Abstract
In inflamed venules, leukocytes use P-selectin glycoprotein ligand-1 (PSGL-1) to roll on P-selectin and E-selectin and to activate integrin αLβ2 (lymphocyte function-associated antigen-1, LFA-1) to slow rolling on intercellular adhesion molecule-1 (ICAM-1). Studies in cell lines have suggested that PSGL-1 requires its cytoplasmic domain to localize in membrane domains, to support rolling on P-selectin, and to signal through spleen tyrosine kinase (Syk). We generated “ΔCD” mice that express PSGL-1 without the cytoplasmic domain. Unexpectedly, neutrophils from these mice localized PSGL-1 normally in microvilli, uropods, and lipid rafts. ΔCD neutrophils expressed less PSGL-1 on their surfaces because of inefficient export from the endoplasmic reticulum. Limited digestion of wild-type neutrophils with O-sialoglycoprotein endopeptidase was used to reduce the PSGL-1 density to that on ΔCD neutrophils. At matched PSGL-1 densities, both ΔCD and wild-type neutrophils rolled similarly on P-selectin. However, ΔCD neutrophils rolling on P-selectin did not trigger Syk-dependent activation of LFA-1 to slow rolling on ICAM-1. These data demonstrate that the PSGL-1 cytoplasmic domain is dispensable for leukocyte rolling on P-selectin but is essential to activate β2 integrins to slow rolling on ICAM-1.
Introduction
During inflammation, leukocytes tether to and roll on the vessel wall. They then roll more slowly until they arrest. Finally, they crawl through or between endothelial cells into the underlying tissues. Interactions of selectins with glycosylated ligands mediate tethering and rolling. Interactions of β2 integrins with ligands, such as intercellular adhesion molecule-1 (ICAM-1), mediate slow rolling and arrest.1,2 These interactions occur in blood flow, which exerts force on adhesive bonds that affects bond lifetimes.3,4 Furthermore, engagement of adhesion receptors transmits signals that intersect with chemokine receptor signals to influence the adhesion cascade.1
Binding of integrin cytoplasmic domains to signaling and cytoskeletal proteins is critical for integrin function.1,5 Interactions of selectin cytoplasmic domains with cytosolic proteins also contribute to their adhesive properties. E-selectin and P-selectin are expressed on activated endothelial cells and/or platelets, whereas L-selectin is expressed on the microvilli of leukocytes.2 The cytoplasmic domains of E-selectin and P-selectin interact with clathrin-coated pits. These interactions cluster E-selectin and P-selectin on the endothelial cell surface, enhancing leukocyte rolling under flow.6,7 The cytoplasmic domain anchors L-selectin to the cytoskeleton by binding to α-actinin8 and to the ezrin/radixin/moesin (ERM) family.9 Mutation of the ERM-binding site in the cytoplasmic domain shifts L-selectin out of microvilli onto the cell body of transfected cells and impairs tethering to L-selectin ligands under flow.10 Removal of the α-actinin-binding site markedly impairs rolling of transfected cells on L-selectin ligands, and deletion of the cytoplasmic domain virtually eliminates rolling.11
Less is known about the contributions of cytoplasmic domains of selectin ligands to adhesion and signaling. P-selectin glycoprotein ligand-1 (PSGL-1), a transmembrane homodimeric mucin on leukocytes,2 mediates tethering to and rolling on P-selectin and L-selectin under flow,12,13 and cooperates with other leukocyte glycoproteins to mediate tethering to and rolling on E-selectin.14,15 The sequence of the cytoplasmic domain of PSGL-1 is conserved across species, suggesting important functions. Like L-selectin, PSGL-1 is concentrated on the tips of microvilli.16,17 In vitro, a juxtamembrane sequence of the cytoplasmic domain of PSGL-1 binds to ERM proteins,18 suggesting that PSGL-1 might also target to microvilli through ERM interactions. On agonist-mediated polarization of leukocytes, PSGL-1 redistributes to uropods,17 but mutation of the ERM-binding sequence prevents a portion of PSGL-1 from redistributing to uropods of transfected cells.18 Deletion of all but 4 residues of the cytoplasmic domain was reported to prevent PSGL-1–mediated rolling of transfected cells on P-selectin.19 These data imply that PSGL-1 must connect its cytoplasmic domain to the cytoskeleton to regulate both its membrane localization and its adhesive properties. However, none of these studies examined the functions of the PSGL-1 cytoplasmic domain in primary leukocytes.
