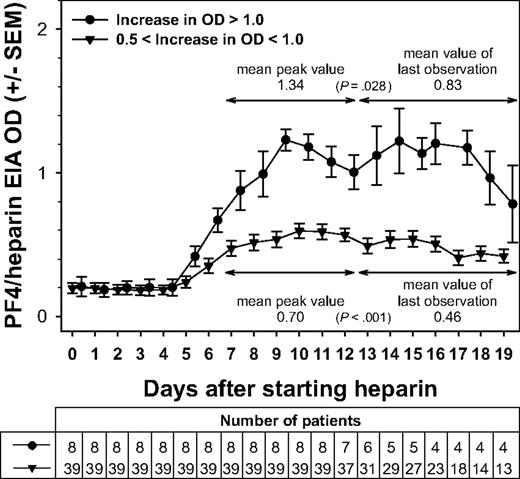
Interestingly, whether the antibody response was relatively weaker or stronger, in all groups, the ODs of PF4/heparin EIA declined after reaching a maximum, despite the UFH or LMWH being continued. Figure 2 shows this pattern for the 2 groups of maximal OD reactivity 0.5 to less than 1.0 and OD more than 1.0. In all groups, the immune response started on average at day 5 and reached its maximum at day 9 to 10. Importantly, the OD values then gradually declined despite continuing the heparin. The OD at the last day of heparin (after day 12) in all groups was significantly lower compared with the peak OD between day 7 and 12. The differences in the means were 0.06 for those with low reactivity (change in OD < 0.1; P = .068), 0.12 for those with OD changes of 0.1 to less than 0.5 (P < .001), 0.24 for those with changes in OD of 0.5 to less than 1.0 (P < .001), and 0.51 for those with strong reactivity (change in OD > 1.0; P = .028; Figure 2). Data on OD levels for patients in whom serial EIAs were performed up to day 10, and for those in whom EIA results were available thereafter, were very similar for the first 10 days (data not shown); this indicates that the pattern of OD changes seen in those patients with heparin treatment beyond day 12 is representative.
Changes in the immune response against PF4/heparin over time. The mean ODs in the anti-PF4/heparin EIA for each day of heparin application are shown. Beginning at approximately day 5, the ODs first increased, peaking between day 7 and day 12, and then decreased again, despite continuing application of heparin. The mean peak OD values during the day 7 to day 12 interval were significantly higher than the ODs observed on the last day of heparin application (P < .001). This pattern is given for intermediate (0.5- < 1.0) and strong (> 1.0) maximal changes in OD. The x-axis represents the days of heparin application; y-axis, the mean ODs measured. All ODs were measured, whereas the respective patients received UFH or LMWH. For 7 of the 8 patients who exhibited a strong immune response (ΔOD ≥ 1.0), antibody levels declined after reaching a peak despite continued heparin administration.
Changes in the immune response against PF4/heparin over time. The mean ODs in the anti-PF4/heparin EIA for each day of heparin application are shown. Beginning at approximately day 5, the ODs first increased, peaking between day 7 and day 12, and then decreased again, despite continuing application of heparin. The mean peak OD values during the day 7 to day 12 interval were significantly higher than the ODs observed on the last day of heparin application (P < .001). This pattern is given for intermediate (0.5- < 1.0) and strong (> 1.0) maximal changes in OD. The x-axis represents the days of heparin application; y-axis, the mean ODs measured. All ODs were measured, whereas the respective patients received UFH or LMWH. For 7 of the 8 patients who exhibited a strong immune response (ΔOD ≥ 1.0), antibody levels declined after reaching a peak despite continued heparin administration.
Anti-PF4/heparin EIAs determined separately for the Ig classes, IgG, IgM, and IgA.
Of the 244 sets of sera showing a change in the OD in the anti-PF4/heparin IgG/A/M EIA, 63 were further analyzed for the individual Ig classes. The patients represented by these studies included: all 8 serum sets showing a large change in OD (> 1.0), 32 of the 48 serum sets showing an intermediate change in OD (0.5- < 1.0), and 23 of the 188 serum sets showing a weak change in OD (< 0.5). The 23 latter serum sets were randomly chosen. Sixteen sets of sera with an intermediate change in OD could not be assessed because of insufficient material.
