Abstract
CD4+ T (Th)–cell help to B lymphocytes requires cognate interaction and CD40 engagement. Invariant natural killer T (iNKT) cells are innate-like T lymphocytes that recognize αgalactosylceramide (αGalCer) presented by CD1d, and can help B-cell responses. We asked whether αGalCer-activated iNKT cells help B lymphocytes through cognate interaction, or indirectly, via enhancement of Th-B–cell interaction. After immunization with protein Ags and αGalCer, antibody titers were assessed in wild-type or splenectomized mice, and in bone marrow radiation chimeras lacking CD1d or CD40 expression on B lymphocytes, or expressing CD1d or MHC II disjointly on antigen-presenting cells (APCs). We find that αGalCer-dependent enhancement of B-cell response (1) can occur when B cells do not express CD1d but express CD40; (2) requires that iNKT and Th cells interact with the same APCs that coexpress both CD1d and MHC-II; and (3) takes place without spleen. These findings demonstrate αGalCer-induced help for antibody responses can occur without cognate iNKT/B-cell interaction, and suggest this help entails activation of APCs by iNKT cells, which in turn activate Th cells and their helper functions for B cells. Thus, the αGalCer-induced help recapitulates the function of classical adjuvants that stimulate the innate immune system to support adaptive immune responses.
Introduction
T cell–dependent (T-D) antibody response to a protein Ag is a dynamic process relying on an ordered series of intercellular interactions, which take place in defined zones of secondary lymphoid organs.1 Ag-specific Th cells expand and acquire helper functions in the T zone upon recognition of cognate peptide–MHC II complexes on dendritic cells (DCs), which have internalized and processed the Ag. In parallel, B cells in the follicles capture the antigen via their B-cell receptors (BCRs). This results both in B-cell activation and in antigen internalization, processing, and surface presentation of peptides in the context of MHC II. Activated Th and B cells meet at the follicle/T-zone boundary and, upon recognition of cognate peptide–MHC II complexes on B cells, Th cells (1) up-regulate CD40L that engages CD40 on B cells, delivering a critical proliferation and differentiation signal; and (2) secrete cytokines required for Ig isotype switching.1 This cognate T-B interaction leads to the development of either short-lived plasma cells (PCs) or, following the germinal center (GC) reaction, to memory B cells and long-lived plasma cells bearing high-affinity and class-switched Ig.2
Successful protective T-D antibody responses can be induced by vaccination with purified protein antigens mixed with immunologic adjuvants, defined as any substance that stimulates early innate immune responses to aid the establishment of protective adaptive responses.3 Adjuvants ultimately target antigen-presenting cells (APCs) and antigen-presenting functions by facilitating their antigen uptake and presentation, or by enhancing their costimulatory functions, or both.4
Invariant natural killer T cells (iNKT cells) are characterized by the expression of the homologue invariant Vα14-Jα18 (iVα14-Jα18) and iVα24-JαQ rearrangements in mice and humans, respectively.5 The iTCRα chains pair with variable TCRβ chains that use a restricted repertoire of V regions: Vβ8.2, Vβ7, and Vβ2 in mice and Vβ11 in humans.6,7 The semi-invariant TCR recognizes CD1d,8,9 a non–MHC-encoded class I–like molecule expressed mainly on cells of hematopoietic origin and by some nonhematopoietic cell type.10,11 iNKT cells recognize glycosphingolipids Ags that specifically bind CD1d, among which is αgalactosylceramide (αGalCer), isolated from marine sponges.12,13
Because of their constitutive effector phenotype and functions, and expression of several NK cell receptors, iNKT cells represent the prototype of innate T lymphocytes.14
