Abstract
Previous work has demonstrated that a subset of macrophages expresses a folate receptor (FR) that can mediate internalization of folate-linked molecules, including imaging and therapeutic agents. To characterize this subset, macrophages were collected from peritoneal cavities of mice injected with saline, thioglycolate, zymosan, heat-killed or live bacteria, and cell-surface markers that coexpress with FR were identified. Virtually no F4/80+ peritoneal macrophages from saline-injected mice expressed FR, whereas numerous macrophages from mice injected with each inflammatory stimulus expressed FR. Examination of cell differentiation antigens that are up-regulated in FR+ macrophages revealed markers characteristic of an activated state (CD80, CD86, Ly-6C/G), whereas macrophages lacking these activation markers expressed few or no FR. FR+ macrophages also produced tumor necrosis factor-α (TNF-α) and reactive oxygen species, and production of reactive oxygen species correlated linearly with expression of FR. Synovial macrophages collected from arthritic patients were found to bind and internalize folate-linked dyes. Moreover, a folate-linked radioimaging agent was shown to image inflamed joints of rheumatoid arthritic patients. These results suggest that FR constitutes a marker for macrophage activation and that FR+ macrophages can be targeted with folate-linked drugs without promoting drug uptake by nonactivated macrophages. This trial was registered at www.clinicaltrials.gov as #NCT00588393.
Introduction
In the absence of inflammatory signals, tissue-resident macrophages participate primarily in tissue homeostasis.1-4 However, when induced with inflammatory stimuli, macrophages become activated and mobilize disease-resistance mechanisms by releasing proinflammatory mediators that both activate defense responses in adjacent cells and recruit immune cells to sites of inflammation.1-4 In most tissues, activated macrophages can be easily distinguished from their tissue-resident counterparts by both the molecules they secrete (eg, cytokines) and the cell-surface antigens they express (eg, CD80 [B7.1], CD86 [B7.2], Ly6C/G [Gr-1]).5-7
Although activated macrophages primarily serve to protect against opportunistic infections,7,8 when they become activated inappropriately, they can participate in the development of autoimmune and inflammatory diseases. Thus, activated macrophages have been shown to play an integral role in the etiology of diseases, such as rheumatoid arthritis,9,10 lupus,11,12 ulcerative colitis,13 atherosclerosis,14,15 psoriasis,16 ischemia reperfusion injury,17,18 sarcoidosis,19 transplantation rejection,20,21 and diabetes,22,23 among others. During progression of these diseases, activated macrophages may release cytokines (interleukin-1 [IL-1], IL-6, tumor necrosis factor-α [TNF-α]), chemokines (eg, monocyte chemotactic protein-1 [MCP-1]), digestive enzymes (eg, collagenases), prostaglandins, and reactive oxygen species (ROS), which can aggravate or accelerate damage to the normal tissues.1,9,21
In earlier studies of rheumatoid arthritis, inflamed joints were observed to accumulate a subpopulation of macrophages that also express a receptor for the vitamin, folic acid.24 Because no other tissues/cell types except the kidneys and certain malignant cells expressed this folate receptor (FR), accumulation of FR+ macrophages in arthritic joints allowed the selective targeting of folate-linked imaging and therapeutic agents to these sites of inflammation.25 Because folate conjugates of therapeutic drugs were also found to selectively kill FR+ macrophages,26,27 and because the degree of articular inflammation could be readily quantitated from the uptake of folate-linked imaging agents,28 the nature and properties of the FR-expressing macrophages became a topic of considerable interest. In the current report, we characterize the subpopulation of macrophages that expresses high levels of FR and demonstrate that this fraction is highly activated. We also show that nonactivated resident macrophages do not express FR and that establishment of FR as a marker of macrophage activation enables the facile labeling, isolation, and treatment of these inflammatory cells with folate conjugates.
Methods
Reagents
The following rat monoclonal antibodies against mouse antigens were purchased from Invitrogen (Carlsbad, CA): phycoerythrin (PE)–conjugated anti-CD80, anti-CD86, anti-CD23, TriColor (PE-Cy5)–conjugated anti-F4/80, and anti–Ly-6C/G. A mouse monoclonal antibody against human CD11b conjugated with TriColor was also from Invitrogen. PE-conjugated rat monoclonal antibodies against mouse TNF-α were purchased from eBioscience (San Diego, CA). Alexa Fluor 647–conjugated rat anti-mouse mannose receptor was obtained from BioLegend (San Diego, CA). Human recombinant growth factors/cytokines, macrophage colony-stimulating factor (M-CSF), IL-1α, IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-17, lipopolysaccharide (LPS), interferon-γ (IFN-γ), TNF-α, muramyl dipeptide, and neopterin were purchased from Sigma-Aldrich (St Louis, MO). Mouse recombinant IFN-γ was purchased from eBioscience. Folate–fluorescein isothiocyanate (folate-FITC) and EC20 (a folate-linked chelator of 99mTc) were kind gifts from Endocyte (West Lafayette, IN). Folate-rhodamine and folate–Oregon Green were synthesized by solid phase using methods similar to those described previously for other dyes.29 3H-Folic acid was obtained from GE Healthcare (Chalfont St Giles, United Kingdom). ROS indicator, carboxy-H2DCFDA, was obtained from Invitrogen. All other chemicals were purchased from Sigma-Aldrich or were reagent grade chemicals from other major suppliers.
Mice
Female 6- to 8-week-old C57BL/6 mice were purchased from Harlan (Indianapolis, IN). Mice were housed in sawdust-lined cages and fed standard rodent chow and water ad libitum. All experiments were approved by the Purdue University Animal Care and Use Committee.
Recruitment of peritoneal macrophages
Peritoneal macrophages were recruited and collected by 1 of 5 methods. Thioglycolate-recruited macrophages were isolated by peritoneal lavage 3 days after intraperitoneal injection of 1.5 mL 3% sterile thioglycolate medium. Briefly, 30 g of dehydrated Brewer thioglycolate medium powder (Sigma-Aldrich) was dissolved in 1000 mL deionized water and autoclaved for 20 minutes at 15 pounds of pressure (121°C). The autoclaved medium was kept in the dark under sterile conditions at room temperature for at least 3 months before use.