Engagement of PSGL-1 transduces signals that are integrated with signaling through chemokine receptors to elicit effector responses.20-25 A limitation of most studies is that signaling was induced by cross-linking PSGL-1 with antibodies or selectins for minutes to hours. Signaling during the rapidly reversible interactions of PSGL-1 with selectins during rolling has received less attention. In vivo, neutrophils that roll on P-selectin and E-selectin transition to slower rolling through interactions of β2 integrins with ICAM-1 and other ligands on endothelial cells.26,27 These signals cooperate with those from chemokine receptors to recruit neutrophils to inflammatory sites.28,29 When murine blood is perfused ex vivo, neutrophils roll slower on P-selectin or E-selectin when either protein is coimmobilized with ICAM-1. Slow rolling requires engagement of PSGL-1, which activates integrin LFA-1 through a Syk-dependent pathway.29 Syk is usually activated by Src family kinases after it is recruited to immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic domains of adaptors.30 The cytoplasmic domain of PSGL-1 lacks conventional ITAM sequences, although the ERM-binding region was reported to recruit Syk indirectly through a cryptic ITAM on moesin or ezrin.23 To date, there is no direct evidence that the PSGL-1 cytoplasmic domain contributes to Syk-mediated signaling in primary leukocytes.
To directly examine the functions of the PSGL-1 cytoplasmic domain in leukocytes, we generated knock-in mice that express PSGL-1 without the cytoplasmic domain. Leukocytes from these mice were used to study the contributions of the PSGL-1 cytoplasmic domain to rolling and signaling under flow.
Methods
Reagents
Rat monoclonal antibodies (mAbs) RB40.34 and 4RA10 were gifts from Dietmar Vestweber (Vrije Universiteit Brussel, Brussels, Belgium). Rat mAb HECA-452 was from ATCC (Manassas, VA). Rat mAb MTH3131 was a gift from Geert Raes (University of Minnesota, St Paul, MN). Murine mAb CHO-13132 was a gift from Bruce Walcheck (University of Minnesota, St Paul, MN). Rabbit polyclonal antiserum against a 19-residue peptide corresponding to the N-terminal sequence of murine PSGL-1 was a gift from Richard Cummings (Emory University School of Medicine, Atlanta, GA). Rabbit polyclonal antiserum, which was raised against a 17-residue peptide corresponding to the cytoplasmic domain of human L-selectin, recognized both human and murine L-selectin. IgG was isolated and biotinylated by standard methods. Streptavidin–Alexa 488 and donkey anti–goat Alexa 546 were from Invitrogen (Carlsbad, CA). Goat anti–mouse CD43 (S-19) was from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-labeled goat anti–rabbit IgG and anti–mouse IgG were from Pierce Chemical (Rockford, IL). Gold-conjugated (15 nm) goat anti–rabbit IgG was from Electron Microscopy Sciences (Hatfield, PA). Mouse anti–flotillin-1 mAb (clone 18) was from BD Biosciences Transduction Laboratories (Lexington, KY). Rat anti–LFA-1 mAb (clone M17/4), rat anti–Mac-1 mAb (clone M1/70), hamster anti–ICAM-1 mAb (clone 3E2), phycoerythrin (PE)–labeled rat anti–murine PSGL-1 mAb 2PH1, and fluorescein isothiocyanate–labeled rat mAbs to murine L-selectin (Mel-14), CD18 (C71/16), CD24 (M1/69), CD43 (S7), CD44 (IM7), and CD45 (30-F11) were from BD Biosciences PharMingen (San Diego, CA). Piceatannol was from Calbiochem (San Diego, CA).
