Abstract
Evidence suggests the transcription factor GATA-2 is a critical regulator of murine hematopoietic stem cells. Here, we explore the relation between GATA-2 and cell proliferation and show that inducing GATA-2 increases quiescence (G0 residency) of murine and human hematopoietic cells. In human cord blood, quiescent fractions (CD34+CD38−HoechstloPyronin Ylo) express more GATA-2 than cycling counterparts. Enforcing GATA-2 expression increased quiescence of cord blood cells, reducing proliferation and performance in long-term culture-initiating cell and colony-forming cell (CFC) assays. Gene expression analysis places GATA-2 upstream of the quiescence regulator MEF, but enforcing MEF expression does not prevent GATA-2–conferred quiescence, suggesting additional regulators are involved. Although known quiescence regulators p21CIP1 and p27KIP1 do not appear to be responsible, enforcing GATA-2 reduced expression of regulators of cell cycle such as CCND3, CDK4, and CDK6. Enforcing GATA-2 inhibited human hematopoiesis in vivo: cells with highest exogenous expression (GATA-2hi) failed to contribute to hematopoiesis in nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice, whereas GATA-2lo cells contributed with delayed kinetics and low efficiency, with reduced expression of Ki-67. Thus, GATA-2 activity inhibits cell cycle in vitro and in vivo, highlighting GATA-2 as a molecular entry point into the transcriptional program regulating quiescence in human hematopoietic stem and progenitor cells.
Introduction
Hematopoietic stem cells (HSCs) select fates, including self-renewal, differentiation, quiescence, and apoptosis. Integration of multiple signals to direct specific fate decisions is mediated at least partially by expression levels of key transcription factors.1,2 Identifying transcription factors for which expression level plays key roles in cell fate choice is of major interest for the understanding of normal and leukemic hematopoiesis.
The relevance and control of HSC quiescence in long-term hematopoiesis is gradually being understood. Under steady state conditions, with no hematologic insult, the majority of HSCs in human and mouse bone marrow enter cycle infrequently at intervals of 1 to 3 months.3-5 Quiescent HSCs may be functionally distinct from those in cycle6 and capable of greater repopulation in vivo,7-9 confirming that physiologically conferred quiescence is reversible to allow repopulation. Much work on HSC growth control has focused on the murine system and requires extension into the human setting. Given the importance of appropriate growth control to HSC function, and the clinical utility of human HSCs, modulating quiescence of human cells may be useful for HSC expansion protocols10 and higher efficiency bone marrow transplantation.
Although quiescence is critical in preventing erosion of HSC numbers,11 and may limit propagation of accumulated DNA damage,12 the mechanisms controlling HSC cycle exit are still poorly understood. Known control points in HSCs include the cell- cycle inhibitor p21CIP111 and the transcription factor MEF (ELF4).13 The Angiopoietin-Tie signaling axis also appears important in conferral of quiescence at the niche by cell-cell contacts,14 and N-cadherin expression in long-term HSCs (LT-HSCs) shows an apparently more quiescent subpopulation.15
Transcriptional profiling has shown high expression of GATA-2 in quiescent hematopoietic cells,16,17 but a causative relation between GATA-2 level and quiescence has not yet been shown in these or other reported studies. Altering GATA-2 levels in mouse bone marrow cells by virally mediated forced expression results in reduced repopulation in a transplantation setting in a manner that could be consistent with enhanced quiescence.18 In this work, however, no defect in cell cycling in GATA-2–overexpressing cells was detected. In contrast, cell-cycle changes have been observed in transformed murine cell lines transduced with expression vectors containing GATA-2/ER protein chimeras. Here, activation of the exogenous GATA-2/ER moiety by addition of estradiol ligand produced accumulation of cells with 2n DNA content. The extent to which these cell-cycle effects were mediated at the level of quiescence was not addressed,16,19 nor was it investigated whether the cycling effects produced by GATA-2/ER could also be elicited by a wild-type GATA-2 molecule. This latter point is of importance, given reported differences in the activities of GATA-2 and GATA-2/ER.20
We have directly addressed a potential causative relation between GATA-2 and quiescence in hematopoietic stem and progenitor cells. We first demonstrate that growth arrest and G0/G1 DNA peak accumulation produced by GATA-2/ER in murine cell line models are due to the reversible conferral of cellular quiescence. Moreover, enforced expression of GATA-2 broadly recapitulates this phenotype, thereby providing evidence that conferral of quiescence is a physiologic property of the native GATA-2 molecule.
Second, given that almost all studies on GATA-2 function to date have used murine models, we went on to test this hypothesis in human cord blood (CB) because of its clinical utility21 and the relevance of growth control to effective human transplantation and chemotherapy,10,22 both areas in which modulating quiescence clinically might be of great advantage.
We show that in human CB cells, high GATA-2 expression correlates with quiescence ex vivo and in vitro, and enforcing high GATA-2 expression on human CB cells inhibits proliferation, confirming this relation. GATA-2–inhibited CB cell function correlates with modulation of multiple genes with roles in proliferation and differentiation. We also show changes in expression of genes with relevance to cell cycle, including MEF and elements of the cell-cycle machinery itself. This transcriptional program may reflect that of wild-type HSCs with innate high expression of GATA-2. Xenotransplantation of human stem and progenitor cells with enforced GATA-2 expression shows that increased quiescence confers a level-dependent block on hematopoietic reconstitution in medium and long-term engraftment assays, and it suggests that the underlying mechanism is delayed proliferative kinetics. Taken together, these studies begin to dissect the mechanisms by which a transcriptional program driven by the activity of GATA-2 acts to control hematopoiesis by increased human CB stem and progenitor cell quiescence.
Methods
GATA-2/ER ligand activation experiments
Ba/F3 cell clones growing under self-renewal conditions as described in “Cell culture and transduction” were exposed to 1 μM β-estradiol (Sigma-Aldrich, St Louis, MO). Proliferation or cell cycle variables were assessed as described. Withdrawal of β-estradiol was performed by washing 3 times in culture media.
Cord blood preparation and cell sorting
Fresh umbilical CB samples were collected and informed consent was obtained in accordance with the Declaration of Helsinki and local ethical guidelines, Oxfordshire Local Research Ethics Committee Approval no. 04/Q1605/22, were processed to mononuclear cells by density gradient centrifugation on FicollPaque Plus (GE Healthcare, Little Chalfont, United Kingdom). Red cells lysed with Gey solution were subfractionated with the use of CD34 progenitor cell isolation kits (Miltenyi Biotec, Auburn, CA, or StemCell Technologies, Vancouver, BC). Cells were stained (CD34APC, LNGFR-APC, CD45APC, CD19PeCy7, CD33PE; all Becton Dickinson, Franklin Lakes, NJ; CD38Biotin; Serotec, Oxford, United Kingdom; and then Streptavidin-PeCy7; Beckman Coulter, Fullerton, CA) for sorting on a MoFlo cell sorter or analysis with a CyAn cell analyser (Dako UK, Ely, United Kingdom); gating was based on unstained control samples.
Lentiviral constructs and packaging
Flag-tagged GATA-2 subcloned into the pHR-SIN-CSGW-EGFP lentiviral expression construct23 under control of the SFFV promoter or MEF similarly cloned with LNGFR replacing eGFP (vector components were kind gifts from Adrian Thrasher and Waseem Qasim, London, United Kingdom) was used to package lentiviral particles essentially as described previously.24
Cell culture and transduction
The derivation of Ba/F3 cells stably expressing GATA-2/ER has been described previously16 ; Ba/F3 cells were cultured in RPMI supplemented with 10% fetal calf serum and 2% WEHI-3B conditioned media as a source of IL-3. CB CD34+ cells and subsets thereof were maintained in either IMDM supplemented with 15% FCS (both Invitrogen, Paisley, United Kingdom), or StemSpan Serum-Free Expansion Medium (StemCell Technologies); both media were supplemented with penicillin-streptomycin and l-glutamine (Cambrex, East Rutherford, NJ) and 50 ng/mL hFLT3L, hSCF, and hTPO (StemCell Technologies). Where described, 5 ng/mL hTGFβ1 (PeproTech, Rocky Hill, NJ), 20 μM roscovitine, or 50 μM olomoucine (both Sigma-Aldrich) were added. Where applicable, cells were γ-irradiated with doses between 6 and 18 Gy and allowed to recover overnight, or they were washed 3 times and cultured overnight without cytokines. Murine Lin−Kit+Sca+ (LKS) cells were cultured in StemSpan Serum-Free Expansion Medium supplemented with penicillin-streptomycin and l-glutamine and with 50 ng/mL rSCF, 50 ng/mL mFlt3L, and 20 ng/mL mTPO (PeproTech). Lentiviral transductions were performed at a MOI of 100 to 200 three days before assay, and transduced cells were washed 3 times less than 24 hours after addition of virus.