Similarly, live bacteria-, heat-killed bacteria-, and zymosan-recruited macrophages were isolated 3 days after injection of 107 colony forming units (CFU) of live Pseudomonas aeruginosa (designations: PAO1024; ATCC, Manassas, VA), 108 CFU of heat-killed P aeruginosa, or 1 mg of sterile zymosan, respectively, in 200 μL sterile phosphate-buffered saline (PBS) suspension. Briefly, P aeruginosa was stored in nutrient broth at 109 CFU/mL at −70°C. When desired, 10 μL stock culture was inoculated into 4 mL broth and incubated overnight at 37°C with shaking. An aliquot was then diluted 1/10 into fresh broth and cultured an additional 4 to 5 hours. Bacteria were either directly injected intraperitoneally into mice after final dilution with sterile PBS or autoclaved at 15 lb of pressure (121°C) for 20 minutes to generated heat-killed bacteria before injection.
Finally, nonactivated macrophages were similarly isolated by peritoneal lavage 3 days after injection of sterile saline. Before further analyses, all cells were resuspended in folate-deficient RPMI 1640 supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin solution.
Flow cytometry
Peritoneal cells from mice or synovial cells from patients with rheumatoid arthritis were stained with the appropriate antibodies for 30 minutes on ice. Samples were washed 3 times in PBS, followed by incubation with folate-FITC or folate–Oregon Green (100 nM) for 30 minutes at 37°C to stain FR. For analysis of coexpression of TNF-α and FR, folate–Oregon Green and F4/80 TriColor-labeled cells were fixed, permeabilized, and incubated with anti–TNF-α (PE-conjugated) before flow cytometry. For analysis of ROS production, cells were incubated with either folate-FITC (100 nM) or carboxy-H2DCFDA (25 μM) for 20 minutes at 37°C after staining with anti-F4/80 for 30 minutes on ice. In competition studies, cells were coincubated with 10 μM folic acid to competitively block all FR. In all cases, appropriate antibody isotype controls were used. Flow cytometry was performed on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). CellQuest (BD Biosciences) was used for acquiring and analyzing the data. For data analysis, the fluorescence gate for FR expression (x-axis) was set so that less than 1% of macrophages were counted as FR+ in the presence of folate-FITC plus 100-fold excess free folic acid (to competitively block binding of folate-FITC to all FR). Similarly, the fluorescence gate for activation markers was set so that less than 1% of the macrophages appeared to be positive when examined with a nonspecific antibody isotype control. Experiments from each group were repeated at least 3 times, and representative data from each group are shown.
Kinetics of 3H-folic acid binding and internalization by macrophages
Peritoneal cells were plated on 24-well plates in RPMI 1640 folate-deficient medium and incubated at 37°C for 2 hours to allow cells to adhere. Nonadherent cells were removed by washing and adherent macrophages were incubated for various time periods at 37°C in the presence of 100 nM 3H-folic acid (1 μCi). In some cases, cells were preincubated with 10 μM folic acid to competitively block all FR, and in other cases cells were subjected to acidic saline wash (pH 3.0) to remove all cell surface bound 3H-folic acid. Samples were washed 3 times with PBS to remove unbound 3H-folic acid, and cells were dissolved in 0.25 M NaOH and transferred to scintillation vials for counting. All experiments were performed in triplicate.
Effect of temperature on 3H-folic acid binding
Folic acid binding and internalization were analyzed at both 4°C and 37°C. Cells were distributed into microcentrifuge tubes and incubated for 2 hours at the appropriate temperature in the presence of 100 nM 3H-folic acid (1 μCi). In some cases, cells were preincubated with 10 μM folic acid to competitively block all FR. Furthermore, after incubation some samples were subjected to an acidic saline wash (pH 3.0) to remove all cell surface bound 3H-folic acid. All samples were washed 3 times with PBS to remove unbound 3H-folic acid. Cell pellets were dissolved in 0.25 M NaOH and transferred to scintillation vials for counting. All experiments were performed in triplicate.
Confocal microscopy
Morphology, ROS production, and the binding and endocytosis of folate conjugates in FR+ peritoneal macrophages were visualized using an IX81 inverted microscope (Olympus America, Center Valley, PA) equipped with an FV1000 confocal unit and a 60×/1.2 NA water objective. A 543-nm HeNe laser was used to excite the folate-rhodamine and a 488-nm Argon laser was used to excite the oxidized fluorescein–derivative carboxy-DCF (from carboxy-H2DCFDA), and PE-Cy5. Three-color imaging was performed with 2 spectral detectors (fluorescein, excitation 488 nm, detector range 500-530 nm; rhodamine, excitation 543 nm, detector range 555-625 nm) and a detector with 655- to 755-nm filter (PE-Cy5, excitation 488 nm). Images were processed using FLUOVIEW software (Olympus America).
For cell imaging, macrophages were plated in chambered coverglass wells (Nalge Nunc International, Rochester, NY) and allowed to adhere for 1 hour at 37°C, followed by washing 2 times with PBS to remove nonadherent cells. For in vitro staining, cells were incubated with folate-rhodamine (100 nM) at 37°C for the desired times before washing 2 times to remove unbound folate conjugate. For in vivo staining, 5 nmol folate-rhodamine in 200 μL PBS was injected intraperitoneally in mice, and after 10 minutes of incubation, peritoneal cells were collected by peritoneal lavage and examined by confocal microscopy. The in vivo competition was carried out by preinjection with 100-fold excess (500 nmol in 200 μL PBS) of folic acid. In the study of ROS detection, macrophages were simultaneously incubated with folate-rhodamine (100 nM), carboxy-H2DCFDA (25 μM), and TriColor (PE-Cy5)–conjugated anti-F4/80 for 30 minutes at 37°C and imaged after washing 2 times with PBS.
Analysis of synovial fluids from patients with rheumatoid arthritis
Rheumatoid arthritic synovial fluid samples were obtained from patients diagnosed with rheumatoid arthritis at Arnett Clinic (Lafayette, IN). All procedures were approved by the Institutional Review Boards of Purdue University and Lafayette Home Hospital and St Elizabeth Medical Center. Patients were recruited into the study after informed consent was obtained in accordance with the Declaration of Helsinki. Synovial fluid was removed from the arthritic joints of patients, and cells were collected after either direct washing 2 times with PBS or Ficoll gradient separation. Expression of FR on synovial macrophages was analyzed by flow cytometry after incubation with folate-FITC, as described in “Flow cytometry.”