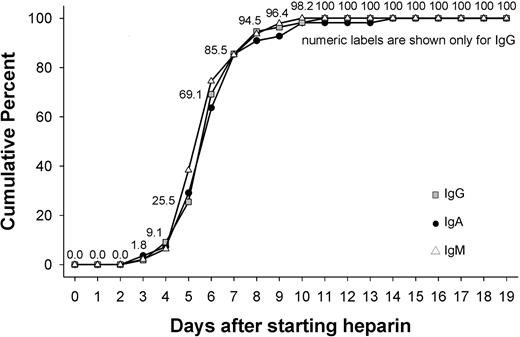
Of the 63 patients, 55 (87.3%) showed a change in OD of at least 15% for IgG, 47 (74.6%) for IgM, and 55 (87.3%) for IgA. The median of first day the immune response was measurable was day 6 for IgG (range, day 3-11), for IgM (range, day 3-10), as well as for IgA (range, day 3-14). More than two-thirds of patients showed an immune response that began between days 3 and 6 (inclusive; Figure 3). The majority of immune responses were weak, with a change in OD less than 0.5 occurring for more than half of patients (IgG, 54.5%; IgM, 76.6%; IgA, 54.5%). An intermediate strength of immune response (change in OD, 0.5- < 1.0) occurred for IgG in 41.8%, for IgM in 12.8%, and for IgA in 27.3% of patients. Strong immune reactions with an increase in OD more than 1.0 were observed for IgG in 3.6%, for IgM in 10.6%, and for IgA in 18.2% of patients.
Day of first onset of immune response against PF4/heparin separately for IgG, IgM, and IgA antibodies. The figure shows the days during application of heparin during which the immune response against PF4/heparin complexes first became detectable, shown separately for IgG, IgM, and IgA antibodies. There was a narrow time period between day 4 and day 12 during which the immune response predominantly occurred. The timing of the onset of the immune response was the same for antibodies of the IgG, IgA, or IgM classes.
Day of first onset of immune response against PF4/heparin separately for IgG, IgM, and IgA antibodies. The figure shows the days during application of heparin during which the immune response against PF4/heparin complexes first became detectable, shown separately for IgG, IgM, and IgA antibodies. There was a narrow time period between day 4 and day 12 during which the immune response predominantly occurred. The timing of the onset of the immune response was the same for antibodies of the IgG, IgA, or IgM classes.
Differences in onset of IgG, IgM, and IgA immune responses in individual patients.
Of the 63 patients, 37 (58.7%) showed an immune response against anti-PF4/heparin involving all 3 Ig classes, 6 (9.5%) showed only a dual IgG and IgM response, 12 (19.0%) showed only a dual IgG and IgA response, 3 (4.8%) showed only a dual IgM and IgA response, and only 4 patients (6.3%) showed a response for only one individual Ig class (IgM 1 [1.6%]; IgA 3 [4.8%]). (One patient who was positive in the IgG/A/M combined EIA was negative for all 3 Ig classes when these were measured individually.)
In the 58 patients showing an immune response for more than one Ig class, we assessed whether there was a difference in the onset of the formation of the different Ig classes, eg, whether IgM class antibodies were detectable 1 or more days before onset of formation of IgG class antibodies. Regardless of whether the IgG response resulted in a small or large increase in the OD (data not shown), antibodies of the other Ig classes, when formed, were usually detectable at the same day (± 1 day). In particular, there was no general tendency for IgM class antibodies to precede formation of either IgG or IgA class antibodies (Table 1).
Observations on antibody waning or disappearance despite continued heparin treatment
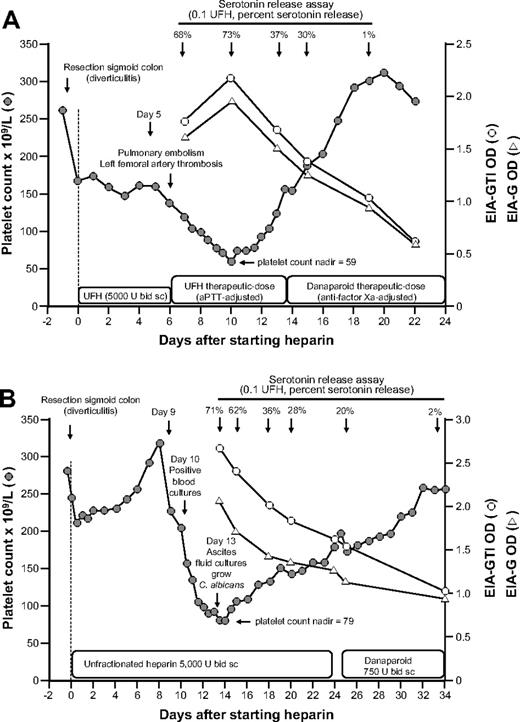
We observed 2 patients with clinical HIT who continued to receive heparin for 8 and 13 days after the onset of thrombocytopenia and in whom serial blood samples were available for analysis of anti-PF4/heparin antibodies by EIA and antibody reactivity in the SRA. Both patients showed a decrease in the OD levels and in the SRA (judged by percentage serotonin release) despite the continuation of heparin (Figure 4). Furthermore, in both patients, the platelet counts recovered despite the continuation of heparin and in parallel with waning antibody levels.