Hoechst/pyronin staining
CD34+ cells were stained essentially as described previously.25 Briefly, cells washed and resuspended in HBSS containing 20 mM HEPES, 1 g/L d-glucose, 10% FCS, and 50 μM verapamil (Sigma-Aldrich) were stained in this solution plus 1 μg/mL Hoechst 33342 (Invitrogen) at 37°C for 90 minutes, then 1 μg/mL Pyronin Y (Polysciences, Warrington, PA) was added for 45 minutes. After staining, washed cells were kept on ice until sorting or analysis. For CD34+CD38−/+ cells, populations were presorted directly into Hoechst staining solution for processing as above. All gates were set, based on unstained control samples. For cytokine withdrawal, cells were fixed for 1 hour at −20°C after dropwise resuspension in cold 70% ethanol. Washed cells were stained in 4 μg/mL Hoechst 33342 at 37°C for 15 minutes, then 1 μg/mL Pyronin Y was added for 20 minutes.
Electrophoretic mobility shift assay
Bandshift experiments were essentially performed as previously described.26 See Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Western blotting
Total protein extracts were prepared from equivalent cell numbers in RIPA buffer plus Complete protease inhibitor cocktail (Roche, Indianapolis, IN) and run on precast sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Novex, San Diego, CA; Invitrogen) before blotting onto PVDF membrane (Immobilon-P; Millipore, Billerica, MA). Blotted proteins were detected in TBST containing 5% milk with GATA-2 H-116, CDK4 H-22, CDK6 C-21, β-tubulin H-235 (Santa Cruz Biotechnology, Santa Cruz, CA).
Proliferation assays
Cells sorted by GFP status and surface marker expression were cultured in 96-well plates. Live cell counts were taken at indicated intervals using Trypan Blue exclusion (Sigma-Aldrich). Adjustment of cell counts was made to account for any feeding of the cultures, with feeding dictated by degree of proliferation.
Apoptosis assays
Early and late apoptotic cells were enumerated by flow cytometry of cells stained according to the manufacturer's instructions with either annexin V–PE and 7-AAD (Becton Dickinson) or with annexin V–APC (Axxora, Nottingham, United Kingdom) and Hoechst 33258 (Invitrogen).
Colony-forming cell assays
Cells sorted on the basis of surface marker and GFP expression were plated in multipotential methylcellulose (MethoCult GF+ H4435; StemCell Technologies) at 500 CD34+ cells or 150 CD34+CD38− cells per dish, and colonies were assessed 14 to 18 days later.
Long-term culture-initiating cell assays
Long-term culture-initiating cell (LTC-IC) assays were performed with the use of MyeloCult H5100 medium (StemCell Technologies) essentially according to the manufacturer's instructions but using a 1:1 mixture of irradiated M2-10B4 and SI/SI cells (StemCell Technologies) as per published protocols.27 GFP+CD34+CD38− cells were sorted into 96-well plates containing irradiated stroma and fed weekly by half-volume media changes. At 5 weeks the medium was replaced with multipotential MethoCult GF+ H4435. Colony formation was scored 14 to 18 days later.
Quantitative reverse transcription–polymerase chain reaction
Approximately equivalent quantities of total RNA prepared with Trizol (Invitrogen) were reverse transcribed with Superscript II (Invitrogen), and diluted cDNA samples were analyzed with TaqMan gene expression assays (ABI, Foster City, CA) according to the manufacturer's protocols. Expression relative to HPRT was determined according to Pfaffl.28 Absolute expression values for HPRT were checked to ensure no gross changes in HPRT expression were due to transduction.
Transplantation assays
All animal studies were approved by our institutional review board at the Weatherall Institute of Molecular Medicine, and animals were maintained in accordance with local regulations. See Document S1 for details.
Ki-67 analysis
GFP+hCD45+ and GFP−hCD45+ cells were recovered by fluorescence-activated cell sorting from bone-injected nonobese diabetic–severe combined immunodeficient (NOD-SCID) or NOD-SCID/IL2rγnull mice 4 or 6 weeks after transplantation as described in “Transplantation assays.” Cells were fixed on ice in 1% paraformaldehyde (Electron Microscopy Services, Hatfield, PA) for 30 minutes before permeabilization on ice in 0.1% Triton X-100 for 15 minutes. Cells were washed and stained for Ki-67 according to the manufacturer's instructions (Becton Dickinson) before staining overnight in 2 μg/mL Hoechst 33342 (Invitrogen).
Statistical analyses
Standard error of the mean (SEM) was calculated as standard deviation/square root of n, where n equals the number of replicates. Sets of replicate experiments with the use of paired samples were compared with 2-tailed t test.
Results
Ligand-activated GATA-2/ER fusions confer quiescence on hematopoietic cells
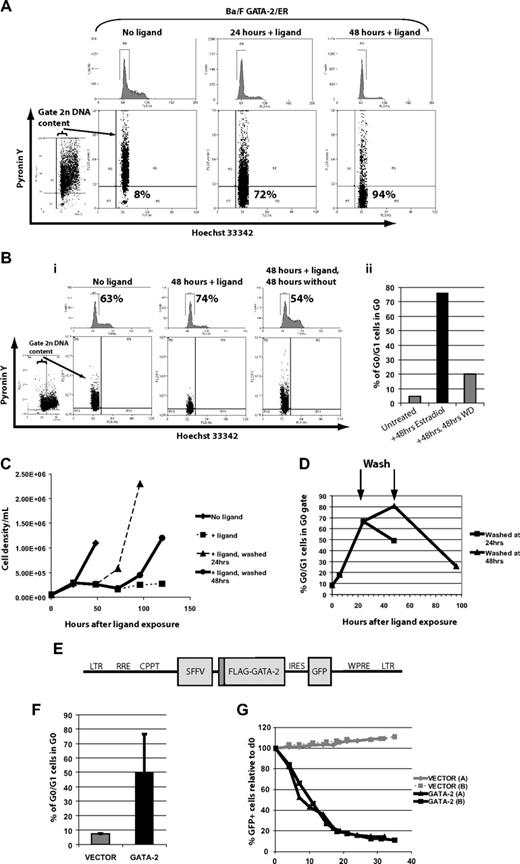
Previous data have shown a profound effect on cell cycling and growth on activation of GATA-2/ER in a murine hematopoietic setting.16,19,20 We examined the apparent G0/G1 accumulation on ligand activation using DNA staining with Hoechst 33342 and RNA staining with Pyronin Y. In this way we were able to define whether this accumulation was due to quiescence or growth arrest (Figure 1A). Ligand activation drove Ba/F3 cells to become more quiescent, with cells in all cycle phases reducing their polysomal RNA content and accumulating in G0 while growth was attenuated. A key feature of quiescence as distinct from growth arrest is that quiescence is reversible. To establish this we washed out the ligand and demonstrated that the G0 accumulation was reversible (Figure 1B), and that as a result cells reentered cycle-reconstituting normal growth kinetics (Figure 1C) and cycle phase distributions (Figure 1D). Thus, the GATA-2/ER-conferred quiescent state was fully reversible.