Administration of αGalCer into mice and humans rapidly activates iNKT cells to release Th1 and Th2 cytokines.15,16 Vaccination of mice with αGalCer mixed with irradiated malaria sporozoites markedly enhances the level of CD8+ T cell–dependent protective immunity against malaria,17 implying helper functions for iNKT cells. Cognate recognition of αGalCer-CD1d complexes presented by DCs in vivo is crucial to trigger helper functions in iNKT cells,18,19 which can then assist the activation of CD4+ and CD8+ T cells specific for the coadministered Ags via licensing of DCs.20-22
We have shown that human iNKT cells help B-cell proliferation and antibody production in vitro upon cognate interaction.23 Furthermore, we have recently demonstrated that vaccination of mice with protein Ags administered with αGalCer greatly enhanced critical features of the T-D B-cell response, such as protection from infections and B-cell memory.24 The expression of CD40L was critical, whereas that of both IFN-γ and IL-4 was dispensable for the αGalCer-dependent enhancement of B-cell responses.24
In the present study, we have investigated the mechanisms by which αGalCer-activated iNKT cells enhance antibody responses specific for protein Ags. To this aim, we constructed different types of bone marrow radiation chimeras (BMCs). First, to study whether iNKT cells enhance Ab responses directly, by provision of cognate help to B cells, we generated BMCs in which B cells do or do not express CD1d. Second, to determine whether iNKT cells help antibody responses indirectly, through the licensing of APCs that in turn improves Th cell activation and subsequent conventional T/B-cell interactions, we constructed BMCs in which CD1d or MHC II molecules are expressed disjointly on all APCs. Third, to assess the role for CD40 triggering on B cells, we generated mice in which B cells do or do not express CD40. Finally, we investigated whether the enhancement of protein Ag-specific antibody responses by iNKT cells depends on cellular interactions occurring in the spleen. BMCs, and splenectomized and control mice were immunized subcutaneously with different protein Ags, with or without αGalCer or alum, and their specific antibody titers were compared.
Methods
Mice
Wt C57BL/6 mice were purchased from Charles River (Calco, Italy). B6.129-CD1tm1Gru CD1d-deficient mice25 (referred to as CD1d−/−) were kindly obtained from Dr H. R. MacDonald (Ludwig Institute for Cancer Research, Lausanne, Switzerland) and were backcrossed at the 12th generation onto C57BL/6 at the San Raffaele Scientific Institute. B6.129S2-Igh-6tm1Cgn B cell–deficient (μMT°),26 B6.129-Tnfrsf5tm1Kik CD40-deficient (CD40−/−),27 and B6.129-H2-Ab1tm1Doi/DoiOrl MHC II–deficient mice (AB0, referred to as MHCII−/−)28 were obtained from the European Mouse Mutant Archive (EMMA, Orleans, France). All mice were housed in a pathogen-free environment and used for experiments at 6 to 8 weeks of age. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee at San Raffaele Scientific Institute.
Generation of mixed BM chimeras
One week before irradiation, recipient mice received antibiotic prophylaxis in the drinking water. BM, extracted from the femurs of donor mice, was T-cell depleted by negative selection using anti-Thy1.2 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and immunomagnetic sorting according to the manufacturer's instructions. To generate mice bearing B cells expressing or not expressing CD1d or CD40, 6- to 8-week-old μMT° mice were lethally irradiated and reconstituted intravenously with 1 × 107 T-cell–depleted BM cells, 80% of which were from μMT° mice and 20% from either wt or CD1d−/− or CD40−/− mice. As described,29 over an 8-week period the 20% BM cells completely repopulated the peripheral lymphoid system with B cells while contributing only 20% to other lineages (data not shown). To generate mice bearing APCs expressing CD1d or MHC II disjointly, CD1d−/− mice were lethally irradiated, and reconstituted by intravenous injection of 107 BM cells, composed of equal numbers of cells taken from CD1d−/− and from MHC II−/− mice.
Mice splenectomy
After anesthesia, the spleen was exposed through the peritoneum and skin and removed after ligation of arteries with unresorbable silk (Biosyn 4-0; Syneture, Mansfield, MA). The peritoneal cavity and skin were closed with silk suture and clips, respectively. Splenectomized mice were vaccinated 7 days after surgery.