Mice
ΔCD knock-in mice were prepared by standard procedures,14 with additional details provided in “Generation of ΔCD mice.” PSGL-1–deficient (Selplg−/−) mice were generated as described.14 All experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Leukocytes
Murine bone marrow leukocytes were isolated by flushing femurs and tibias with Hank's balanced salt solution (HBSS) without Ca2+ or Mg2+. Peripheral blood was obtained from isoflurane-anesthetized mice by retroorbital bleed into microvette tubes coated with ethylenediaminetetraacetic acid (Sarsted, Numbrecht, Germany). Red blood cells were lysed by adding 5 mL 150 mM NH4Cl, 10 mM of NaHCO3, 1 mM ethylenediaminetetraacetic acid to 0.5 mL leukocytes for 20 seconds and then immediately adding 5 mL HBSS. After centrifugation at 100g for 5 minutes, cells were resuspended at 2 × 106/mL in HBSS containing 1.26 mM Ca2+, 0.81 mM Mg2+, and 0.5% human serum albumin (HSA).
Transfected cells
Chinese hamster ovary (CHO) cells stably expressing FucT-VII and core 2 β1-6-N-acetylglucosaminyltransferase-I were described previously.33 The cells were transfected with cDNAs encoding wild-type (WT) human PSGL-1 or ΔCD human PSGL-1 in pZeoSV (Invitrogen). Cells were placed into low-salt Dulbecco modified Eagle medium, 10% FBS, 100 μM hypoxanthine, 16 μM thymidine, nonessential amino acids, 600 μg/mL G418, 100 μg/mL hygromycin, 250 μg/mL Zeocin (Invitrogen), and 2 mM glutamine 48 hours after transfection. Clones expressing matched surface densities of WT and ΔCD PSGL-1 were identified by flow cytometry using anti–human PSGL-1 mAb.16
Generation of recombinant proteins
A construct encoding glutathione-S-transferase (GST) fused to the PSGL-1 cytoplasmic tail (GST-CD) was generated using synthetic oligonucleotides encoding the human PSGL-1 cytoplasmic domain with BamHI and EcoRI sites appended, respectively, at the 5′ and 3′ ends. Oligonucleotides were annealed, phosphorylated using T4 polynucleotide kinase (US Biochemical, Cleveland, OH), and ligated into the pGEX-5x-3 vector (GE Healthcare Life Sciences, Little Chalfont, United Kingdom). To make a construct with GST fused to 2 arginines at its C terminus (GST-RR), pGEX-5x-3 was used as a template to introduce a second arginine and stop codon in the BamHI cleavage sequence of the multiple cloning site, using the QuikChange Mutagenesis kit (Stratagene, La Jolla, CA). A similar approach was used to make a construct with GST fused to the PSGL-1 cytoplasmic domain in which 4 residues required for binding to ERM proteins were replaced with alanine (GST-CD-mutated). Constructs were confirmed by DNA sequencing.
BL21 Escherichia coli (Stratagene) were transformed with vectors encoding GST-CD, GST-CD-mutated, or GST-RR and induced with 1 mM isopropyl beta-D-thiogalactopyranoside for 4 hours at 37°C. Bacteria were harvested by centrifugation, washed with phosphate-buffered saline (PBS), and frozen at −80°C. Thawed bacteria were lysed with Bacterial Protein Extraction Reagent (Pierce Chemical) supplemented with 5 mM benzamidine, 5 mM phenylmethylsulfonyl fluoride, and Complete Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN) for 30 minutes at room temperature. After lysis, cell debris was removed by centrifugation (20 000g, 15 minutes, 4°C). The supernatant was mixed with glutathione-Sepharose 4B beads (GE Healthcare Life Sciences) and incubated for 30 minutes at room temperature with gentle mixing. The beads were washed 4 times in 50 mL PBS. GST fusion proteins attached to beads were stored in PBS with 0.02% NaN3 and protease inhibitors at 4°C.