Ligand-activated GATA-2/ER chimeric form reversibly induced quiescence in a manner reproduced with constitutive lentiviral expression of FLAG–GATA-2. (A) β-Estradiol (1 μM) induces G0/G1 accumulation of Ba/F3 cells expressing GATA-2/ER (top) concomitant with increased quiescence as measured by DNA staining with Hoechst 33342 and polysomal RNA staining with Pyronin Y (bottom). One of 3 representative experiments is shown, with the percentage of G0/G1 cells falling in the G0 gate indicated. (Bi) Withdrawal of ligand allows cells to progress into S phase and to exit quiescence, as assessed by reduced G0/G1 proportions indicated (top) and increased staining with Pyronin Y (bottom). (Bii) Proportion of G0/G1 cells falling in the G0 gate from panel Bi are plotted. One of 2 representative experiments is shown. (C) Cell growth, attenuated by GATA-2/ER activation, resumes on ligand withdrawal. (D) Return to normal cell growth lags behind reduction in the proportion of quiescent cells as measured by Hoechst/Pyronin Y. (E) Bicistronic lentiviral expression construct used to drive enforced expression of FLAG-tagged GATA-2 and GFP. (F) Constitutive expression of FLAG–GATA-2 also increases quiescence of Ba/F3 cells growing in IL-3. The mean of 2 representative experiments is shown. Error bars indicate SEM. (G) The GATA-2–transduced cells are outgrown by untransduced cells in a GATA-2–dependent manner; n = 2 experiments.
Ligand-activated GATA-2/ER chimeric form reversibly induced quiescence in a manner reproduced with constitutive lentiviral expression of FLAG–GATA-2. (A) β-Estradiol (1 μM) induces G0/G1 accumulation of Ba/F3 cells expressing GATA-2/ER (top) concomitant with increased quiescence as measured by DNA staining with Hoechst 33342 and polysomal RNA staining with Pyronin Y (bottom). One of 3 representative experiments is shown, with the percentage of G0/G1 cells falling in the G0 gate indicated. (Bi) Withdrawal of ligand allows cells to progress into S phase and to exit quiescence, as assessed by reduced G0/G1 proportions indicated (top) and increased staining with Pyronin Y (bottom). (Bii) Proportion of G0/G1 cells falling in the G0 gate from panel Bi are plotted. One of 2 representative experiments is shown. (C) Cell growth, attenuated by GATA-2/ER activation, resumes on ligand withdrawal. (D) Return to normal cell growth lags behind reduction in the proportion of quiescent cells as measured by Hoechst/Pyronin Y. (E) Bicistronic lentiviral expression construct used to drive enforced expression of FLAG-tagged GATA-2 and GFP. (F) Constitutive expression of FLAG–GATA-2 also increases quiescence of Ba/F3 cells growing in IL-3. The mean of 2 representative experiments is shown. Error bars indicate SEM. (G) The GATA-2–transduced cells are outgrown by untransduced cells in a GATA-2–dependent manner; n = 2 experiments.
Given the nonphysiologic nature of estrogen receptor fusions,20 we went on to examine the effect of constitutive GATA-2 expression in Ba/F3 cells with the use of a lentiviral expression construct driven by the SFFV promoter (Figure 1E), and we found that quiescence was also increased compared with cells transduced with empty vector (Figure 1F). Taken together, these data suggested that this increased quiescence correlated with reduced proliferation (as assessed by overgrowth of untransduced cells in the GATA-2 but not the empty vector transductions; Figure 1G). Thus, for the first time we demonstrate that increased quiescence underlies the growth defect observed on increased GATA-2 activity.
High expression of GATA-2 in quiescent human stem and progenitor cells
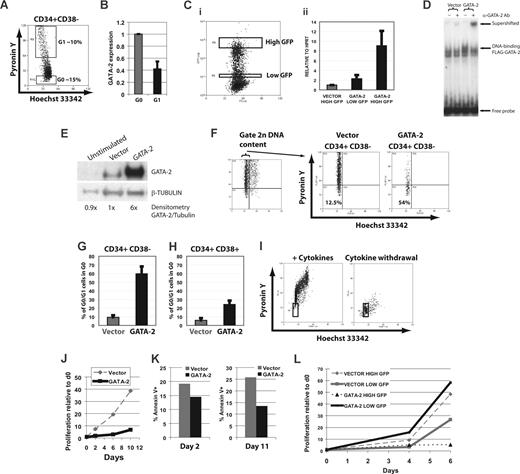
We hypothesized from this work and previous studies that high GATA-2 expression in vivo might be associated with quiescence of human stem and progenitor cells.17 To this end we isolated cycling (G1) and quiescent (G0) cells from CD34+CD38− CB cells based on staining with Hoechst 33342 and Pyronin Y (Figure 2A) and assessed GATA-2 expression by quantitative reverse transcription–polymerase chain reaction (RT-PCR). This analysis showed greater than 2-fold higher expression of GATA-2 in the quiescent subset (Figure 2B). Similar data were obtained with CD133+ cells (data not shown). We therefore tested this correlation functionally.
High expression of endogenous GATA-2 correlates with quiescence of human cord blood (CB) cells. (A) Quiescent and cycling CD34+CD38− cells were sorted from freshly thawed CB on the basis of low and high Pyronin Y staining, respectively. (B) Quantitative RT-PCR analysis from 2 independent samples showed higher expression of GATA-2 relative to HPRT in the quiescent G0 cells. HPRT was expressed at similar levels in these subsets (data not shown). Error bars indicate SEM. (C) The bicistronic lentiviral expression construct in Figure 1E was used to drive enforced expression of FLAG-tagged GATA-2 and GFP. Transduced CD34+ cells selected on the basis of GFP expression (i) were used for quantitative RT-PCR for GATA-2 (ii) to show that expression of GATA-2 is higher in GFPhi cells. (D) DNA-binding competency and expression level of FLAG–GATA-2 expressed in sorted GFP+ total CD34+ CB cells as assessed by electrophoretic mobility shift assay using 32P-labeled GATA oligonucleotide (sequence, 5′ to 3′ TATTTTTATCTGATAGGAAGT). (E) Western blot showing expression level of lentivirally expressed GATA-2 in freshly isolated unstimulated CB CD34+ cells, or after transduction with vector or GATA-2. Expression relative to β-tubulin (normalized to ratio in vector-transduced cells) is shown below the blot. (F) CB CD34+ samples were split, infected with lentiviral vectors, and sorted 3 days later on CD34 and CD38 before staining with Hoechst and Pyronin Y. Hoechst 33342 and Pyronin Y staining profiles are shown for the indicated populations from a representative sample. The mean percentage of quiescent GFP+CD34+CD38− (G) and quiescent GFP+CD34+CD38+ cells (H) falling in the G0 gates is presented from 3 independent samples. Error bars indicate SEM. (I) CB CD34+ cells in culture in SCF, TPO, and Flt3L were washed to remove cytokines and left in culture overnight; a control culture had cytokines added back. Eighteen hours later the cells were fixed in ethanol and stained with Hoechst and Pyronin Y. (J) Enforced GATA-2 expression confers a profound growth defect. Transduced GFP+CD34+CD38− cells were put into culture in SCF, TPO, and Flt3L and counted at intervals. Cell density per milliliter is shown from 1 of 3 representative experiments. (K) Cells were sampled at day 2 and day 11 and stained for annexin V by flow cytometry. Cells with enforced expression of GATA-2 were not more apoptotic than those expressing empty vector. Similar data were obtained throughout 4 replicate in vitro proliferation experiments. (L) The proliferative defect is predominantly observed in cells expressing high levels of GATA-2. Samples of transduced CD34+ cells with the highest and lowest levels of GFP expression were sorted and cultured as before. Cell density per milliliter is shown from a representative experiment.