Antibodies and flow cytometry
The following mAb conjugates (BD Biosciences, San Jose, CA) were used to stain the cells: anti–CD3-FITC (145-2C11), anti–I-Ab-FITC (25-9-17), anti–CD11c-FITC (HL3), anti–CD1d-PE (1B1), anti–CD40-PE (3/23), anti–CD4-PerCP (RM4-5), anti–NK1.1-PerCP (PK136), anti–CD19-APC (1D3), and anti–TCR-β-APC (H57-597). iNKT cells were specifically identified by αGalCer-loaded mouse CD1d-IgG1 dimers (BD Biosciences), as described previously.30 Samples were acquired on a FACScanto flow cytometer (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, Ashland, OR).
The reconstitution of T, B, iNKT, and NK cells was determined by flow cytometry analysis 8 weeks after the BM transplantation. Peripheral blood mononuclear cells (PBMCs), obtained from all mice that received a BM transplant, and splenic and hepatic mononuclear cells, obtained from 2 mice per group, were stained using anti-CD3, anti-CD4, and anti-CD19 mAbs (T and B cells) or αGalCer-mCD1d dimers and anti-TCRβ and anti-NK1.1 mAbs (iNKT and NK cells). CD1d, MHC-II, and CD40 expression on circulating B lymphocytes was assessed by triple staining with anti-CD19, anti-CD1d, anti-IAβ, and anti-CD40 mAbs. To determine the disjoint expression of CD1d and MHC II on splenic DCs, spleens were minced and digested with collagenase D, and single-cell suspensions were stained with anti-CD19, anti-CD1d, and anti-MHC II mAbs (B cells) or with anti-CD11c, anti-CD1d, and anti–MHC II mAbs (DCs).
Immunization protocol
Mice were immunized with the following antigens and doses: tetanus toxoid (TT, 3 μg/dose), hemoagglutinin/neuroaminidase subunits from the human influenza viruses A/Panama/2007/99-RESVIR17 (H3N2, 3 μg/dose) (from Novartis Vaccines), and 4-hydroxy-3-nitrophenyl-chicken gamma globulin (NP-CGG, 50 μg/dose; Biosearch Technologies). Antigens dissolved in 100 μL PBS, alone or with 1 μg/mL αGalCer (0.1 μg/dose; Alexis, Lausen, Switzerland), were injected subcutaneously in the left flank on day 0 in 5 mice per group. On day +14, a second dose of the same antigen without αGalCer was given to all mice.
Titration of antigen-specific antibodies
Sera samples were collected from mice on days +13 and +27 after immunization to titrate antigen-specific antibodies. Individual sera were titrated in parallel at the same time for their content in circulating antigen-specific antibodies by end-point enzyme-linked immunosorbent assay (ELISA) as described.24
Antigen-specific cytokine production in vitro
Wt and BMC mice (3 per group) were immunized subcutaneously with PBS, NP-CGG, or NP-CGG and αGalCer at day 0. At day +10, splenocytes were obtained from mice and plated in duplicated wells at 2 × 106/well in 0.2 mL RPMI medium 1640, 5% FCS, and increasing concentrations of CGG. Secreted IFN-γ was quantified by standard ELISA (BD Pharmingen, San Diego, CA) in culture supernatants obtained after 72 hours.
Statistics
Antibody titers were log10 transformed and analyzed for statistically significant differences by 2-tailed Student t test for unpaired samples, applying correction for unequal variances when required. When more than 2 groups were compared, the one-factor ANOVA with the LSD correction for multiple comparisons was applied. For all tests, a P value less than .05 was considered significant.