Clinical course, platelet counts, and anti-PF4/heparin antibody reactivity in the EIA and the SRA of 2 patients with prolonged heparin application despite clinical HIT. (A) A 74-year-old man developed thrombocytopenia, pulmonary embolism, and left femoral artery thrombosis on day 6 of postoperative UFH thromboprophylaxis. Despite increased (therapeutic) heparin dosing, the platelet count recovered from 59 × 109/L (nadir, day 10) to 155 × 109/L (day 13). On day 13, the positive SRA test result became available, and heparin was replaced by danaparoid. (B) An 81-year-old woman developed thrombocytopenia beginning on day 9 of postoperative UFH thromboprophylaxis after perforated sigmoid colon resection. Blood cultures on day 10 were positive for Enterococcus faecium, and the platelet count decline was considered to be related to “line sepsis.” Despite continued UFH thromboprophylaxis, the platelet count recovered from 79 × 109/L (nadir) to 157 × 109/L (day 23). On day 23, the positive SRA test result became available, and UFH was replaced by danaparoid.
Clinical course, platelet counts, and anti-PF4/heparin antibody reactivity in the EIA and the SRA of 2 patients with prolonged heparin application despite clinical HIT. (A) A 74-year-old man developed thrombocytopenia, pulmonary embolism, and left femoral artery thrombosis on day 6 of postoperative UFH thromboprophylaxis. Despite increased (therapeutic) heparin dosing, the platelet count recovered from 59 × 109/L (nadir, day 10) to 155 × 109/L (day 13). On day 13, the positive SRA test result became available, and heparin was replaced by danaparoid. (B) An 81-year-old woman developed thrombocytopenia beginning on day 9 of postoperative UFH thromboprophylaxis after perforated sigmoid colon resection. Blood cultures on day 10 were positive for Enterococcus faecium, and the platelet count decline was considered to be related to “line sepsis.” Despite continued UFH thromboprophylaxis, the platelet count recovered from 79 × 109/L (nadir) to 157 × 109/L (day 23). On day 23, the positive SRA test result became available, and UFH was replaced by danaparoid.
Study on relevance of asymptomatic seroconversion for PF4/heparin antibodies for long-term outpatient thrombosis prophylaxis
Of the 242 patients who participated in the outpatient extended LMWH thrombosis prophylaxis study, 16 (6.6%) tested positive by HIPA test at day 16 and 31 of 237 (13.1%) tested positive by IgG/A/M EIA with an OD more than 0.5 to 1.0 in 27 patients and an OD more than 1.0 in 4 patients (5 patient sera were not tested by EIA). None of the antibody-positive patients developed clinically symptomatic thrombosis within the next 3 months, whereas thrombosis occurred in 3 of the 209 patients who had been antibody-negative at discharge (P > .999 by Fisher exact test, 2-tailed, for both comparisons of antibody-positive vs antibody-negative status). We did not retest these patients at the time of thrombosis.
Discussion
This study assessing systematically the timing of the immune response against PF4/heparin complexes in patients undergoing major orthopedic and trauma surgery provides evidence that the immune reaction against PF4/heparin complexes differs from other “classic” immune reactions, eg, alloimmunization after blood transfusion, or vaccination.10,14 Although more than half of the patients exposed to heparin showed an immune response toward PF4/heparin, they did not show the pattern of a typical primary immune response, ie, one that is characterized by the initial formation of IgM class antibodies followed by a more delayed, and relatively weak, IgG immune response. However, the patterns we observed were also not typical for an anamnestic (secondary) immune response, with stronger and persistent formation of IgG antibodies. Indeed, we observed a decline of antibody reactivity in the EIA that occurred despite continuing application of heparin, even in 2 patients who had strong evidence for clinical HIT (Figures 2, 4). These data corroborate a previous study8 that showed HIT antibodies to be very transient, although the presumption of the previous study was that antibody waning resulted from discontinuation of the heparin.
Nevertheless, we did observe certain features that are characteristic of a secondary immune response of B cells, which have previously undergone Ig-class switch. In the majority of patients who showed anti-PF4/heparin seroconversion, 2 or even all 3 antibody classes were detected. Further, these were formed between days 5 and 14, with a median of day 6 seen for all 3 immunoglobulin classes (IgG, IgA, IgM). This profile was seen regardless of whether the immune response was weak or strong, as judged by ΔOD values (Figure 1B; Table 1). In addition, when we considered the clinically more relevant responses (ΔOD > 0.5), more patients showed an IgG response than an IgM response (39.7% IgG vs 17.5% IgM; P = .02).