High expression of endogenous GATA-2 correlates with quiescence of human cord blood (CB) cells. (A) Quiescent and cycling CD34+CD38− cells were sorted from freshly thawed CB on the basis of low and high Pyronin Y staining, respectively. (B) Quantitative RT-PCR analysis from 2 independent samples showed higher expression of GATA-2 relative to HPRT in the quiescent G0 cells. HPRT was expressed at similar levels in these subsets (data not shown). Error bars indicate SEM. (C) The bicistronic lentiviral expression construct in Figure 1E was used to drive enforced expression of FLAG-tagged GATA-2 and GFP. Transduced CD34+ cells selected on the basis of GFP expression (i) were used for quantitative RT-PCR for GATA-2 (ii) to show that expression of GATA-2 is higher in GFPhi cells. (D) DNA-binding competency and expression level of FLAG–GATA-2 expressed in sorted GFP+ total CD34+ CB cells as assessed by electrophoretic mobility shift assay using 32P-labeled GATA oligonucleotide (sequence, 5′ to 3′ TATTTTTATCTGATAGGAAGT). (E) Western blot showing expression level of lentivirally expressed GATA-2 in freshly isolated unstimulated CB CD34+ cells, or after transduction with vector or GATA-2. Expression relative to β-tubulin (normalized to ratio in vector-transduced cells) is shown below the blot. (F) CB CD34+ samples were split, infected with lentiviral vectors, and sorted 3 days later on CD34 and CD38 before staining with Hoechst and Pyronin Y. Hoechst 33342 and Pyronin Y staining profiles are shown for the indicated populations from a representative sample. The mean percentage of quiescent GFP+CD34+CD38− (G) and quiescent GFP+CD34+CD38+ cells (H) falling in the G0 gates is presented from 3 independent samples. Error bars indicate SEM. (I) CB CD34+ cells in culture in SCF, TPO, and Flt3L were washed to remove cytokines and left in culture overnight; a control culture had cytokines added back. Eighteen hours later the cells were fixed in ethanol and stained with Hoechst and Pyronin Y. (J) Enforced GATA-2 expression confers a profound growth defect. Transduced GFP+CD34+CD38− cells were put into culture in SCF, TPO, and Flt3L and counted at intervals. Cell density per milliliter is shown from 1 of 3 representative experiments. (K) Cells were sampled at day 2 and day 11 and stained for annexin V by flow cytometry. Cells with enforced expression of GATA-2 were not more apoptotic than those expressing empty vector. Similar data were obtained throughout 4 replicate in vitro proliferation experiments. (L) The proliferative defect is predominantly observed in cells expressing high levels of GATA-2. Samples of transduced CD34+ cells with the highest and lowest levels of GFP expression were sorted and cultured as before. Cell density per milliliter is shown from a representative experiment.
Enforced expression of GATA-2 also confers quiescence on human stem and progenitor cells
Our lentiviral expression construct allows correlation of GATA-2 expression level with GFP intensity (Figure 2C). This construct or empty vector (expressing only eGFP) was used to infect human CB CD34+ cells, and expression of full-length protein capable of direct GATA-site binding was confirmed (Figure 2D). Expression level of mRNA (Figure 2C) and protein (Figure 2E) was assessed and indicated that our lentiviral strategy maintains functional protein expression at a level slightly higher than endogenous GATA-2 in primary CD34+ cells.
By Hoechst staining alone we did not reproducibly detect enhanced accumulation of cells in the G0/G1 DNA peak in our CD34+CD38− samples, consistent with previous studies18 (data not shown). However, effects on cell cycle were observed in CD34+CD38− cells when we further resolved these DNA peaks with Hoechst 33342 and Pyronin Y to assess the relative quiescence of subsets of transduced cells (Figure 2F). In both CD34+CD38− cells and the less-primitive CD34+CD38+ subset (Figure 2F-H; Figure S1B), GATA-2 expression increased quiescence relative to vector-transduced cells, with more marked conferral of quiescence in the more primitive subset, possibly reflecting their greater propensity for quiescence in vivo. The lack of observable G0/G1 accumulation contrasts with the GATA-2/ER experiments and suggests that, although G0 accumulation is observed in both settings, the GATA-2/ER model may be more potent in cycle inhibition.
To confirm that reduced Pyronin Y staining reflects quiescence and not general cycle arrest, we treated CB CD34+ cells with agents that cause growth inhibition. Neither roscovitine or olomoucine (CDK inhibitors inhibiting cell-cycle progression), nor TGFβ1 or γ-irradiation reproducibly induced a reduction in Pyronin Y staining, similar to that described here (Figure S1A). However, removing cytokines for approximately 18 hours yielded a massive reduction in Pyronin Y staining preceding frank cell death (Figure 2I). These data suggest that the reduced Pyronin Y staining described herein is not an inevitable marker of growth arrest, but a relatively specific surrogate marker of cellular quiescence, in agreement with previous studies.5 Taken together, our results suggest that enforced GATA-2 expression increases CB cell quiescence.
Enforced expression of GATA-2 inhibits proliferation and cell-cycle entry
GATA-2–transduced CD34+CD38− cells failed to proliferate with the same kinetics as vector-transduced cells, showing a marked growth defect (Figure 2J). Annexin V staining throughout in vitro proliferation culture showed no increase in apoptosis conferred by enforced GATA-2 expression (Figure 2K). Thus, despite the presence of cytokines, cells with artificially raised levels of GATA-2 survive but fail to proliferate well, whereas vector-transduced cells proliferate well and show higher Pyronin Y staining. Moreover, CD34+CD38− cells sorted with high enforced GATA-2 expression proliferated poorly, whereas cells with the lowest levels proliferated essentially as if vector transduced (Figure 2L). These results indicate that there is a level-dependent effect of GATA-2 expression on stem and progenitor cell proliferation.
Level of GATA-2 expression inhibits stem and progenitor function in vitro
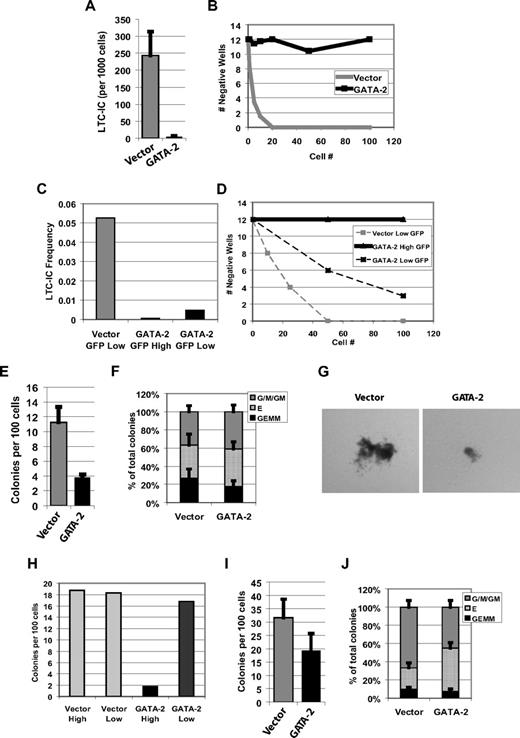
CD34+CD38− human CB cells transduced with empty vector or GATA-2 were assessed for in vitro stem and progenitor cell activity in LTC-IC assays. Strikingly, GATA-2–transduced cells almost totally failed to read-out in LTC-IC assay (Figure 3A,B) despite the relatively high LTC-IC frequency in this primitive population. Moreover, cells with high enforced GATA-2 expression (high GFP) exhibit less LTC-IC activity than do cells with low expression (Figure 3C,D). Visual inspection suggested that enforcing GATA-2 expression inhibits proliferation during culture on supporting stromal cells (data not shown). Thus, the effect of GATA-2 in LTC-IC assay involves a failure to establish long-term cultures capable of producing colony-forming cells. To investigate a possible defect in the second phase of the assay when progenitor potential is assessed, we examined the effect of GATA-2 on colony formation.
In vitro stem and progenitor cell function is inhibited by constitutive expression of GATA-2. (A) Transduced CD34+CD38− cells show a GATA-2–specific loss of LTC-IC activity in vitro. Mean frequency (± SEM) of LTC-ICs in the transduced populations is shown for 5 independent experiments. (B) Data from 1 representative experiment shown in panel A is presented as the cell number plated per well versus the number of wells that did not contain an LTC-IC. (C) LTC-IC frequency in transduced CB CD34+CD38− cells sorted by GFP expression level. A single representative experiment is shown. (D) Same data as in panel C, plotted as the cell number plated per well versus the number of wells that did not contain an LTC-IC. (E) CFC frequency in transduced CB CD34+ cells. (n = 4; error bars indicate SEM). (F) Same data as in panel E, plotted for colony type distribution. Mean proportions of total colonies of myeloid (G/M/GM), erythroid (E), or mixed (GEMM) types are shown from 4 independent experiments. G indicates granulocyte; M, macrophage; GM, granulocyte/macrophage; GEMM, granulocyte/erythroid/monocyte/macrophage; n = 4; error bars indicate SEM. (G) Photomicrograph of representative erythroid colonies from transduced CB CD34+ cells (Nikon SMZ1500, 1× objective, Nikon DXM1200F camera using ACT-1 v.2.12; Nikon UK Limited, Kingston Upon Thames, United Kingdom). (H) Total CFC colony number in transduced CB CD34+ cells sorted by GFP expression level. A single representative experiment is shown. (I) Enforced expression of GATA-2 in CD34+CD38− cells similarly inhibits colony formation but (J) here leads to a modest increase in erythroid colony output; n = 4; error bars indicate SEM.