Results
αGalCer-activated iNKT cells can enhance B-cell responses to protein Ags in a noncognate fashion
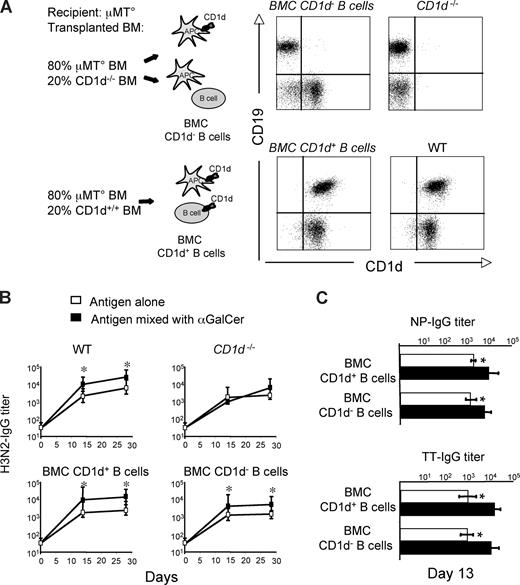
We have recently shown that immunization of mice with protein Ag and αGalCer elicits iNKT-cell helper functions that enhanced primary and secondary antibody responses.24 To determine whether iNKT cells enhance antibody responses by providing cognate help to B cells, we constructed a set of mixed BMCs in which iNKT cells would be activated by wild-type APCs, but would interact with B cells expressing or lacking CD1d. Lethally irradiated immunodeficient RAG−/− mice were reconstituted with 80% BM cells from mice lacking B lymphocytes (μMT°) mixed with 20% BM cells from mice either bearing (CD1d+/+) or lacking (CD1d−/−) CD1d. Two months later, normal numbers of circulating NK, T, and B cells were found in both groups of mixed BMCs, whereas iNKT cells were reduced about 3-fold in comparison with wt animals (data not shown). In the group of mice reconstituted with mixed μMT° and CD1d−/− BM cells, B lymphocytes did not express CD1d, whereas in the group reconstituted with mixed μMT° and CD1d+/+ BM cells, all B lymphocytes displayed normal levels of CD1d (Figure 1A). Both groups of mice were immunized with H3N2, or TT or NP-CGG with or without αGalCer, following the schedule given in Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), and their antibody responses induced after each immunization were assessed. As shown in Figure 1B and C, in both mixed BMC groups, the Ag-specific antibody titers induced by immunization with αGalCer were significantly higher than those induced by the Ag alone. These results suggested that cognate-dependent help did not account for the observed iNKT cell–dependent enhancement of antibody responses to protein or hapten-carrier antigens.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines in a noncognate fashion. Specific IgG responses induced in BMCs and wt mice upon vaccination with Ags mixed or not mixed with αGalCer, according to the schedule shown in Figure S1. (A) Expression of CD1d on circulating B cells (CD19+) from mixed BMCs, and CD1d−/− and wt mice. (B) H3N2-specific antibody titers induced by immunization of wt mice, CD1−/− mice, or mixed BMCs expressing or not expressing CD1d on B cells. (C) Primary TT- and NP-CGG–specific antibody titers induced by vaccination in BMCs expressing or not expressing CD1d on B cells. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines in a noncognate fashion. Specific IgG responses induced in BMCs and wt mice upon vaccination with Ags mixed or not mixed with αGalCer, according to the schedule shown in Figure S1. (A) Expression of CD1d on circulating B cells (CD19+) from mixed BMCs, and CD1d−/− and wt mice. (B) H3N2-specific antibody titers induced by immunization of wt mice, CD1−/− mice, or mixed BMCs expressing or not expressing CD1d on B cells. (C) Primary TT- and NP-CGG–specific antibody titers induced by vaccination in BMCs expressing or not expressing CD1d on B cells. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
iNKT cells enhance B-cell response to protein Ags by facilitating Th-cell activation through the licensing of the same APC
The results obtained in the previous set of experiments led us to hypothesize that αGalCer-activated iNKT cells helped antibody responses to protein or hapten-carrier Ag indirectly, through an adjuvant-like mechanism that stimulates APCs to promote the establishment of Th-cell responses and, in turn, of conventional T-B–cell interactions. To test this hypothesis, we constructed mixed BMCs in which iNKT and Th cells could not recognize the same APC because of the disjoint expression of CD1d or MHC-II on all hematopoietic cells. The mixed BMCs were constructed by transplanting an equal number of BM cells from MHC-II−/− and CD1d−/− mice into lethally