Imaging of patient with rheumatoid arthritis
After obtaining the approval from the institutional review board of Mayo Clinic (Rochester, MN) and patient informed consent, the folate-targeted 99mTc-based radioimaging agent, EC20, was administered to a 67-year-old female patient with a 7-year history of seropositive rheumatoid arthritis with active synovitis and joint swelling. The extent of active joint involvement was assessed clinically by a rheumatologist (E.L.M.). After this assessment, the patient underwent imaging by a 2-mL injection of 0.1 mg of EC20 labeled with 20 to 25 mCi technetium-99m followed by a FolateScan. Head-to-toe anterior and posterior planar scintigrams (whole body imaging) were acquired with a dual-detector, large-field-of-view gamma camera equipped with low-energy high-resolution parallel-hole collimators 2 hours after the injection. Blinded assessment of radiolabeled conjugate uptake in the joints was then performed, and the results were compared with the clinical examination.
Statistical analysis
Analysis of the statistical significance of FR expression in response to different stimuli was performed by pairwise comparison with sterile saline-treated controls using t tests with pooled SD. Individual flow cytometric, confocal microscopic, and folate-binding assays were repeated at least 3 times, and representative data are shown.
Results
FRs are expressed on macrophages recruited by inflammatory stimuli but not on resident peritoneal macrophages in mice
Previous work has shown that FR+ macrophages accumulate in the joints of patients with rheumatoid arthritis. However, this subset of macrophages has never been characterized. To obtain large quantities of FR+ macrophages for detailed analysis, C57BL/6 mice were injected intraperitoneally with thioglycolate broth and peritoneal cells were isolated. The cell suspension was then treated with folate-FITC plus a murine macrophage-specific antibody, anti-F4/80, and analyzed by flow cytometry for folate conjugate binding (Figure 1A; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Side scatter (measuring cell granularity) and expression of F4/80 were used to distinguish a macrophage region (R1) from cell populations enriched in granulocytes (R2), lymphocytes, or erythrocytes (R3). Importantly, only cells in the macrophage region displayed significant uptake of folate-FITC, as evidenced by their shift to higher fluorescence in the associated flow cytometry histogram (open black histogram). Little evidence of folate conjugate binding was seen in the granulocyte, lymphocyte, or erythrocyte-enriched populations. Further, folate-FITC uptake by the macrophage-enriched population was quantitatively inhibited by 100-fold excess free folic acid (filled gray histogram), indicating that folate-FITC binding to these cells was FR-specific. These data demonstrate the selective expression of FR on a subpopulation of macrophages and the apparent lack of FR expression on other peritoneal cells.
Flow cytometric analysis of FR+ peritoneal cells. Representative flow cytometric analyses of FR expression on (A) thioglycolate-recruited, (B) bacteria-recruited, and (C) quiescent peritoneal cells. Three days after intraperitoneal injection of thioglycolate, live P aeruginosa, or sterile PBS, peritoneal cells were removed and analyzed by flow cytometry. On the basis of side scatter and F4/80 fluorescence, 3 regions (R1-R3) of density plots were defined. Cells in R1 are macrophages; cells in R2 granulocytes; cells in R3 lymphocytes and a few erythrocytes. The cell suspension was stained with 100 nM folate-FITC in the absence (solid black histogram) or presence of an excess (10 μM) of free folic acid to competitively occupy FR (filled gray histogram). The percentage of FR+ cells (average from at least 3 independent experiments) within each gate is shown.
Flow cytometric analysis of FR+ peritoneal cells. Representative flow cytometric analyses of FR expression on (A) thioglycolate-recruited, (B) bacteria-recruited, and (C) quiescent peritoneal cells. Three days after intraperitoneal injection of thioglycolate, live P aeruginosa, or sterile PBS, peritoneal cells were removed and analyzed by flow cytometry. On the basis of side scatter and F4/80 fluorescence, 3 regions (R1-R3) of density plots were defined. Cells in R1 are macrophages; cells in R2 granulocytes; cells in R3 lymphocytes and a few erythrocytes. The cell suspension was stained with 100 nM folate-FITC in the absence (solid black histogram) or presence of an excess (10 μM) of free folic acid to competitively occupy FR (filled gray histogram). The percentage of FR+ cells (average from at least 3 independent experiments) within each gate is shown.
In an attempt to recruit macrophages using a more physiologic stimulus, mice were injected intraperitoneally with a sublethal dose of a live pathogenic bacterium (P aeruginosa), and 3 days later peritoneal cells were retrieved by lavage (Figures 1B, S1). As seen with the thioglycolate-treated mice, bacteria-recruited macrophages were found to constitute the only population of FR-expressing cells, as evidenced by their appearance in the macrophage population selected by both side scatter and anti-F4/80 staining (open black histogram). Competition studies also confirmed that folate-FITC uptake was FR-mediated and not a consequence of nonspecific phagocytosis (filled gray histogram).
Because thioglycolate and live bacteria can both cause inflammation, it was important to compare FR expression on macrophages harvested in the absence of an inflammatory stimulus. For this purpose, resident macrophages were collected by lavage from the peritoneal cavities of mice after injection of sterile PBS. As seen in Figures 1C and S1, analysis of cell staining patterns revealed that only approximately 2% of the F4/80+-resident peritoneal macrophages expressed FR, a value significantly lower than that seen in thioglycolate- or bacteria-injected mice (Figure 2).
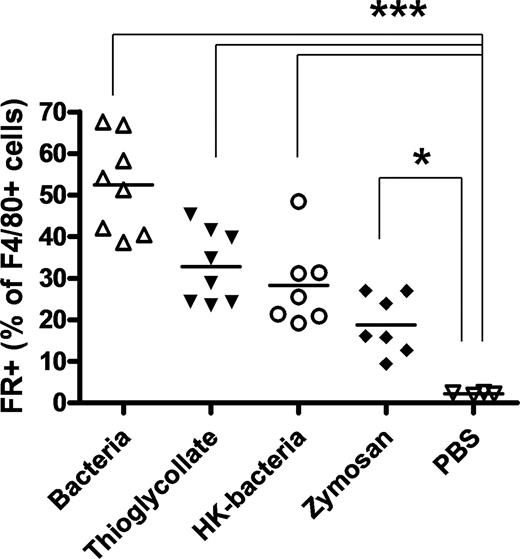
FRs are expressed on F4/80+ macrophages recruited by inflammatory stimuli, but not on resident peritoneal F4/80+ macrophages. The percentage of FR+ cells within the F4/80+ macrophage population isolated from the peritoneal cavities of mice treated with thioglycolate, live bacteria, heat-killed bacteria (P aeruginosa), zymosan, or sterile PBS is shown. Pairwise comparisons using t tests with pooled SD were used for statistical analysis; ***P < .001, *P < .05. HK-bacteria indicates heat-killed bacteria.