In vitro stem and progenitor cell function is inhibited by constitutive expression of GATA-2. (A) Transduced CD34+CD38− cells show a GATA-2–specific loss of LTC-IC activity in vitro. Mean frequency (± SEM) of LTC-ICs in the transduced populations is shown for 5 independent experiments. (B) Data from 1 representative experiment shown in panel A is presented as the cell number plated per well versus the number of wells that did not contain an LTC-IC. (C) LTC-IC frequency in transduced CB CD34+CD38− cells sorted by GFP expression level. A single representative experiment is shown. (D) Same data as in panel C, plotted as the cell number plated per well versus the number of wells that did not contain an LTC-IC. (E) CFC frequency in transduced CB CD34+ cells. (n = 4; error bars indicate SEM). (F) Same data as in panel E, plotted for colony type distribution. Mean proportions of total colonies of myeloid (G/M/GM), erythroid (E), or mixed (GEMM) types are shown from 4 independent experiments. G indicates granulocyte; M, macrophage; GM, granulocyte/macrophage; GEMM, granulocyte/erythroid/monocyte/macrophage; n = 4; error bars indicate SEM. (G) Photomicrograph of representative erythroid colonies from transduced CB CD34+ cells (Nikon SMZ1500, 1× objective, Nikon DXM1200F camera using ACT-1 v.2.12; Nikon UK Limited, Kingston Upon Thames, United Kingdom). (H) Total CFC colony number in transduced CB CD34+ cells sorted by GFP expression level. A single representative experiment is shown. (I) Enforced expression of GATA-2 in CD34+CD38− cells similarly inhibits colony formation but (J) here leads to a modest increase in erythroid colony output; n = 4; error bars indicate SEM.
Colony formation is reduced by high GATA-2 expression
In total CD34+ cells, enforced GATA-2 expression caused an approximate 3-fold reduction in colony number relative to vector (Figure 3E), and colonies that did form were smaller (Figure 3G). Numbers of all colony types were reduced proportionately (Figure 3F). We also noted a level-dependent effect of enforced GATA-2 expression on CFC activity of CD34+ cells (Figure 3H). From the more primitive CD34+CD38− cell population, GATA-2 expression caused a more modest reduction in colony number (Figure 3I), and, although GATA-2 colonies were smaller, they were less markedly so than observed from total CD34+ cells. In CD34+CD38− cells GATA-2 skewed colony output, producing a selective approximate 5-fold reduction in myeloid colony formation and causing a mild increase in erythroid output (Figure 3J). Broadly, these findings confirm previous data showing inhibition of CFC activity by GATA-218 ; however, examining lineage suggests that GATA-2 has varying effects on different progenitor classes.
GATA-2 overexpression regulates genes encoding transcription factors with roles in stem and progenitor cell function
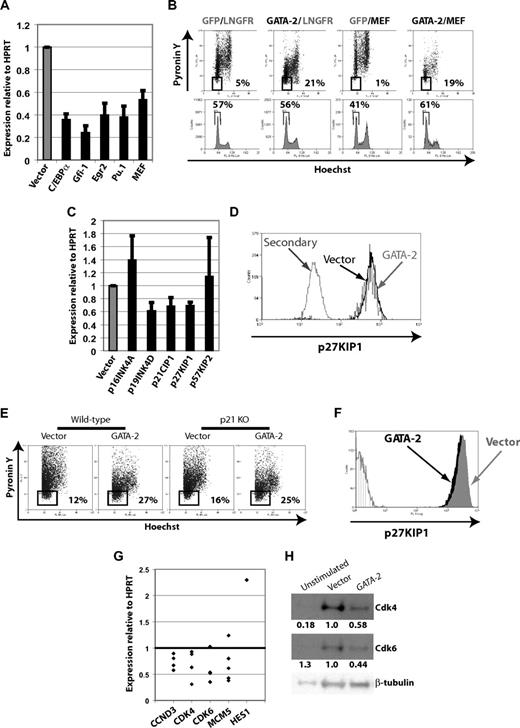
We used quantitative RT-PCR of candidate genes in GATA-2–transduced CD34+CD38− cells to investigate the mechanistic basis of effects on cell cycle. We looked for induction of genes in which high expression was known to negatively affect cell cycle, such as C/EBPα29 and Gfi-1,30,31 but neither gene was induced by GATA-2; indeed both were repressed (Figure 4A). EGR2/KROX20, an immediate-early gene induced on stimulation of quiescent cells,32 was repressed on enforced GATA-2 expression (P < .01; Figure 4A). Down-regulation of PU.1 (P < .01; Figure 4A), a known GATA-2 target,20 may underlie this repression.33
Enforced GATA-2 expression alters expression of genes involved in cell-cycle control but does not confer quiescence by MEF repression or CKI induction. (A) Quantitative RT-PCR of C/EBPα, Gfi-1, Egr2, Pu.1, and MEF in CB CD34+CD38− cells on enforced expression of GATA-2. Expression relative to HPRT (n ≥ 4; error bars indicate SEM). P < .01 for all. (B) Enforced expression of MEF from an LNGFR-expressing bicistronic construct does not prevent the conferral of quiescence by enforced expression of GATA-2 in CD34+ cells. Cells cotransduced with all combinations of GFP- and LNGFR-marked expression vector, empty or encoding GATA-2 or MEF, respectively, were stained with Hoechst and Pyronin Y. Enforced MEF expression has positive effects on cell cycle independently of GATA-2, but the effect of enforced GATA-2 expression is dominant to this. (C) CKIs p16INK4A, p19INK4D, p21CIP1, p27KIP1, and p57KIP2 are not induced by enforced GATA-2 expression in CB CD34+CD38− cells by quantitative RT-PCR relative to HPRT (n ≥ 4l error bars indicate SEM). (D) Enforced expression of GATA-2 in CB CD34+ cells does not cause induction of p27KIP1 as measured by intracellular flow cytometry. (E) Enforced expression of GATA-2 induces quiescence in p21−/− mouse Lin−Kit+Sca+ cells as measured by Hoechst/Pyronin staining, but (F) p27KIP1 is not induced in these cells. (G) Expression of CCND3, CDK4, CDK6, and MCM5 is reduced by enforced GATA-2 expression in CB CD34+CD38− cells, whereas HES1 expression is induced (quantitative RT-PCR relative to HPRT). (n ≥ 4 except n = 1 for HES1; P < .05 for all except MCM5 and HES1. MCM5 expression is reduced in 4 of 5 independent experiments and as a result P > .05). (H) Expression of CDK4, CDK6, and β-tubulin by Western blot in CD34+ cell extracts prepared from freshly isolated unstimulated cells and cells transduced with empty vector and GATA-2 after 3 days in SCF, TPO, and Flt3L. Densitometric analysis of expression relative to β-tubulin, normalized to the ratio in vector-transduced cells, is shown below each panel.