irradiated CD1d−/− recipients (Figure 2A). Control BMCs were generated by reconstituting lethally irradiated wt mice with wt BM cells. After immune reconstitution, mixed BMCs expressing MHC-II or CD1d disjointly on APCs (Figure 2A) were immunized with TT or NP-CGG, mixed or not mixed with αGalCer (according to the schedule in Figure S1) to determine the specific antibody titers induced after each immunization. As shown in Figure 2B, the disjoint expression of CD1d or MHC-II on APCs completely abrogated the αGalCer-induced enhancement of antibody responses. By contrast, BMCs generated with wt donor BM, therefore bearing APCs coexpressing both CD1d and MHC-II, mounted significantly higher antibody responses when vaccinated with antigen and αGalCer, achieving antibody titers substantially similar to that of wt animals. The 2 types of BMCs responded with comparable antibody titers to immunization with NP-CGG mixed with alum (data not shown), indicating that the disjoint expression of CD1d or MHC II on APCs did not abrogate the effect of a benchmark adjuvant, but specifically impaired that of αGalCer.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines by facilitating Th-cell priming via licensing of the same APC. Specific IgG and T-cell responses induced in mixed BMCs and wt mice by vaccination with Ags mixed or not mixed with αGalCer, according to the schedule in Figure S1. (A) Expression of MHC-II and CD1d on circulating B cells (CD19+) and, in 2 representative animals displaying the expected expression on circulating B cells, on splenic DCs (CD11c+) of BMCs. (B) Ag-specific IgG titers induced in BMCs and wt mice by vaccination. (C) IFN-γ secretion by splenic T cells upon restimulation in vitro with CGG 10 days after immunization of mice with NP-CGG with or without αGalCer. Asterisks indicate statistically significant differences: * indicates P values less than or equal to .05; **P ≤ .01. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines by facilitating Th-cell priming via licensing of the same APC. Specific IgG and T-cell responses induced in mixed BMCs and wt mice by vaccination with Ags mixed or not mixed with αGalCer, according to the schedule in Figure S1. (A) Expression of MHC-II and CD1d on circulating B cells (CD19+) and, in 2 representative animals displaying the expected expression on circulating B cells, on splenic DCs (CD11c+) of BMCs. (B) Ag-specific IgG titers induced in BMCs and wt mice by vaccination. (C) IFN-γ secretion by splenic T cells upon restimulation in vitro with CGG 10 days after immunization of mice with NP-CGG with or without αGalCer. Asterisks indicate statistically significant differences: * indicates P values less than or equal to .05; **P ≤ .01. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
To verify that, in the mixed BMCs, the lack of linked recognition of APCs by iNKT and Th cells had affected their licensing, resulting in a consequent reduction of the Ag-specific Th-cell response, mixed BMCs or wt mice were immunized with NP-CGG with or without αGalCer. After 10 days, their spleen cells were restimulated in vitro using CGG as recall Ag. As shown in Figure 2C, separation of CD1d and MHC-II on APCs significantly reduced the in vitro recall response of Th cells from mixed BMCs immunized with Ag plus αGalCer, making it comparable with that of Th cells from the same mixed BMCs vaccinated without αGalCer. By contrast, immunization with Ag plus αGalCer of either mixed BMCs generated with wt BM, or wt mice, significantly enhanced the in vitro recall response to the Ag in comparison with the immunization with Ag alone.
Together, these findings indicate that αGalCer exerts its adjuvant activity for antibody responses to protein or hapten-carrier Ags by activating iNKT cells that, in turn, facilitate Th-cell priming via licensing the same DCs upon cognate interaction.