Full-length human moesin cDNA was amplified with primers that introduced a C-terminal 8-residue epitope for a Ca2+-dependent mAb.34 The PCR product was digested and ligated into the bacterial expression vector pKK223-3 (GE Healthcare Life Sciences). The sequence was confirmed by DNA sequencing. Recombinant moesin was expressed in JM109 E coli (Promega, Madison, WI) after induction with 1 mM of isopropyl beta-D-thiogalactopyranoside for 4 hours at 37°C. Recombinant moesin was purified from cell lysates by immunoaffinity chromatography.34 The purified protein was dialyzed in 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 50 mM NaCl. Protein concentration was determined using the BCA assay (Pierce Chemical).
Pull-down assay
GST fusion proteins bound to glutathione-Sepharose 4B beads were washed 3 times with 1 mL buffer A (10 mM of N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5, 150 mM of NaCl, 0.1% Triton X-100) and resuspended in 200 μL of the same buffer. Recombinant moesin was incubated with the beads at room temperature for 2 hours with occasional mixing and then washed 4 times with buffer A. Bound proteins were eluted by boiling in 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for 5 minutes. Precipitated moesin was detected by Western blotting with antimoesin mAb.
Western blots and flow cytometry
Plasma PSGL-1 measurement
Cell-free plasma generated from acid-citrate-dextrose-anticoagulated blood35 was diluted 3-fold in PBS and incubated at 4°C for 2 hours with 2 μg anti–PSGL-1 IgG and then with 50 μL protein A-agarose (Santa Cruz Biotechnology) at 4°C for 2 hours. The protein A-agarose beads were washed 3 times in PBS, and bound protein was eluted by boiling the pellets in SDS sample buffer. To separate microparticles, cell-free plasma was diluted 3-fold in PBS and ultracentrifuged at 150 000g for 2 hours at 4°C. Lysis buffer was added to the pellet and the supernatant, and PSGL-1 was immunoprecipitated. Immunoprecipitates were analyzed by Western blots probed with 1 μg/mL rat anti–murine PSGL-1 mAb MTH31.31 A second immunoprecipitation did not yield additional PSGL-1, confirming that the first immunoprecipitation was quantitative. Beads coated with nonimmune rabbit immunoglobulin G (IgG) did not precipitate PSGL-1 (data not shown). Immunoprecipitates from leukocyte lysates were analyzed in parallel. To quantify the amount of PSGL-1 in plasma, the number of murine PSGL-1 molecules on transfected CHO cells36 was determined by calibrating the fluorescence of bound PE-conjugated anti–PSGL-1 mAb 2PH1 with Quantibrite beads bearing known quantities of PE (BD Biosciences, San Jose, CA). The number of PSGL-1 molecules on the cell surface was converted to the mass of PSGL-1 in a given volume of cell lysate, assuming a molecular weight of 230 000 for dimeric PSGL-1. Western blots of plasma and of serial dilutions of lysates from transfected CHO cells were probed with anti–PSGL-1 IgG. Calibration of the densitometric ratios of the PSGL-1 in cell lysates and plasma was used to determine the amount of PSGL-1 in plasma.
Whole blood clotting time
Whole blood clotting times were performed as described.37
Scanning electron microscopy
Leukocytes were fixed for 5 minutes in 1% paraformaldehyde and then labeled with 15 μg/mL rabbit anti–PSGL-1 IgG and gold-conjugated (15 nm) goat anti–rabbit IgG (1:30 dilution). Samples were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate. After washing, cells were dehydrated using a graded ethanol series and dried in a critical point dryer using carbon dioxide as transitional fluid (Autosamdri-814). Fixed cells were mounted on bulk specimen holders (JEOL), coated with carbon using a Hummer VI sputter coater, and viewed with a JEOL JSM-880 scanning electron microscope under 15-kV accelerating voltage. Backscattered and secondary electron images were acquired simultaneously and separately because some gold particles from backscattered images were masked by secondary electrons. Images were processed using Photoshop (Adobe Systems). Pseudocolor was applied to better visualize immunogold and cell surfaces. Brightness/contrast adjustment was applied equally to the entire image.