Enforced GATA-2 expression alters expression of genes involved in cell-cycle control but does not confer quiescence by MEF repression or CKI induction. (A) Quantitative RT-PCR of C/EBPα, Gfi-1, Egr2, Pu.1, and MEF in CB CD34+CD38− cells on enforced expression of GATA-2. Expression relative to HPRT (n ≥ 4; error bars indicate SEM). P < .01 for all. (B) Enforced expression of MEF from an LNGFR-expressing bicistronic construct does not prevent the conferral of quiescence by enforced expression of GATA-2 in CD34+ cells. Cells cotransduced with all combinations of GFP- and LNGFR-marked expression vector, empty or encoding GATA-2 or MEF, respectively, were stained with Hoechst and Pyronin Y. Enforced MEF expression has positive effects on cell cycle independently of GATA-2, but the effect of enforced GATA-2 expression is dominant to this. (C) CKIs p16INK4A, p19INK4D, p21CIP1, p27KIP1, and p57KIP2 are not induced by enforced GATA-2 expression in CB CD34+CD38− cells by quantitative RT-PCR relative to HPRT (n ≥ 4l error bars indicate SEM). (D) Enforced expression of GATA-2 in CB CD34+ cells does not cause induction of p27KIP1 as measured by intracellular flow cytometry. (E) Enforced expression of GATA-2 induces quiescence in p21−/− mouse Lin−Kit+Sca+ cells as measured by Hoechst/Pyronin staining, but (F) p27KIP1 is not induced in these cells. (G) Expression of CCND3, CDK4, CDK6, and MCM5 is reduced by enforced GATA-2 expression in CB CD34+CD38− cells, whereas HES1 expression is induced (quantitative RT-PCR relative to HPRT). (n ≥ 4 except n = 1 for HES1; P < .05 for all except MCM5 and HES1. MCM5 expression is reduced in 4 of 5 independent experiments and as a result P > .05). (H) Expression of CDK4, CDK6, and β-tubulin by Western blot in CD34+ cell extracts prepared from freshly isolated unstimulated cells and cells transduced with empty vector and GATA-2 after 3 days in SCF, TPO, and Flt3L. Densitometric analysis of expression relative to β-tubulin, normalized to the ratio in vector-transduced cells, is shown below each panel.
We found that MEF expression was reduced by approximately 47% in GATA-2–transduced CD34+CD38− CB cells (Figure 4A), placing GATA-2 upstream of MEF. Chromatin immunoprecipitation showed reproducible weak (∼2-fold) enrichment of GATA-2 upstream of the MEF locus in multipotential mouse cells (data not shown), suggesting that GATA-2 may directly affect MEF regulation. Recently, MEF/ELF4 has been described to play a role in HSC growth control because MEF knockout HSCs are more quiescent, cycle less, and proliferate less.13 Knockdown of MEF in CBs to approximately 60% to 70% of normal levels increased quiescence,13 suggesting a role for MEF repression in the GATA-2–induced growth defect.
We tested this mechanistic relation by enforcing expression of both GATA-2 and MEF in CB CD34+ cells. Enforced MEF expression alone caused mildly increased Pyronin Y staining and a major increase in the G2/M fraction, although this was overridden with enforced GATA-2 expression (Figure 4B), suggesting dominance of the GATA-2 program. Notably, GATA-2–induced quiescence was not blunted by enforced expression of MEF (Figure 4B), and the performance of GATA-2–transduced CB CD34+ cells in CFC assays was not improved (data not shown). Thus, although these data place GATA-2 upstream of MEF, they imply that GATA-2 confers quiescence by additional pathways.
GATA-2 overexpression regulates genes involved in cell-cycle progression
In addition to candidate transcription factors, we assessed expression of components and regulators of the cell-cycle machinery. Cyclin-dependent kinase inhibitors (CKIs) inhibit the cyclin D–dependent kinases CDK4 and CDK634 ; thus, we looked for induction of these genes in response to GATA-2. Expression of p16INK4A, p19INK4D, p21CIP1, p27KIP1, or p57KIP2 was not induced (Figure 4C). Prior studies with GATA-2/ER chimeras had suggested a role for p21CIP1 and p27KIP1 in growth inhibition,16 perhaps explaining the more potent phenotype and G0/G1 accumulation observed in that setting. Given these prior data, we investigated these CKIs further. Intracellular flow cytometry in CB CD34+ cells showed enforced GATA-2 expression did not significantly affect expression of p27KIP1 (Figure 4D). To formally exclude a role for p21CIP1 and p27KIP1, we enforced expression of GATA-2 in LKS (Lin−Kit+Sca+) cells from wild-type and p21−/− mice.35 Enforced GATA-2 expression also reduced Pyronin Y staining in the murine setting, and deletion of p21CIP1 did not prevent this (Figure 4E). Finally, we assessed p27KIP1 expression by flow cytometry in transduced LKS cells from wild-type and p21−/− mice and showed that p27KIP1 was not increased in the absence of p21CIP1 (Figure 4F). Thus, growth inhibition by enforced GATA-2 expression does not appear to be mediated by either CKI alone or in combination.
We assessed expression of cyclins and D-type cyclin-dependent kinases important in G0/G1 control and HSC quiescence. CCND3 is expressed at low levels in the relatively quiescent LT-HSC pool9 and repressed in quiescent murine T-lymphoma cells.36 In CB CD34+CD38− cells, reduced proliferation conferred by GATA-2 correlated with repression of CCND3 (Figure 4G). We found no significantly altered expression of cyclins D1 or E1 in response to enforced GATA-2 expression (data not shown). We also assessed expression of the kinase partners of D-type cyclins, CDK4 and CDK6, rate-limiting factors in cell-cycle reentry from quiescence by phosphorylation of Rb.34 Enforced GATA-2 expression significantly reduced levels of CDK4 and CDK6 (Figure 4G), and CD34+ cells transduced with GATA-2 showed reduced levels of CDK4 and CDK6 protein (Figure 4H). Moreover, levels of CDK4 (but not CDK6) were lower in freshly isolated CB CD34+ cells than in cycling vector-transduced cells (Figure 4H), supporting the relevance of CDK4 expression to cellular quiescence. In 4 of 5 CB CD34+CD38− samples, we detected repression of MCM5 by GATA-2 (Figure 4G). Similar data using GATA-2/ER activation in cell lines suggest repression of multiple MCM genes by GATA-2 in a manner reducing entry into S phase, presumably by inhibiting replication origin licensing (A.J.T. and T.E., unpublished data, 2008). Thus, our data suggest that multiple pathways relevant to cell-cycle entry and progression are modulated by GATA-2.
Preliminary evidence suggests higher HES1 expression in GATA-2–transduced CD34+CD38− cells (Figure 4G), reminiscent of reduced expression of HES1 observed in the heterozygous mouse model where it is a directly bound target of GATA-2.37 HES1 appears important in the reversibility of quiescence,38 and it may be that GATA-2 acts via HES1 in this regard.
Effects of enforced GATA-2 expression in vivo
Although our in vitro data suggested level of GATA-2 conferred quiescence, it remained possible that in vivo cues may override this. Thus, we enforced GATA-2 expression in human CB CD34+CD38− cells and transplanted these into NOD-SCID mice. We assessed engraftment of vector- and GATA-2–transduced cells in small bone marrow aspirates at 4 weeks, killing the same animals at 8 weeks. Transduced cells were injected 18 hours after infection, before GFP expression is observed. Aliquots of the transplanted cells were reserved in vitro and assessed for level of GFP expression and transduction efficiency. Gating showed the proportion of GFP+ cells with high- and low-GFP expression (Figure 5A), allowing estimation of GATA-2 expression on a population basis.