αGalCer-induced enhancement of B-cell response to protein Ags requires CD40 expression by B cells
Engagement of CD40 on B cells by activated Th cells is crucial for T-D antibody responses. To determine whether the expression of CD40 on B cells was required for the αGalCer-induced enhancement of antibody response to protein vaccines, mixed BMCs in which B cells were expressing or lacking CD40, while all other BM-derived cells were CD40+, were constructed by reconstituting lethally irradiated μMT° mice with 80% BM cells from the same mice, mixed with 20% BM cells from mice either bearing (CD40+/+) or lacking (CD40−/−) CD40. In mice reconstituted with mixed μMT° and CD40+/+ BM cells, all APCs (MΦ, DCs, B cells) expressed CD40 on the surface, whereas in animals reconstituted with mixed μMT° and CD40−/− BM cells, all APCs except B cells expressed CD40 on their surface (Figure 3A and data not shown). Mixed BMCs were immunized with NP-CGG or TT with or without αGalCer, according to the schedule in Figure S1, to determine the specific antibody titers induced after each immunization. As shown in Figure 4B, antigen-specific antibody responses were completely abrogated in mixed BMCs that lacked CD40 expression on B cells, whether or not αGalCer was used as adjuvant.
Expression of CD40 by B cells is critical for the αGalCer-induced enhancement of T-D B-cell response to protein vaccines. Specific IgG responses induced in mixed BMCs and wt mice upon vaccination with Ags mixed or not mixed with αGalCer, according to the schedule shown in Figure S1. (A) Comparative expression of CD40 on circulating B lymphocytes (CD19+) of BMCs and, in 2 animals displaying the expected expression on circulating B cells, on splenic DCs (CD11c+) of BMCs. (B) NP-specific antibody titers induced by vaccination of mixed BMCs and CD40−/− mice. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 3 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
Expression of CD40 by B cells is critical for the αGalCer-induced enhancement of T-D B-cell response to protein vaccines. Specific IgG responses induced in mixed BMCs and wt mice upon vaccination with Ags mixed or not mixed with αGalCer, according to the schedule shown in Figure S1. (A) Comparative expression of CD40 on circulating B lymphocytes (CD19+) of BMCs and, in 2 animals displaying the expected expression on circulating B cells, on splenic DCs (CD11c+) of BMCs. (B) NP-specific antibody titers induced by vaccination of mixed BMCs and CD40−/− mice. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 3 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines in splenectomized mice. Splenectomized mice were vaccinated with NP-CGG with or without αGalCer 7 days after surgery, following the schedule in Figure S1. NP-specific IgG titers were determined. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
αGalCer-activated iNKT cells enhance T-D B-cell response to protein vaccines in splenectomized mice. Splenectomized mice were vaccinated with NP-CGG with or without αGalCer 7 days after surgery, following the schedule in Figure S1. NP-specific IgG titers were determined. Asterisks indicate statistically significant differences (P ≤ .05) in antibody titers between groups of mice immunized with Ag alone or mixed with αGalCer. Shown are the results from 1 experiment representative of 2 in which 5 mice per group were tested. Results are shown as mean plus or minus SEM.
αGalCer enhances T-D B-cell response to protein Ags in splenectomized mice
We finally investigated whether the adjuvant effect of iNKT cells triggered by αGalCer requires the involvement of innate and adaptive effectors homing to the spleen. To test this hypothesis, splenectomized wt mice were immunized subcutaneously with NP-CGG with or without αGalCer, according to the schedule in Figure S1. Even in the absence of the spleen, mice immunized with NP-CGG and αGalCer displayed antibody titers significantly higher than those immunized with NP-CGG alone (Figure 4). The stronger adjuvant effect of αGalCer found at day 13 then day 27 in asplenic mice may reflect a lower primary B-cell response induced by the Ag alone, suggesting a contribution of splenic B cells to the primary B-cell response induced by less immunogenic Ags. Similar results were obtained when splenectomized mice were vaccinated with TT with or without αGalCer (data not shown). These findings suggest that the lack of spleen does not preclude the helper function of αGalCer-activated iNKT cells when animals are immunized via the subcutaneous route, although the spleen may be necessary when Ags are delivered through other routes. Furthermore, this result also suggests that the αGalCer-activated iNKT cells help conventional T-B–cell interactions that may take place in peripheral LNs.