Detergent-resistant membrane preparation
Triton X-100 leukocyte lysates were centrifuged at 4°C in OptiPrep (Sigma-Aldrich, St Louis, MO) as described.7 Fractions collected from the top of the tube were analyzed by Western blotting.
Immunofluorescence
Leukocytes were stimulated with 1 μM of formyl-methionyl-leucyl-phenylalanine (fMLP) and settled on poly-L-lysine–coated coverslips at 37°C for 10 minutes. The polarized cells were washed, fixed in 1% paraformaldehyde for 10 minutes at 4°C, blocked with 1% bovine serum albumin in HBSS for 1 hour, and incubated with 15 μg/mL biotinylated rabbit anti–mouse PSGL-1 IgG in HBSS plus 0.5% bovine serum albumin for 45 minutes at 4°C, followed by streptavidin–Alexa 488 (1:2000 dilution) for 45 minutes at 4°C. Cells were washed and incubated with 15 μg/mL goat anti–mouse CD43 IgG for 45 minutes at 4°C, followed by donkey anti–goat Alexa 546 (1:1000 dilution) for 45 minutes at 4°C. As controls for primary antibody binding, PSGL-1–deficient leukocytes were incubated with biotinylated rabbit anti–mouse PSGL-1 followed by streptavidin-Alexa 488, or WT and ΔCD leukocytes were incubated with irrelevant polyclonal goat antibody followed by donkey anti–goat Alexa 546. As controls for secondary antibody binding, WT leukocytes were incubated with rabbit anti–mouse PSGL-1 followed by donkey anti–goat Alexa 546, or with goat anti–mouse CD43 followed by streptavidin Alexa 488. Immunofluorescence was detected with a Leica TCS NT confocal microscope (Leica Microsystems, Deerfield, IL) equipped with a krypton/argon laser.
Intravital microscopy
Intravital microscopy of leukocyte rolling in postcapillary venules of murine cremaster muscle was performed as described.14
Flow chamber assay
Leukocyte adhesion under flow was measured as described.14,38 Briefly, a mAb to the Fc portion of human IgM was adsorbed on a demarcated area of 35-mm polystyrene dishes. For some experiments, ICAM-1-Ig (R&D Systems, Minneapolis, MN) was also adsorbed. After blocking the dishes for 2 hours in 1% HSA, murine P-selectin-IgM was captured. Site densities were measured by binding of 125I-labeled anti–P-selectin mAb RB40.34 or anti-ICAM-1 mAb 3E2. Leukocytes (106/mL in HBSS with 0.5% HSA) were perfused over P-selectin in dishes mounted in a parallel-plate flow chamber at the indicated wall shear stresses. In some experiments, leukocytes were suspended in citrate-anticoagulated plasma from WT or ΔCD mice rather than in buffer. After 5 minutes, cells were analyzed using a videomicroscopy system coupled to a digital analysis system. Velocities of rolling cells were measured over a 5-second interval.39 To reduce PSGL-1 density on WT leukocytes to the level found on ΔCD leukocytes, we treated 107 WT leukocytes/mL with 28 μg/mL O-sialoglycoprotein endopeptidase (OSGE) for 20 minutes at 37°C. Leukocyte membrane tether extrusion was visualized by differential interference contrast microscopy.40 Transfected CHO cells expressing WT or ΔCD human PSGL-1 (3 × 105 cells/mL in HBSS with 0.5% HSA) were perfused over immobilized P-selectin from human platelets. Rolling velocities and the number of rolling cells were assessed as for leukocytes.
Statistics
The unpaired Student t test was used to determine P values as indicated in the figures.