FRs are expressed on F4/80+ macrophages recruited by inflammatory stimuli, but not on resident peritoneal F4/80+ macrophages. The percentage of FR+ cells within the F4/80+ macrophage population isolated from the peritoneal cavities of mice treated with thioglycolate, live bacteria, heat-killed bacteria (P aeruginosa), zymosan, or sterile PBS is shown. Pairwise comparisons using t tests with pooled SD were used for statistical analysis; ***P < .001, *P < .05. HK-bacteria indicates heat-killed bacteria.
Finally, because a distinction in FR expression seemed to be emerging between inflammation-recruited and quiescent resident macrophage, 2 other inflammatory stimuli were also exploited to recruit macrophages and their levels of FR expression on F4/80+ macrophages were evaluated. As seen in Figure 2, a substantial fraction of both zymosan- and heat-killed bacteria-recruited F4/80+ macrophages also expressed FR. Collectively, these data demonstrate that FR is selectively expressed on the macrophages recruited by inflammatory stimuli but not on their resting counterparts.
FR constitutes an activation marker for macrophages
Activated macrophages are observed to display a larger and more irregular morphology than resting macrophages.5 To begin to assess the activation status of FR-expressing macrophages, peritoneal macrophages were recruited by sterile saline (Figure S2A), thioglycolate (Figure S2B), or live P aeruginosa (Figure S2C) and labeled with folate-rhodamine before imaging by confocal microscopy. Cells showing significant folate-rhodamine uptake (Figure S2B,C) were seen to be larger and more echinocytic than their resting nonfluorescent counterparts (Figure S2A). Indeed, within the field of cells (eg, Figure S2C), macrophages with greater folate-rhodamine uptake invariably displayed a morphology indicative of a greater degree of activation.
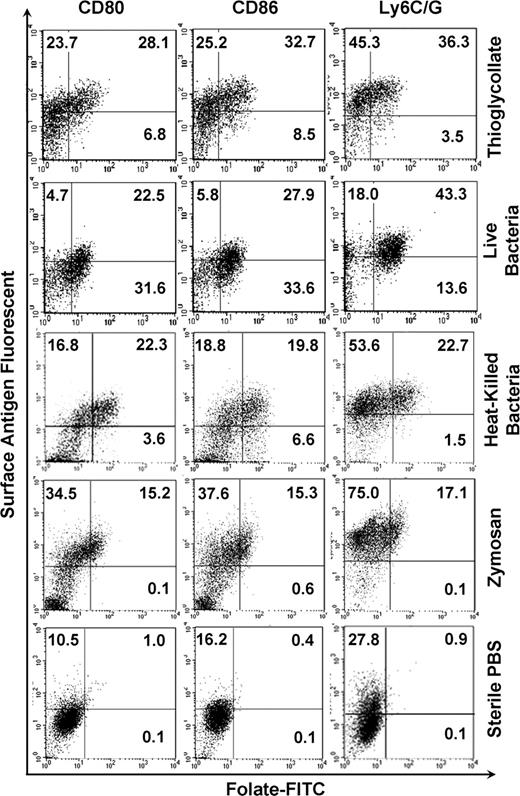
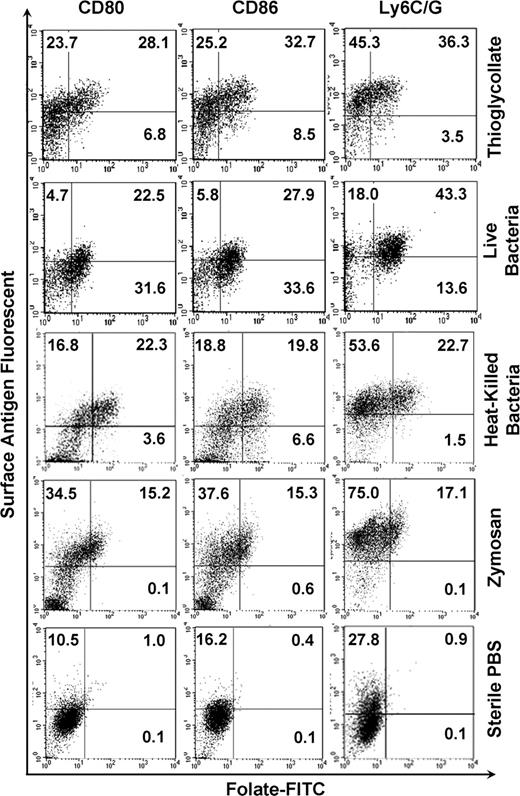
In addition to the aforementioned morphologic changes, activated macrophages also up-regulate certain surface antigens, including Ly6C/G, CD80, and CD86.5-7 To explore whether FR+ macrophages also up-regulate these markers, cells isolated from the peritoneal cavities of mice treated with inflammatory stimuli were labeled with both folate-FITC and an antibody to Ly6C/G, CD80, or CD86. Figure 3 shows that, in contrast to quiescent peritoneal macrophages, FR+ macrophages express high levels of all 3 activation markers. Furthermore, macrophages lacking these activation markers are predominantly FR−. Although not all cells expressing Ly6C/G, CD80, and CD86 are FR+, the data nevertheless confirm that the FR is primarily expressed on macrophages that also display standard activation markers.
FR is coexpressed with macrophage activation markers, CD80, CD86, and Ly6C/G. Peritoneal macrophages gated in the region defined by forward and side scatter were examined by flow cytometry for expression of both FR and antigens characteristic of activated macrophages, CD80, CD86, and Ly6C/G. Note that FR+ macrophages express high levels of these markers.