Thus, in most patients, the immune response against PF4/heparin complexes seems to lack features of either a classic primary or a classic secondary response, based on the following rationale. A primary response should not evince IgG class antibodies as early as day 5 (something that should only be possible if previous antigen contact had occurred). On the other hand, a secondary immune response should not be characterized by such a rapid decline of antibody levels, particularly given the continuation of heparin.
These considerations raise the question as to how these unusual features of the immune response against PF4/heparin complexes might be explained. One speculation is that the pattern of antibody formation might be more compatible with a non–T cell–dependent immune reaction, in which B cells are stimulated by the PF4/heparin antigenic complexes without the help of T cells. This type of reactivity has been described for immune reactions against antigens with repetitive epitopes, which is typical for certain viruses.24 Such an immune response is characterized by rapid onset, as well as a rapid decline, of antibodies with no memory cells being formed. In line with this hypothesis, we have shown recently that the antigenic PF4/heparin complexes are linear, ridge-like clusters of 100 to 150 nm size in which PF4 tetramers expose repetitive epitopes.25 Within these complexes, the single PF4 tetramers have a distance of approximately 4 to 6 nm, which is within the range of repetitive viral epitopes found to cause T-independent B-cell activation.26 Furthermore, PF4 and heparin can form rather large amounts of complexes, particularly given the degree of platelet activation that occurs during major surgery. Indeed, large amounts of antigen represent another factor that predisposes to a T-cell-independent B-cell response. This would also explain why, after major orthopedic surgery and after cardiac surgery, the immune response to PF4/heparin is much more frequent than in medical patients.27
However, there are also several strong arguments for a T cell–dependent immune reaction. First, T cell–independent B-cell responses should be primarily IgM, whereas in our study there were even more patients who formed IgG than IgM class antibodies. This suggests that there may have been previous contact(s) between the immune system and the “HIT antigens,” which induced antibody class switching, thus allowing for the subsequent rapid formation of IgG and IgA antibodies on recapitulation of the “HIT antigens” through pharmacologic heparin exposure. In line with our hypothesis that previous contact of the immune system with “HIT antigens” could have occurred and thereby predispose to “secondary” stimulation of anti-PF4/heparin antibodies despite “primary” exposure to pharmacologic heparin are several observations that arise from studies28,29 of heparin-induced immunization in children. For example, we found, in a recent prospective trial in the neonatal intensive care unit, that none of the more than 100 heparin-exposed neonates who received heparin formed anti-PF4/heparin antibodies.28 Because neonates do not have contact with foreign antigens before birth, this lack of antibody formation could reflect the absence of preceding exposure to environmental or other factors that might have resulted in primary immunization against PF4-dependent antigens. However, we analyzed the neonates up until day 28 after start of heparin, and we did not find any evidence even for a delayed primary immune response. Thus, the opportunity during the fetal/neonatal period for formation of PF4/heparin-reactive B cells with an Ig-class switch seems to be low. In addition, in support of a low risk of neonatal immunization by heparin, a recent study of pediatric cardiac surgery found a much lower rate of immunization of neonates and infants compared with older children.29,30
In addition, previous analysis of the sequences encoding CDR3 domains of individual Vβ families showed that T cells obtained from patients with recent HIT, and which were cultured in the presence of PF4/heparin complexes, preferentially expressed T cell receptor–containing β chains of the Vβ 5.1 family and shared a common tetrapeptide motif. This indicates that the humoral immune response associated with HIT could involve helper T cells recognizing PF4 peptides.31
Another argument for a T cell–dependent B-cell response against PF4/heparin complexes arises from a mouse model in which the immune response against PF4/heparin was found to be T cell-dependent.32 However, the antibody response in this mouse model occurred between days 20 and 25, a time period much later than we observed in our studies of humans exposed to heparin. Moreover, this later time period represents the typical time window for a primary immune response.