Results
Generation of ΔCD mice
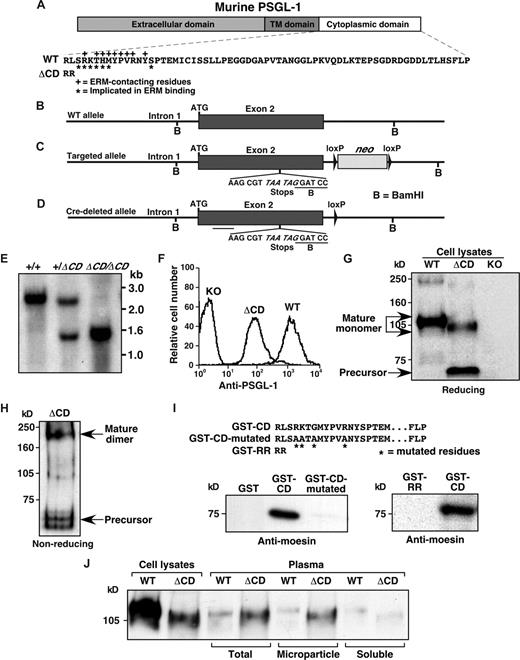
We produced knock-in ΔCD mice that express PSGL-1 without the cytoplasmic domain. The entire 67-residue cytoplasmic domain was deleted except for the membrane-proximal arginine. A second arginine was added to ensure anchorage in the membrane (Figure 1A). The deletion includes the sequences involved in binding to ERM proteins18,41 (Figure 1A). We made a targeting construct that introduced 2 stop codons immediately 3′ to the sequence encoding the transmembrane domain and the 2 cytoplasmic arginines, plus a BamHI site to facilitate screening of targeted embryonic stem cells (Figure 1B,C). A neomycin cassette flanked by loxP sites was introduced downstream of exon 2 (Figure 1C). The targeting construct was transfected into embryonic stem cells. After identifying clones with correct homologous recombination, the neomycin cassette was removed by transient in vitro expression of Cre recombinase (Figure 1D). Embryonic stem cells were injected into C57BL/6J blastocysts. Chimeric offspring were bred for germ line transmission of the targeted allele, which was confirmed by Southern blot (Figure 1E). ΔCD mice homozygous for the targeted allele have been backcrossed over 10 generations in the C57BL/6J congenic background. ΔCD mice develop normally, and both sexes are fertile. ΔCD mice have normal peripheral blood counts, and histologic examination of multiple organs revealed no abnormalities. Like PSGL-1–deficient mice,14 ΔCD mice appear healthy and have normal lifespans.
Generation of ΔCD mice. (A) Schematic of the extracellular, transmembrane (TM), and cytoplasmic domains of murine PSGL-1. The amino acid sequence of the WT cytoplasmic domain is depicted. *Residues implicated by mutagenesis as contributing to binding to ERM proteins.18 +Residues that directly contact an ERM protein in a crystal structure.41 The 2 arginines in the ΔCD cytoplasmic domain are shown. (B-D) Diagrams of the WT Selplg allele, the targeted allele, and the targeted allele after Cre-mediated deletion of the neo cassette. The probe used for Southern blotting is underlined. (E) Southern blot of BamHI-digested genomic DNA from WT mice and from mice heterozygous or homozygous for the targeted allele. (F) Flow cytometric analysis of PSGL-1 expression on peripheral blood neutrophils from WT mice, ΔCD mice (homozygous for the targeted allele), and PSGL-1 knockout (KO) mice. Cells were incubated with PE-conjugated anti–PSGL-1 mAb 2PH1. (G,H) Western blots of leukocyte lysates electrophoresed under reducing or nonreducing conditions. Blots were probed with rabbit anti–PSGL-1 IgG. Arrows mark the precursor and mature forms of PSGL-1. (I) Recombinant moesin was incubated with GST fused to the entire cytoplasmic domain of PSGL-1 (GST-CD), the cytoplasmic domain in which 4 residues required for binding to ERM proteins were substituted with alanines (GST-CD–mutated), or to the 2 arginines in the cytoplasmic domain of ΔCD mice (GST-RR). Precipitated protein was eluted and probed by Western blot with antimoesin antibody under reducing conditions. Equivalent loading was confirmed by reprobing with anti-GST antibody (data not shown). Results are representative of 3 experiments. (J) Leukocyte lysates or PSGL-1 immunoprecipated from total plasma or from microparticle or soluble fractions of plasma were probed by Western blot with anti–PSGL-1 mAb MTH31 under reducing conditions. Results are representative of 5 experiments.