FR is coexpressed with macrophage activation markers, CD80, CD86, and Ly6C/G. Peritoneal macrophages gated in the region defined by forward and side scatter were examined by flow cytometry for expression of both FR and antigens characteristic of activated macrophages, CD80, CD86, and Ly6C/G. Note that FR+ macrophages express high levels of these markers.
A third characteristic of activated macrophages lies in their ability to produce cytotoxic agents, such as TNF-α1,2 and ROS.30 To test FR+ macrophages for production of TNF-α, bacteria-recruited F4/80+ macrophages were analyzed by intracellular flow cytometry for coexpression of FR and TNF-α. As shown in Figure S3, virtually all FR+ macrophages also express TNF-α.
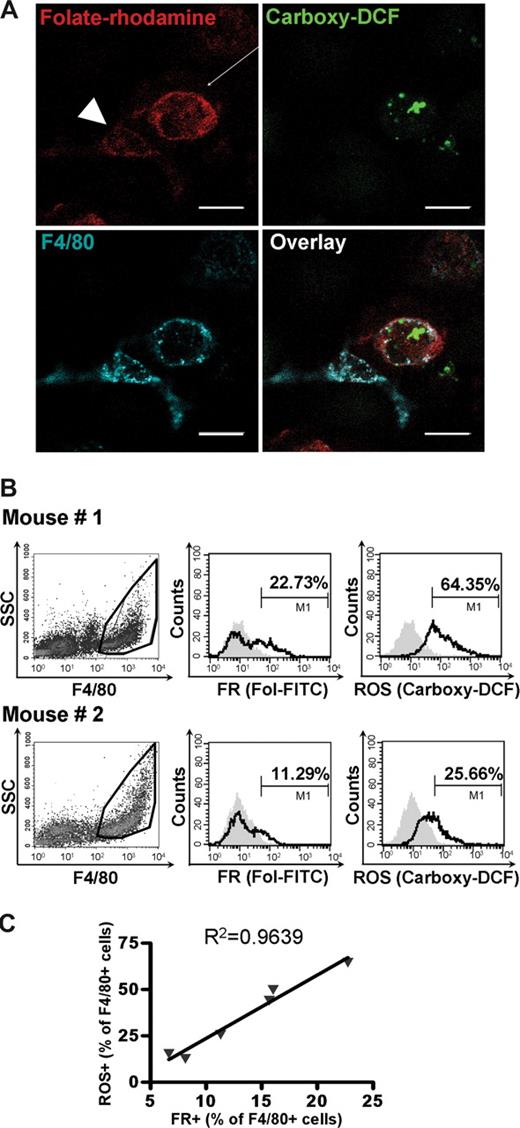
To test FR+ macrophages for production of ROS, peritoneal macrophages from live bacteria-injected mice were incubated with folate-rhodamine, anti-F4/80, and carboxy-H2DCFDA, a cell-permeant dye that becomes fluorescent on reaction with ROS.31 As shown in Figure 4A, ROS was consistently detected inside FR+ macrophages using 3-color confocal microscopy. Moreover, cells that were actively producing ROS invariably had brighter folate-rhodamine fluorescence (arrow) than macrophages generating no ROS (arrowhead). It should be noted that folic acid levels had no effect on ROS or TNF production (data not shown).
ROS production correlates with expression of FR on macrophages. (A) FR+ macrophages actively produce ROS. Macrophages isolated from bacteria-treated mice were simultaneously incubated with (1) carboxy-H2DCFDA to image ROS (green), (2) folate-rhodamine to image FR (red), and (3) anti-F4/80 to stain macrophages (blue) and then examined by confocal microscopy. The overlay of all the 3 colors within the same microscope field is also shown. (B) Cells collected from the peritoneal cavities of mice injected 3 days earlier with different doses of bacteria were analyzed by flow cytometry for the binding of folate-FITC and the production of ROS. The fraction of FR+ and ROS+ cells is indicated. (C) The percentage of ROS+ cells correlate linearly with the percentage of FR+ macrophages. Scale bar represents 20 μm.
ROS production correlates with expression of FR on macrophages. (A) FR+ macrophages actively produce ROS. Macrophages isolated from bacteria-treated mice were simultaneously incubated with (1) carboxy-H2DCFDA to image ROS (green), (2) folate-rhodamine to image FR (red), and (3) anti-F4/80 to stain macrophages (blue) and then examined by confocal microscopy. The overlay of all the 3 colors within the same microscope field is also shown. (B) Cells collected from the peritoneal cavities of mice injected 3 days earlier with different doses of bacteria were analyzed by flow cytometry for the binding of folate-FITC and the production of ROS. The fraction of FR+ and ROS+ cells is indicated. (C) The percentage of ROS+ cells correlate linearly with the percentage of FR+ macrophages. Scale bar represents 20 μm.
To further establish a possible relationship between FR expression and ROS production, F4/80+ macrophages from mice treated intraperitoneally with various concentrations of live bacteria were analyzed simultaneously for FR expression and ROS production by flow cytometry. An example of the raw flow data is shown in Figure 4B, where both FR expression and ROS production are greater in mouse 1 than mouse 2. More importantly, when similar data from multiple mice were analyzed (Figure 4C), a linear correlation was found between FR expression and ROS production. Together with the data on the stimuli that induce FR expression, cell morphology, and cell differentiation antigen expression, the ROS data argue that FR constitutes an activation marker for macrophages.
Unfortunately, even among activated macrophages, significant phenotypic variation can exist.1,2 Because all of the markers examined (CD80, CD86, Ly6C/G, TNF-α, and ROS) are used solely to identify “classically activated” macrophages, 2 markers of “alternatively activated” macrophages (mannose receptor2 and CD235 ) were also evaluated. As shown in Figure S3, macrophages from the variously stimulated mice expressed only low levels of these “alternatively activated” macrophage markers. And although a fraction of the stimulated macrophages were found to express both FR and either CD23 or the mannose receptor, especially in bacteria-activated and zymosan-recruited macrophages, the overall correlation of FR expression with markers thought to be up-regulated on “alternatively activated” macrophages was not strong.