However, a T cell-dependent immune response with Ig-class switching should induce longer-lasting immunity, such as is observed in immune responses to red blood cell or platelet alloantigens after transfusion. This is apparently not the case in the immune response to PF4/heparin. A T cell-dependent immune response should also induce formation of memory B cells. However, there is increasing evidence that patients who have a history of clinical HIT do not show a typical anamnestic immune response when reexposed to heparin.8,11,33 On the contrary, other reports34,35 suggested that there might sometimes be a more rapid formation of heparin antibodies in the case of reexposure. But neither of these studies34,35 established that their patients had had 2 distinct episodes of HIT. Moreover, 2 reports in which patients did clearly have 2 distinct episodes of serologically confirmed HIT several years apart found that the onset of thrombocytopenia during the second episode of HIT appeared to be no sooner than that observed during the first episode of HIT.36,37
Potentially, in patients exposed to heparin, there are important additional, and as yet unknown, cofactors that could contribute to the induction of such an atypical immune response that differs from the one seen in the animal model of HIT. A recent study of anti-PF4/heparin immunization in postcardiac surgery patients found evidence that proinflammatory factors support the costimulation of B cells.38 This might also explain the decrease of antibody titers despite maintaining heparin, as any proinflammatory costimuli will typically decline soon after surgery.
There is a possible model that is compatible with both the empirical observations we have made in our current study regarding the immune response against PF4/heparin as well as our speculations regarding the possible immunobiologic basis for the immune response in HIT. Exposure to PF4 complexes, perhaps induced earlier in life by factors other than heparin yet leading to clustering of PF4, induces formation of B cells and, with the help of T cells, an antibody class switch from IgM to IgG and IgA. These B cells behave differently than normal memory B cells and are usually not activated. Only in case of concomitant presentation of PF4/heparin clusters, and a proinflammatory response leading to alteration of the autoregulated network of the immune system, do these B cells expand within a narrow time frame, start to produce antibodies of the IgG, IgM, and IgA classes (singly or in any combination), and then become rapidly inactivated. It is increasingly evident that major surgery is a risk factor for the immune response of HIT.39 Major surgery causes inflammation as well as platelet activation with subsequent increased release of PF4. In this context, a very interesting experiment showed that at least in vitro CD4+CD25+ regulatory T cells exposed to PF4 lose the ability to inhibit the proliferative response of CD4+CD25− T cells. Thus, PF4 by itself might facilitate induction of the immune response toward PF4/heparin complexes by impairing regulatory T-cell function.40
The “secondary” response of B cells producing anti-PF4/heparin antibodies that occurs at the time of (even first-time) pharmacologic heparin could even be T cell–independent. Recently, Groom et al provided evidence that it is possible in mice to induce T cell–independent B-cell activation that results in formation of IgG antibodies.41 A potential class of B cells showing this behavior could be marginal zone B cells.42 Interestingly, and in line with the aforementioned studies,28,29 these B cells are not present in neonates. This could explain why, in a prospective neonatal study, none of the neonates developed anti-PF4/heparin antibodies within 1 month after receiving UFH for a mean of 6.5 days. Our hypothesis could be tested in an animal model of HIT. As marginal zone B cells are restricted to the spleen in rodents, splenectomized mice should not be able to show the typical anti-PF4/heparin immune response within its usual 5- to 14-day time period. Our data, however, do not exclude that, in a subset of patients, a more classic type of immune response against PF4/heparin occurs with longer-lasting antibody persistence and a typical anamnestic response.
In conclusion, our study assessing serial samples of a large number of heparin-exposed patients shows that the development of anti-PF4/heparin antibodies does not exhibit features typical of a primary immune response; however, the serologic features do not show typical features of a secondary immune response either. Further, we have provided a novel hypothesis of marginal zone B cells being involved in the immune response toward PF4/heparin, which could be tested in an animal model. Clinically, our study provides further evidence that the critical time period for the immune response toward PF4/heparin during UFH is between days 5 and 14 of heparin application, that antibody levels can wane despite continued heparin exposure, and that prolonged treatment with LMWH in prophylactic doses after day 14 does not cause an increased risk for new thrombosis in patients who developed anti-PF4/heparin antibodies during the preceding 2 weeks.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by German Federal Ministry for Education and Research (CAN04/006); Landesförderungsprogramm Mecklenburg-Vorpommern EFRE; within the Fellows-Program “Life Sciences” of the Alfried Krupp Wissenschaftskollegs Greifswald; by the Alfried Krupp von Bohlen und Halbach-Stiftung, Forschungsverbund Molekulare Medizin of the Ernst-Moritz-Arndt-University Greifswald (FOMM 2007-06); and by the Heart and Stroke Foundation of Ontario (operating grant T6157).
Authorship
Contribution: A.G. and T.E.W. designed the study, analyzed results, and wrote the manuscript; U.S. and J.-A.I.S. performed the laboratory experiments; T.K. performed the statistical analyses; and all authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Greinacher, Institut für Immunologie und Transfusionsmedizin, Ernst-Moritz-Arndt Universität Greifswald, Klinikum/Sauerbruchstraße, D 17475 Greifswald, Germany; e-mail: greinach@uni-greifswald.de.