Discussion
Understanding the mechanisms of action of adjuvants is fundamental to improve both the design and the efficacy of protein-based vaccines. We have recently shown that αGalCer, the iNKT-cell Ag, is a potent adjuvant for the induction of protective antibody responses and B-cell memory specific for protein Ags derived from common human pathogens.24 In the present study, we have investigated the mechanisms by which iNKT cells help the establishment of antibody responses induced upon immunization of mice with protein or hapten-carrier Ags mixed with αGalCer. The results demonstrate that αGalCer-induced enhancement of B-cell response can occur without cognate iNKT/B-cell interaction, and suggest this help entails licensing of APCs by iNKT cells, which in turn improves Th-cell activation and subsequent helper functions for B cells.
Mixed BMCs in which MHC-II or CD1d is expressed disjointly on APCs are unable to mount antibody responses to protein antigen admixed with αGalCer, suggesting that iNKT and Th cells interact sequentially with the same APCs in vivo. It is likely that DCs are the key APCs that transduce iNKT cell–dependent signals into Th-cell activation. It has indeed been previously demonstrated that intravenous injection of αGalCer and protein antigens into mice leads to DC maturation and, in turn, to enhanced activation of CD4+ and CD8+ T cells specific for the coadministered antigen.20,21 Furthermore, in another study, experiments with cultured DCs showed that induction of Ag-specific CD4+ and CD8+ T-cell response was greatly improved by pulsing DCs with both peptides and αGalCer in vitro.22
The demonstration that iNKT cells can help B cells in a noncognate fashion, provided that B lymphocytes express CD40, is intriguing in the light of a recent study, suggesting the requirement for cognate iNKT/B-cell interaction to induce antibody responses to a chemical hapten, upon immunization with the NP-KLH hapten-carrier in combination with αGalCer.31 The discrepancy between this result and our study may derive from the different experimental models (B-cell transfer in B cell–deficient mice, versus bone marrow chimeras in our study), doses of αGalCer (more than a log difference, 4 versus 0.1 μg in our study), and routes of immunization (intraperitoneal versus subcutaneous in our study).
It is possible that both cognate as well as indirect help by iNKT cells coexist and are selectively displayed depending on the involvement of conventional Th cells. In conditions in which conventional Th cells are limiting or absent, the direct cognate help by iNKT cells to B lymphocytes could become necessary. Indeed, we have shown this is the case in vitro when highly purified human iNKT and B cells interact in the absence of Th cells,23 and it is suggested to occur also in vivo in mice lacking MHC-II expression and conventional Th cells.24
Three recent studies suggest a direct iNKT/B-cell cognate interaction in the induction of antibody responses elicited in mice upon infection with bacteria containing the target protein Ag and an αGalCer analog,32 or immunization with protein Ag and αGalCer both linked to bead particles,33 or with the NP hapten directly conjugated to αGalCer.34 We think the differences in Ag formulation and composition account for the difference between these results and our present study. Indeed, our immunogens were free protein antigens or hapten-carriers simply admixed with αGalCer, whereas these studies use complex or even particulated immunogens made of Ag physically linked to αGalCer. It is likely that in the case of Ag physically linked to αGalCer, the BCR binds the nominal Ag and internalizes it inside B cells together with the linked αGalCer, which can now bind CD1d and be presented by B cells directly to iNKT cells, soliciting their help.