FRs expressed on macrophages are fully functional
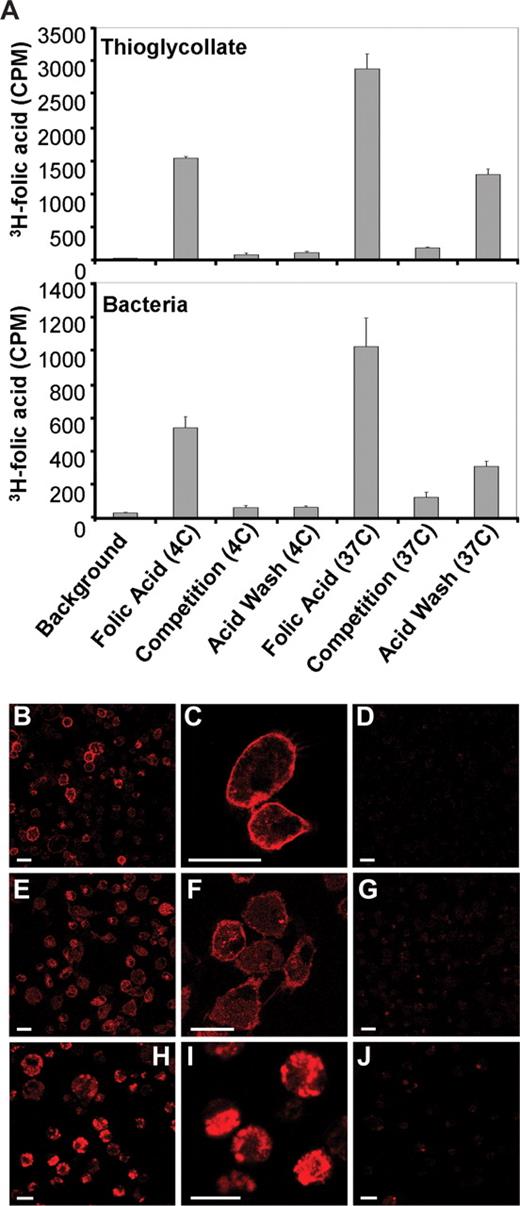
For reasons unknown, some FRs (eg, those expressed on hematopoietic stem/progenitor cells) cannot bind or internalize either folic acid or any of its conjugates.32,33 The potential for using the FR expressed on macrophages to deliver imaging and therapeutic agents necessitates that those FRs be capable of both binding and internalizing folate-linked molecules. To test the functional status of FR on activated peritoneal macrophages, thioglycolate- and bacteria-recruited macrophages were incubated with 3H-folic acid for 2 hours at either 4°C (ie, a temperature at which ligand binding can proceed, but endocytosis is not permitted) or 37°C (a temperature at which both binding and endocytosis occur freely).34,35 Samples were then either washed to remove unbound ligand or stripped with acidic buffer to remove both unbound and externally bound 3H-folic acid. As illustrated in Figure 5A (top panel, thioglycolate-recruited macrophages; and bottom panel, bacteria-recruited macrophages), cells incubated at 4°C bound the radioligand at neutral pH but failed to retain it during acid wash, suggesting that the 3H-folic acid was externally bound. In contrast, macrophages incubated with radioligand at 37°C retained approximately 40% of their total 3H-folic acid after acid wash, demonstrating that much of their folic acid had been internalized. These data suggest that FR is retained at the cell surface at 4°C but internalized and inaccessible after a 2-hour incubation at 37°C. This behavior is typical of receptor-mediated endocytosis.34
FR+ macrophages bind and internalize 3H-folic acid and folate conjugates. (A) Thioglycolate- (top panel) and bacteria-recruited (bottom panel) macrophages were examined for binding and internalization of 3H-folic acid under permissive (37°C) and nonpermissive (4°C) conditions for receptor-mediated endocytosis. (B,C) Confocal images show strong surface binding of folate-rhodamine after 10 minutes of incubation in thioglycolate-recruited FR+ macrophages. (D) Surface binding of folate-rhodamine can be blocked by coincubation with 100-fold excess of folic acid. (E,F) A fraction of bound folate-rhodamine is internalized by 2 hours at 37°C. (G) The binding and internalization of folate-rhodamine can be blocked by coincubation with 100-fold excess of folic acid. (H,I) In vivo binding and internalization of folate-rhodamine by FR+ macrophages. (J) In vivo binding and internalization of folate-rhodamine can be blocked by preinjection with 100-fold excess of folic acid, which indicates the uptake is FR-mediated. Scale bar represents 20 μm.
FR+ macrophages bind and internalize 3H-folic acid and folate conjugates. (A) Thioglycolate- (top panel) and bacteria-recruited (bottom panel) macrophages were examined for binding and internalization of 3H-folic acid under permissive (37°C) and nonpermissive (4°C) conditions for receptor-mediated endocytosis. (B,C) Confocal images show strong surface binding of folate-rhodamine after 10 minutes of incubation in thioglycolate-recruited FR+ macrophages. (D) Surface binding of folate-rhodamine can be blocked by coincubation with 100-fold excess of folic acid. (E,F) A fraction of bound folate-rhodamine is internalized by 2 hours at 37°C. (G) The binding and internalization of folate-rhodamine can be blocked by coincubation with 100-fold excess of folic acid. (H,I) In vivo binding and internalization of folate-rhodamine by FR+ macrophages. (J) In vivo binding and internalization of folate-rhodamine can be blocked by preinjection with 100-fold excess of folic acid, which indicates the uptake is FR-mediated. Scale bar represents 20 μm.
To obtain information on the kinetics of binding and internalization of 3H-folic acid by FR+ macrophages, the time course of 3H-folate uptake at 37°C was determined. As shown in Figure S5, surface binding was half-maximal by approximately 10 minutes and essentially saturated at approximately 2 hours. Internalization, in contrast, increased linearly with time, suggesting that surface FR can continually recycle to the cell interior and deliver bound folates to the cell cytoplasm. Again, both cell surface binding and internalization were fully competable with excess folic acid, suggesting that binding and endocytosis are FR-mediated.
Surface binding and internalization of folate conjugates by FR+ macrophages were also visualized by confocal microscopy. As seen in Figure 5B and C, surface localization of folate-rhodamine was prominent within 10 minutes of conjugate addition, in agreement with the kinetic studies described in the previous paragraph. In further agreement with the kinetics data (Figure 5A), a fraction of the bound folate conjugate was also internalized by 2 hours at 37°C (Figure 5E,F). In addition, no folate-rhodamine fluorescence was detected in macrophages incubated with excess folic acid to block all empty FR (Figure 5D,G). We therefore conclude that folate-rhodamine binding and internalization are specific for FR-expressing macrophages.