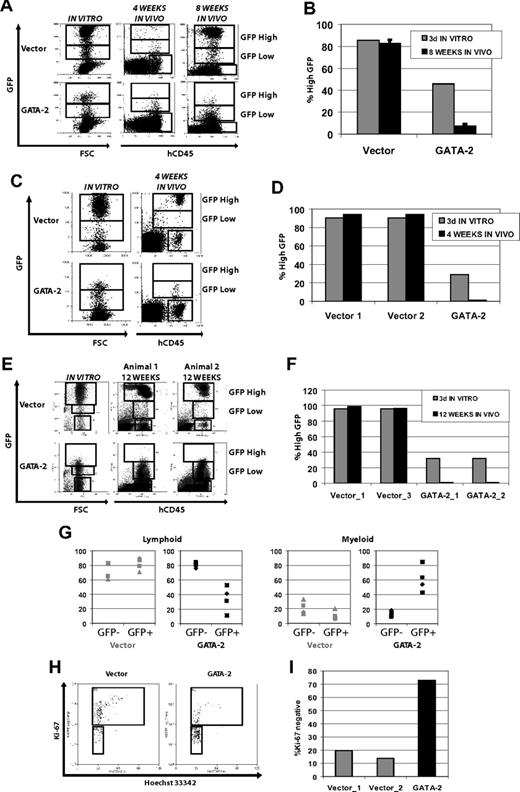
Engraftment and in vivo reconstitution of NOD-SCID animals by CD34+CD38− CB cells is inhibited by enforced GATA-2 expression in a level-dependent manner. (A) GFP expression level after 3 days in vitro culture, showing GFP intensity and transduction efficiency of transplanted GATA-2–transduced populations. Staining for human CD45 and GFP signal are shown for 1 representative animal from each group at 4 and 8 weeks (same animal sampled at 4 weeks is shown at 8 weeks). All animals are represented in Figure S1C. (B) High GATA-2–mediated expansion defect in NOD-SCID animals. Mean proportions of engrafted GFP+ cells falling in the upper gate shown in panel A, with high expression of GFP, are plotted for all engrafted animals at 8 weeks (n = 4 for each transgene). Error bars indicate SEM. (C) Expression level-dependent inhibition of hematopoiesis in NOD-SCID mice is not due to alterations in homing. GFP expression level after 3 days in vitro culture and in vivo 4 weeks after intraosseous injection. Engrafted cells expressing high levels of GATA-2 are not observed at the same frequency as in the initial transplant material. Cells were injected directly into the bone marrow cavity after only 5 hours of exposure to lentivirus. Staining for human CD45 and GFP signal are shown. (D) Proportions of engrafted human GFP+ cells falling in the upper GFP gate shown in panel C are plotted for the populations given as a transplant (dark gray) and from all engrafted animals at 4 weeks after intraosseous injection (black; n = 2 for vector-transduced cells; n = 1 for GATA-2). (E) Similar to panel C, cells were injected directly into the bone marrow cavity after 5 hours of transduction, and engraftment was assessed 12 weeks later. (F) Similar to panel D, proportions of engrafted human GFP+ cells falling in the upper GFP gate shown in panel E are plotted for the transplant (dark gray) and from all engrafted animals at 12 weeks after intraosseous injection; n = 2 for both groups. (G) Relative lymphoid and myeloid reconstitution is affected by enforced GATA-2 expression. For each animal, the proportion of human CD45+GFP− and human CD45+ GFP+ cells falling in CD19+ lymphoid and CD33+ myeloid gates are plotted; n = 4 animals in each group. Vector-transduced and GFP− cells show no significant difference in lineage distribution, whereas GATA-2–transduced GFP+ cells read out predominantly in the myeloid lineage. (H) Ki-67 expression of engrafted GFP-positive cells sorted from the bone marrow of NOD-SCID mice. Many more vector-transduced cells express the proliferation marker Ki-67 than GATA-2–transduced cells. Plots are shown from 1 of 2 representative experiments, where 1 of 2 vector-engrafted animals is compared with a single GATA-2–engrafted animal. (I) Plots showing proportions of quiescent cells lacking expression of Ki-67 from all animals in the experiment represented in panel H.
Engraftment and in vivo reconstitution of NOD-SCID animals by CD34+CD38− CB cells is inhibited by enforced GATA-2 expression in a level-dependent manner. (A) GFP expression level after 3 days in vitro culture, showing GFP intensity and transduction efficiency of transplanted GATA-2–transduced populations. Staining for human CD45 and GFP signal are shown for 1 representative animal from each group at 4 and 8 weeks (same animal sampled at 4 weeks is shown at 8 weeks). All animals are represented in Figure S1C. (B) High GATA-2–mediated expansion defect in NOD-SCID animals. Mean proportions of engrafted GFP+ cells falling in the upper gate shown in panel A, with high expression of GFP, are plotted for all engrafted animals at 8 weeks (n = 4 for each transgene). Error bars indicate SEM. (C) Expression level-dependent inhibition of hematopoiesis in NOD-SCID mice is not due to alterations in homing. GFP expression level after 3 days in vitro culture and in vivo 4 weeks after intraosseous injection. Engrafted cells expressing high levels of GATA-2 are not observed at the same frequency as in the initial transplant material. Cells were injected directly into the bone marrow cavity after only 5 hours of exposure to lentivirus. Staining for human CD45 and GFP signal are shown. (D) Proportions of engrafted human GFP+ cells falling in the upper GFP gate shown in panel C are plotted for the populations given as a transplant (dark gray) and from all engrafted animals at 4 weeks after intraosseous injection (black; n = 2 for vector-transduced cells; n = 1 for GATA-2). (E) Similar to panel C, cells were injected directly into the bone marrow cavity after 5 hours of transduction, and engraftment was assessed 12 weeks later. (F) Similar to panel D, proportions of engrafted human GFP+ cells falling in the upper GFP gate shown in panel E are plotted for the transplant (dark gray) and from all engrafted animals at 12 weeks after intraosseous injection; n = 2 for both groups. (G) Relative lymphoid and myeloid reconstitution is affected by enforced GATA-2 expression. For each animal, the proportion of human CD45+GFP− and human CD45+ GFP+ cells falling in CD19+ lymphoid and CD33+ myeloid gates are plotted; n = 4 animals in each group. Vector-transduced and GFP− cells show no significant difference in lineage distribution, whereas GATA-2–transduced GFP+ cells read out predominantly in the myeloid lineage. (H) Ki-67 expression of engrafted GFP-positive cells sorted from the bone marrow of NOD-SCID mice. Many more vector-transduced cells express the proliferation marker Ki-67 than GATA-2–transduced cells. Plots are shown from 1 of 2 representative experiments, where 1 of 2 vector-engrafted animals is compared with a single GATA-2–engrafted animal. (I) Plots showing proportions of quiescent cells lacking expression of Ki-67 from all animals in the experiment represented in panel H.
Absolute numbers of engrafted human GFP+ cells were similar in 4-week aspirates from animals that received a transplant with GATA-2– and vector-transduced cells (Figure S1B), suggesting enforced expression of GATA-2 does not reduce initial engraftment in NOD-SCID mice.
At 8 weeks we counted engrafted GFP+ cells falling in high- and low-intensity GFP gates and compared these data with the GFP intensity profile of the transplanted cells. For the vector-transduced cells, after 8 weeks in vivo the percentage of GFPhi cells was essentially unchanged (Figure 5B; Figure S1B). However, for the GATA-2–transduced cells, the GFPhi fraction was sharply reduced, consistent with our predictions based on level-dependent antiproliferative effects. These findings are thus compatible with an in vivo antiproliferative effect of GATA-2 similar to that seen in vitro, and extend into the human stem and progenitor cell setting previous engraftment data that used mouse bone marrow mononuclear cells.18
Effects of enforced GATA-2 expression on reduced hematopoiesis are durable and not due to homing defects
Although transduced cells were presumably injected before frank expression of GATA-2, cells expressing high levels of GFP (and hence GATA-2) might have been selected against in their ability to home. To address this, we repeated the experiment with cells transduced for 5 hours and injected directly into the bone marrow cavity, excluding an effect of GATA-2 expression on homing by time and by site of injection. As before, after 4 weeks the GATA-2–transduced cells showed a clear overgrowth by cells expressing low levels of GFP (and hence low GATA-2; Figure 5C,D). These data confirm that the eroded function of GATA-2hi cells after tail vein injection was not due to impaired homing. Moreover, after 12 weeks of engraftment mice that received a similar transplant show persistence of rare GFPhi cells in the bone marrow, albeit with the same overgrowth of GFPlo cells in the graft (Figure 5E,F).
Enforced expression of GATA-2 is associated with reduced production of lymphoid cells in NOD-SCID mice
More mature progenitors are considered to have exhausted after 8 weeks in NOD-SCID mice, and human cells present are presumed to be derived from transplanted stem and primitive progenitor cells. Typically, at this time point most human cells present are lymphoid.39,40 In tail vein–injected mice 8 weeks after transplantation, a predominantly lymphoid distribution was observed from engrafted vector-transduced cells, with approximately 82% of the cells expressing CD19 and approximately 11% expressing CD33 (Figure 5G). By contrast, GATA-2–transduced cells were only approximately 34% lymphoid, with approximately 61% expressing CD33 (Figure 5G); underlying this was a reduction in the number of CD19+ cells but with CD33+ cell number unaffected. In the same animals, approximately 81% of the nontransduced human cells expressed CD19 and approximately 13% expressed CD33, confirming that the bias toward myeloid output was due to enforced GATA-2 expression. However, because GATA-2 is modulating CB cell-cycle kinetics, altered lineage output here is potentially due to retarded output from myeloid progenitors that have exhausted after 8 weeks in the absence of enforced GATA-2 expression.