Generation of ΔCD mice. (A) Schematic of the extracellular, transmembrane (TM), and cytoplasmic domains of murine PSGL-1. The amino acid sequence of the WT cytoplasmic domain is depicted. *Residues implicated by mutagenesis as contributing to binding to ERM proteins.18 +Residues that directly contact an ERM protein in a crystal structure.41 The 2 arginines in the ΔCD cytoplasmic domain are shown. (B-D) Diagrams of the WT Selplg allele, the targeted allele, and the targeted allele after Cre-mediated deletion of the neo cassette. The probe used for Southern blotting is underlined. (E) Southern blot of BamHI-digested genomic DNA from WT mice and from mice heterozygous or homozygous for the targeted allele. (F) Flow cytometric analysis of PSGL-1 expression on peripheral blood neutrophils from WT mice, ΔCD mice (homozygous for the targeted allele), and PSGL-1 knockout (KO) mice. Cells were incubated with PE-conjugated anti–PSGL-1 mAb 2PH1. (G,H) Western blots of leukocyte lysates electrophoresed under reducing or nonreducing conditions. Blots were probed with rabbit anti–PSGL-1 IgG. Arrows mark the precursor and mature forms of PSGL-1. (I) Recombinant moesin was incubated with GST fused to the entire cytoplasmic domain of PSGL-1 (GST-CD), the cytoplasmic domain in which 4 residues required for binding to ERM proteins were substituted with alanines (GST-CD–mutated), or to the 2 arginines in the cytoplasmic domain of ΔCD mice (GST-RR). Precipitated protein was eluted and probed by Western blot with antimoesin antibody under reducing conditions. Equivalent loading was confirmed by reprobing with anti-GST antibody (data not shown). Results are representative of 3 experiments. (J) Leukocyte lysates or PSGL-1 immunoprecipated from total plasma or from microparticle or soluble fractions of plasma were probed by Western blot with anti–PSGL-1 mAb MTH31 under reducing conditions. Results are representative of 5 experiments.
Leukocytes from ΔCD mice express less PSGL-1 on the cell surface
Leukocytes from both ΔCD and WT mice expressed PSGL-1 on the cell surface (Figure 1F). Unexpectedly, the level of PSGL-1 on ΔCD leukocytes was reduced to approximately 10% of the level on WT leukocytes. This reduced surface expression was observed for neutrophils (Figure 1F) and all other leukocytes, including hematopoietic precursors, using 3 different mAbs and a polyclonal antibody to murine PSGL-1 (data not shown). Cell-surface expression of L-selectin, β2 integrins, CD24, CD43, and CD45 was equivalent on ΔCD and WT leukocytes (data not shown). WT leukocytes express PSGL-1 as a disulfide-linked homodimer, which was detected as an approximate 110-kD monomer in immunoblots of leukocyte lysates resolved by SDS-PAGE under reducing conditions (Figure 1G). Leukocytes from ΔCD mice expressed a form of PSGL-1 that migrated slightly faster under reducing conditions, consistent with deletion of the cytoplasmic domain (Figure 1G). Under nonreducing conditions, ΔCD PSGL-1 migrated as a disulfide-linked dimer (Figure 1H). ΔCD leukocytes also expressed an immunoreactive 65-kD species that migrated identically under reducing and nonreducing conditions (Figure 1G,H). In studies to be described elsewhere, we found that this species is an underglycosylated precursor of PSGL-1. The precursor accumulates intracellularly because of inefficient export from the endoplasmic reticulum, causing lower steady-state levels of ΔCD PSGL-1 on the cell surface (manuscript in preparation). The ΔCD PSGL-1 molecules that exit the endoplasmic reticulum are normally glycosylated as they traffic through the Golgi complex to the cell surface.