To verify that FR is functional on activated macrophages in vivo, folate-rhodamine was injected intraperitoneally into mice previously stimulated with thioglycolate. Ten minutes after folate-rhodamine injection, peritoneal cells were collected by lavage and examined by confocal microscopy. Within the brief incubation period, peritoneal macrophages were observed to both bind and internalize the folate conjugates. Thus, as seen in Figure 5H,I, the majority of the fluorescence has already entered the cell by this 10-minute time point. Because measurable internalization of folate-rhodamine by peritoneal macrophages cultured in vitro required approximately 30 minutes (Figure S5), we suspect that factors within the peritoneal cavity may accelerate FR-mediated surface binding and endocytosis in vivo.
Delivery of folate-conjugated imaging agents to FR+ macrophages in rheumatoid arthritis patients
Activated macrophages have been described as a key mediator of rheumatoid arthritis, and folate-targeted immunotherapy has been applied to effectively treat both adjuvant-induced arthritis in rats and collagen-induced arthritis in mice.25,27 To evaluate whether natural sites of inflammation might also accumulate FR+ macrophages, cells collected from the synovial fluid of patients with clinically diagnosed rheumatoid arthritis were incubated with folate-FITC and TriColor-conjugated anti-CD11b, and expression of functional FR on the synovial macrophages was assessed by dual-color flow cytometry. A substantial fraction of CD11b+ macrophages were found to express FR (Figure 6A left). Furthermore, as indicated by the competition study, folate-FITC binding was specific to FR, as demonstrated by inhibition of binding by incubation with free folic acid (Figure 6A right). This confirms that activated macrophages in the inflamed joints of RA patients express a functional FR in human.
FR expressed on activated macrophages in patients with rheumatoid arthritis can be exploited to deliver folate-conjugated imaging agents to inflamed joints. (A) Synovial cells from 4 patients with rheumatoid arthritis were labeled with anti-CD11b to stain human macrophages and then incubated with folate-FITC in the absence (left panel) and presence (right panel) of 100-fold excess free folic acid before examination by flow cytometry. A representative flow plot is shown here. (B) Radioimage of the hands (left) and feet (right) of a patient with active rheumatoid arthritis, showing sites of uptake of EC20, a folate-targeted 99mTc-based radioimaging agent, in multiple joints of the hands and feet. (C) Radio-image with EC20 of the hands (left) and feet (right) of a nonarthritic patient obtained under identical conditions to those shown in panel B.
FR expressed on activated macrophages in patients with rheumatoid arthritis can be exploited to deliver folate-conjugated imaging agents to inflamed joints. (A) Synovial cells from 4 patients with rheumatoid arthritis were labeled with anti-CD11b to stain human macrophages and then incubated with folate-FITC in the absence (left panel) and presence (right panel) of 100-fold excess free folic acid before examination by flow cytometry. A representative flow plot is shown here. (B) Radioimage of the hands (left) and feet (right) of a patient with active rheumatoid arthritis, showing sites of uptake of EC20, a folate-targeted 99mTc-based radioimaging agent, in multiple joints of the hands and feet. (C) Radio-image with EC20 of the hands (left) and feet (right) of a nonarthritic patient obtained under identical conditions to those shown in panel B.
To evaluate the potential of folate-conjugated imaging agents to assist in the diagnosis of inflammatory diseases, rheumatoid arthritis patients were imaged with EC20, a folate-targeted 99mTc-based radioimaging agent.36,37 As seen in Figure 6B, EC20 uptake was prominent in multiple joints of the hands and feet of arthritic patients, whereas neither hands nor feet from nonarthritic patients showed EC20 uptake (Figure 6C; additional data on 40 rheumatoid patients will be published). Curiously, of the 7 joints seen to display marked EC20 uptake, only 4 were independently identified as arthritic by a clinical rheumatologist blinded to the image analysis, suggesting that the FR-targeted imaging agent may detect different aspects of the inflammatory disease than the clinical examination. Although additional studies remain before an unequivocal interpretation of the human imaging can be offered, the data nevertheless demonstrate that a folate-linked molecule can target sites of active inflammation in human patients with rheumatoid arthritis.
Finally, in an effort to generate larger quantities of FR+ macrophages, we explored methods to induce them in vitro. For this purpose, human peripheral blood monocytes were plated in 6-well plates, induced to differentiate into macrophages by incubation with M-CSF, and stimulated with a variety of cytokines and toll-like receptor ligands to promote their activation. After various incubation periods, adherent macrophages were analyzed for FR expression by flow cytometry. Regardless of the stimulus used (including M-CSF, granulocyte M-CSF (GM-CSF), IL-1, IL-2, IL-4, IL-6, IL-10, IL-12, IL-13, IL-17, TNF-α, IFN-γ, IFN-α, receptor activator of NF-κB ligand (RANKL), neopterin, LPS, zymosan, muramyl dipeptide, CpG oligonucleotides, immune complexes, immune stimulating saponins, methotrexate, and all-trans-retinoic acid), FR expression could not be promoted in any of the macrophage cultures examined (Figure S6). Even multiple combinations of the aforementioned stimulants failed to induce FR expression on the adherent macrophages. In addition, because FR expression was mainly observed in “classically activated” macrophages generated in murine peritonitis models, we also attempted to generate activated FR+ macrophages in vitro by using murine bone marrow–derived macrophages and stimulating them with IFN-γ plus LPS. As expected (Figure S7), IFN-γ plus LPS-treated bone marrow–derived macrophages expressed a similar level of the macrophage marker, F4/80, but a much higher level of the classical activation markers CD86 and TNF-α. However, no folate-FITC binding could be detected in the same cells, indicating that IFN-γ plus LPS stimulation is also unable be induce FR expression in murine bone marrow–derived macrophages in vitro.