Enforced GATA-2 expression affects cell cycle in vivo
A plausible interpretation of our data is that effects of engraftment observed in vivo are due to conferral of quiescence by GATA-2. There are limited reports of quiescence detection in NOD-SCID models. Moreover, our mice were predominantly engrafted with progeny from GFPlo (and hence GATA-2lo) cells that may show a milder phenotype than the unexpanded GFPhi cells. Despite these issues, we sorted GFP+ human cells from NOD-SCID animals 4 weeks after transplantation and stained for nuclear proliferation antigen Ki-67. This analysis showed that fewer GATA-2–transduced cells express Ki-67 than do vector-transduced cells (Figure 5H,I), confirming the relative quiescence of GATA-2–transduced cells in vivo. Similar data were obtained after 6 weeks of engraftment in NOD-SCID/IL2rγnull animals (data not shown). Thus, maintenance of relatively high expression of GATA-2 confers quiescence on human CB cells in vitro and in vivo in a manner that does not appear to prevent their retention in the bone marrow in the medium-to-long term.
Discussion
For the first time, we formally demonstrate here that sustaining high GATA-2 expression in human hematopoietic stem and progenitor cells confers increased quiescence and consequently limits their performance in vitro and in vivo.
Quiescence is an important hallmark property of HSCs, distinct from growth arrest, and critical for maintenance of hematopoiesis throughout life. Methods to distinguish quiescence from growth arrest in live cells are limited, but Pyronin Y staining is a widely accepted technique that has been critical in the prospective isolation and comparison of cycling and quiescent cells.5,11,13,25 In our study we have made extensive use of Pyronin Y staining and have validated the method to show that changes in Pyronin staining reflect quiescence and not growth arrest.
Another operational feature of quiescence is reversibility, and, indeed, if this were not the case, then long-term maintenance of hematopoiesis would not be possible. With the use of a ligand-activated GATA-2/ER chimera, we find that unmasking GATA-2 activity confers reversible quiescence in murine cell line models. The reversibility of these effects on cycle (and hence proliferation) confirm that modulating GATA-2 activity affects quiescence rather than inducing irreversible cycle arrest. Moreover, our data indicate that a return to normal proliferative rate lags behind increased staining with Pyronin Y, confirming that reduced Pyronin Y staining is not merely marking cells that have arrested. Taken together, these results clearly indicate for the first time that GATA-2 acts to limit cycling of human and murine cells in part through increased residency in G0.
Previous data have shown effects of GATA-2 on growth16,19,20 or correlated GATA-2 expression with quiescence.17 This novel appreciation that GATA-2 regulates quiescence provides insight into prior studies. In key work, Persons et al18 showed that enforced GATA-2 expression in murine bone marrow cells blocked hematopoiesis without preventing engraftment or retention. Our data are consistent with this conclusion with the use of primary human CB cells in a NOD-SCID transplantation setting. However, Persons et al18 did not directly examine proliferation of these cells and did not detect altered cell-cycle distribution by DNA staining. We also failed to detect increased G0/G1 accumulation on enforced expression of GATA-2 in subsets of CB CD34+ cells. However, we resolved the G0/G1 peaks by Pyronin staining to show the relative quiescence induced by GATA-2 and to show directly that cells with enforced expression of GATA-2 are less proliferative. It is worth noting in our data that modulating GATA-2 activity (whether by GATA-2/ER or constitutive expression) reduces Pyronin staining of cells irrespective of position in cycle; however, only in the case of GATA-2/ER did we observe G0/G1 accumulation by Hoechst alone. Moreover physiologically, primitive stem and progenitor cells reside in G0/G1 with quiescent cells resolved by low Pyronin Y staining; thus, reduced Pyronin Y staining reflects a physiologically relevant phenomenon.
If increasing GATA-2 expression increases quiescence, then a simple prediction might be that decreasing GATA-2 expression should decrease quiescence. The haploinsufficient GATA-2+/− mouse model provides the opportunity to examine this question, but in fact it shows mildly increased quiescence in the stem and early progenitor cell compartment (detected by decreased Pyronin Y staining and increased survival after 5-FU exposure).41 Moreover, GATA-2+/− bone marrow exhibited reduced repopulation capacity in transplantation,41 mirroring to some degree at least the phenotype exhibited by our more quiescent enforced GATA-2 cells. Certainly, it is not unprecedented for increased and decreased expression of a transcription factor to confer similar outcomes.42,43 If indeed, increased and decreased GATA-2 expression both increase quiescence, then it is not necessarily true that these effects on quiescence are mediated by the same pathways.
Analysis of the mechanisms by which GATA-2 confers quiescence suggests the picture may be complex. We show that GATA-2–enforced cells repress MEF, a transcriptional change that confers quiescence on CB CD34+ cells.13 These data do not provide the complete mechanism of cell-cycle exit, because simultaneously enforcing MEF and GATA-2 expression did not ameliorate quiescence, and we did not detect significant involvement of CKIs. Although our data are not exhaustive in respect with other CKIs, they do suggest that GATA-2 exerts its effects independently of p21CIP1 and p27KIP1, at least in our model system.
Our data show a correlation between quiescence conferred by GATA-2 and reduced expression of CCND3, CDK4, and CDK6. Levels of cyclinD:CDK4/6 complexes are important in cell-cycle progression. CDK6−/− knockout mice displayed mild hematopoietic defects, with decreased cellularity in the thymus and spleen and with reduced peripheral blood cellularity in some animals. Although CDK4−/− knockout mice show no overt hematopoietic phenotype, CDK4/CDK6−/− double knockout mice die in utero with reduced HSC and progenitor counts.44 Thus, reductions in CCND3, CDK4, and CDK6 betray effects of GATA-2 activity on expression of key components of the cell-cycle machinery, in a manner that is known to affect hematopoiesis in vivo. Moreover, our data suggest that GATA-2 represses at least one component of the origin recognition complex (ORC) and reduces cell-cycle kinetics. In agreement with this, we observe down-regulation of multiple ORC components on GATA-2/ER activation in differentiation-competent cell line studies, implying coordinated inhibition of origin licensing by GATA-2 (A.J.T. and T.E., unpublished data, 2008).
As this work shows, modulating GATA-2 activity by increased expression has a marked effect on stem and progenitor cell function via an altered transcriptional profile and conferral of a more quiescent phenotype. Modifying effects (posttranslational modifications or partner proteins), triggered by niche components45 or other in vivo signals, may contribute to these effects and act to maintain high GATA-2 activity.46 Understanding the molecular control of quiescence in human HSCs is particularly relevant to clinical settings because quiescent cells have increased capacity for bone marrow engraftment and reconstitution.6-9 Thus, elucidation of the transcriptional network around GATA-2 may suggest other novel regulators or effectors of these processes (as well as growth control) in hematopoietic stem and progenitor cells. Regulation upstream of GATA-2 expression at the signaling level may allow reversible modulation of human HSC quiescence for HSC expansion protocols10 or eliminating drug-resistant leukemic clones.22
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ann Atzberger, Craig Waugh, Kevin Clark, Anna Fossum, and Zhi Ma for flow sorting assistance and Jacek Toporski and Lilian Wittman for assistance with NOD/SCID assays.
This work was supported by the Medical Research Council (MRC), United Kingdom, Leukaemia Research Fund UK, Hemato-Linné, Sweden, by the Oxford Partnership Biomedical Research Centre (UK), and by Stem Expand (EU).
Authorship
Contribution: A.J.T. contributed to the design, experimental performance, interpretation, and writing; C.P. contributed to experimental performance and interpretation; A.C., D.H., and G.E.M. contributed to experimental performance; N.P.R., L.L., and S.E.W.J. contributed reagents; and T.E. contributed to the design and interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tariq Enver, MRC Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, Oxford OX3 9DS, United Kingdom; e-mail: tenver@gwmail.jr2.ox.ac.uk.