On the one side, it is tempting to speculate that our immunization strategy would be immediately suitable for an adjuvant use of αGalCer as it recapitulates the situation with most of the presently used human vaccines, where purified or recombinant proteins are simply admixed with, or adsorbed to, an adjuvant (as in the case of oil-in-water emulsions or alum, respectively). On the other side, the use of complex or particulated Ag may better mimic natural infection, where it is likely that the direct cognate help provided by iNKT cells to B cells facilitates an early onset of antibody responses.32,33 Consistent with the innate nature of such an early antibody response, it is also suggested that the innate-like MZ B cells, which reside in the spleen and express high levels of membrane CD1d and costimulatory molecules,35 might be the B-cell subset directly helped by iNKT cells.33,34
The results obtained with splenectomized mice imply the possibility that activated iNKT cells stimulate DCs to induce adaptive immune responses in the peripheral LNs draining the site of immunization. Consistent with a possible sentinel role,14 it may be that iNKT cells survey tissues and meet with immature DCs at the site of Ag and αGalCer injection. iNKT cells would then activate in a cognate fashion DCs that have internalized both αGalCer and the protein Ag. The licensed DCs could then reach the draining LN and be fully competent to prime effector Th cells that, in turn, provide optimal cognate help for B cells that have been concomitantly activated by the same Ag. Alternatively, and not mutually exclusive, soluble protein Ag and αGalCer would reach the draining LN, where resident DCs take them up and present αGalCer to resident iNKT cells, which now license DCs to induce Ag-specific Th- and B-cell responses. The frequency of iNKT cells in LNs is low but detectable (about 0.5% of total lymphocytes36 and E.T., unpublished results, 2008), although their precise intranodal location and helper functions are still unknown. Deciphering the overall geography of the iNKT-cell adjuvancy for adaptive T- and B-cell responses is a key issue that warrants further investigation.
The fact that the adjuvant effect of αGalCer is displayed also in splenectomized mice rules out that this Ag formulation activates innate-like marginal zone (MZ) B cells, which home essentially to the spleen.35 It is likely that protein or hapten-carrier Ags mixed with αGalCer induce activated B cells that reside not only in the spleen but also in lymph nodes. This point is also supported by our finding24 (and E.T., unpublished results, 2008), that immunization of mice with protein or hapten-carrier Ags mixed with αGalCer elicits class-switched antibody responses, predominantly of IgG1 and IgG2c isotype, which are characteristic of follicular B cells and require CD4+ T-cell help.2
The indirect adjuvant mechanism of αGalCer would thus be somewhat analog to the one recently described for alum (aluminum hydroxide), an adjuvant widely used in vaccine formulations. The alum adsorbed with the Ag promotes in fact Ag uptake by monocyte precursors, and induces their extranodal differentiation into mature DCs and subsequent migration into draining LNs through the release of the endogenous “innate signal” uric acid from local tissue,37 and by directly activating the Nalp3 inflammasome in APCs.38
In conclusion, αGalCer recapitulates the effect of classical adjuvants that enhance antibody responses to protein antigens by stimulating the innate immune system to induce adaptive immune responses.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded by grants from Associazione Italiana per la Ricerca sul Cancro-AIRC, the Italian Ministry of Health, 1 Novartis Vaccines, (Siena, Italy) and the Italian Ministry of University and Research FIRB-RBNE017. E.T. is supported by fellowships from the PhD Program in Molecular Medicine, University of Vita-Salute San Raffaele (Milano, Italy).
Authorship
Contribution: E.T, S.A., G.C., and P.D. designed research; E.T., G.G., and C.M. performed research; E.T., G.G., S.A., G.C., and P.D. analyzed data; and E.T., S.A., G.C., and P.D. wrote the paper.
Conflict-of-interest disclosure: G.G. is an employee of Novartis Vaccines. S.A. is scientific consultant to Novartis Vaccines. The remaining authors declare no competing financial interests.
Correspondence: Giulia Casorati, Paolo Dellabona, Experimental Immunology Unit, H. San Raffaele Scientific Institute, via Olgettina 58, 20132 Milano, Italy; e-mail: casorati.giulia@hsr.it; dellabona.paolo@hsr.it.