Discussion
In this report, we have demonstrated that FR expression is observed on a subset of peritoneal and synovial macrophages, but not on other infiltrating immune cells. We suggest that these FR+ macrophages are activated based on (1) their accumulation in the peritoneal cavities of mice treated with inflammatory stimuli but not in mice treated with sterile saline, (2) their enlarged and irregular morphologies, (3) their coexpression of multiple activation antigens, (4) their production of both reactive oxygen species and TNF-α, and (5) their enrichment in the joints of rheumatoid arthritic patients but not in the joints of healthy patients. Based on these considerations, we conclude that FR constitutes a new activation marker for macrophages. Whether alternatively activated macrophages also express a FR will require further investigation.
Macrophages are equipped with a plethora of receptors that enable them to sense an enormous variety of danger signals in their environment.38,39 Toll-like receptors permit detection of LPS, bacterial cell wall fragments, foreign DNA, double-stranded RNA, flagella, and many other bacterial, fungal, and viral products. Fc receptors alert the macrophage to immune complexes of foreign antigens that have formed as a consequence of a previously developed immunity. Cytokine, chemokine, and tachykinin receptors inform the macrophage when other cells in the host have detected a pathogen. Cell contact receptors also mediate warning signals from other immune cells (eg, T cells, natural killer [NK] cells) and certain mesenchymal cells, only in this case cell contact is required for signal transmission. Taken together, the macrophage is seen to be endowed with a variety of receptors that act through diverse signaling pathways to prepare the cell to respond to foreign pathogens. With this perspective in mind, it is perhaps not surprising that multiple forms of activated macrophages exist1,2 and that each form is characterized by a somewhat different set of activation markers. As shown in Figure 3, flow cytometric data reveal that most FR+ macrophages also express CD80, CD86, and Ly6C/G, but a few do not. Moreover, as seen in Figure 1, macrophages recruited by thioglycolate appear to homogeneously express higher levels of FR, whereas macrophages recruited by live bacteria separate into distinct populations of strong and nonresponders. Although the molecular causes of these differences remain unknown, the observed diversity in FR+ macrophages should not be surprising because the stimuli used to promote their recruitment are known to induce a variety of activation pathways.
Along this same line of reasoning, whereas all inflammatory stimuli tested were found to induce a population of FR+ peritoneal macrophages, the molecular stimulus required for FR expression was never identified. In a frustrating attempt to define this stimulus, multiple factors (eg, IFN-γ, bacterial cell wall fragments, LPS) known to induce human monocyte-derived macrophage activation40 were tested for their abilities to promote FR expression in vitro (Figure S6). Unfortunately, none of these stimuli, either alone or in combination, were found to up-regulate FR in cultured M-CSF differentiated human macrophages (Figure S6). Similarly, classic activation stimuli, IFN-γ and LPS, were unable to induce FR expression in murine bone marrow–derived macrophages (Figure S7), although various inflammatory stimuli were able to up-regulate FR expression in vivo. Possible explanations for our failure to replicate the in vivo up-regulation of FR on macrophage activation might include the fact that multiple stimuli for macrophage activation exist and the stimulus or combination of stimuli required for induction of FR expression were not replicated in our in vitro experiments. Alternatively, M-CSF may select for a type of macrophage in vitro that differs from naturally matured macrophages in vivo, or FR may be expressed transiently on activated macrophages and the window of FR expression may not have been sampled in our cell-culture experiments.
Because activated macrophages are not thought to be actively proliferating, the question naturally arises why activated macrophages might up-regulate a receptor for a vitamin required primarily for DNA synthesis. Although many hypotheses can be offered, we speculate that macrophages might primarily undergo activation to equip themselves with tools to prevent pathogen invasion; that is, as suggested by their accumulation at sites of inflammation and regions of chronic exposure to pathogens (eg, airways in lungs, outer layers of skin). In this scenario, the macrophage might contribute to pathogen elimination by depleting nutrients essential for pathogen growth. Thus, it has been recently established that macrophages rapidly accumulate iron during an acute infection,41 leading to the commonly observed drop in serum iron levels.42 Perhaps depletion of folate from the nutrient-rich intercellular milieu via expression of a high affinity FR can also contribute to limiting pathogen proliferation.
Flow cytometry data revealed that FR is only observed on cells that display common macrophage markers, including F4/80, CD11b, and CD44, confirming the myelocytic origin of the FR+ cells (data not shown). However, unlike some myeloid cells that express a nonfunctional beta isoform of FR,32,43 FR on activated macrophages was shown to be fully capable of internalizing folic acid and folate-linked molecules. This observation is important because attachment of drugs to folic acid has already been demonstrated to enable selective targeting of the linked drugs to FR-expressing cancers. Indeed, 5 folate-conjugated drugs are currently undergoing human clinical trials for treatment/imaging of cancer (www.ClinicalTrials.gov). Because recent studies have demonstrated that inflamed joints can be similarly imaged and treated with folate-targeted drugs in animal models of rheumatoid arthritis,27 our data showing folate targeting of an imaging agent to the inflamed joints of a rheumatoid arthritis patient raise optimism that FR targeting can also be exploited for the diagnosis and treatment of inflammatory arthritis in humans. Indeed, the observation that activated macrophages contribute to multiple types of inflammatory diseases also opens the need to explore uses of folate-conjugated drugs to image/treat other inflammatory diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Erina Vlashi for providing folate-rhodamine, Walter A. Henne for providing folate–Oregon Green, and Hongtao Chen for helping with the confocal microscopy.
This work was supported in part by a grant from Endocyte (West Lafayette, IN).
Authorship
Contribution: W.X. and A.R.H. performed the research and prepared the first draft of the manuscript; E.L.M. obtained the images of rheumatoid arthritis patients with EC20; M.B.L. collected the synovial fluid from rheumatoid arthritis patients; J.X.C. assisted with the confocal microscopy studies; and P.S.L. conceived of the project, supervised the research, and edited the manuscript.
Conflict-of-interest disclosure: P.S.L. owns stock in Endocyte, a company that is developing folate-targeted drugs for treatment of folate receptor expressing cancers. Demonstration of folate receptor expression on activated macrophages may enable use of folate to target drugs to these cells. The remaining authors declare no competing financial interests.
Correspondence: Philip S. Low, Department of Chemistry, Purdue University, 560 Oval Drive, West Lafayette, IN 47907; e-mail: plow@purdue.edu.
References
Author notes
*W.X. and A.R.H. contributed equally to this study.