To exclude residual binding of ERM proteins to the 2 arginines in the cytoplasmic domain, we compared binding of recombinant moesin to GST fusion proteins containing the entire PSGL-1 cytoplasmic domain, the cytoplasmic domain in which 4 residues required for binding to ERM proteins were replaced with alanines, or just the 2 arginines. When bound to glutathione beads, only the GST fusion protein with the entire cytoplasmic domain precipitated moesin (Figure 1I).
To determine whether deletion of the cytoplasmic domain affected plasma levels of PSGL-1, we immunoprecipitated PSGL-1 from WT and ΔCD plasma. Western blots demonstrated that plasma PSGL-1 from each genotype comigrated with the corresponding protein from leukocyte lysates (Figure 1J), suggesting that it represented the transmembrane protein rather than a proteolytic fragment of the extracellular domain. Consistent with this interpretation, ultracentrifugation sedimented virtually all of the plasma PSGL-1, presumably as membrane microparticles (Figure 1J). Densitometry revealed that ΔCD plasma contained 4.5- plus or minus 0.5-fold (mean ± SEM, n = 5) more PSGL-1 than WT plasma. However, the absolute levels of plasma PSGL-1 remained low. Based on calibration with known quantities of PSGL-1 in lysates from transfected CHO cells (“Plasma PSGL-1 measurement”), the PSGL-1 concentration was approximately 13 ng/mL in WT plasma and approximately 72 ng/mL in ΔCD plasma (data not shown).
Binding of P-selectin to PSGL-1 can generate procoagulant microparticles that decrease whole blood clotting times, a measure of tissue factor-dependent blood clotting.37 Although ΔCD plasma had more PSGL-1–containing microparticles than WT plasma, it was less procoagulant. Whole blood clotting times (mean ± SEM) were 217 plus or minus 17 seconds in WT plasma (n = 11), 353 plus or minus 29 seconds in PSGL-1–deficient plasma (n = 10, P < .001 compared with WT plasma), and 293 plus or minus 24 seconds in ΔCD plasma (n = 13, P < .05 compared with WT plasma).
ΔCD PSGL-1 is concentrated normally on leukocyte microvilli
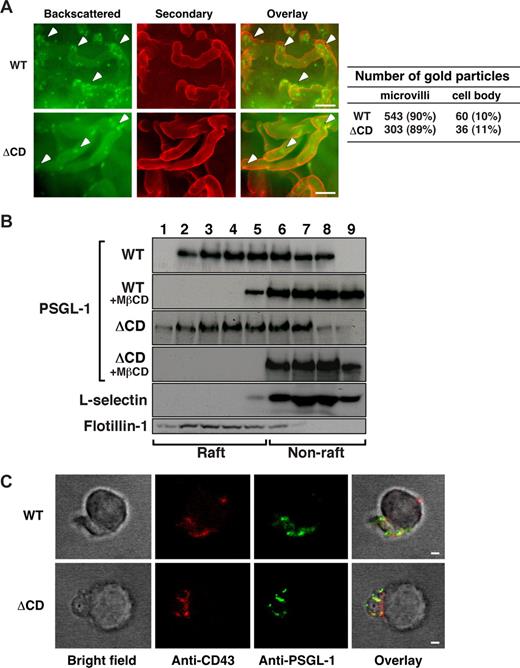
Mutation of the ERM-binding site in the cytoplasmic domain prevents clustering of L-selectin in microvilli of transfected cells.10 The cytoplasmic domain of PSGL-1 has a putative ERM-binding site that might maintain PSGL-1 in microvilli.18 To test this hypothesis, we used immunogold labeling and scanning electron microscopy to compare the distribution of PSGL-1 on WT and ΔCD leukocytes. The density of gold particles on ΔCD leukocytes was lower than on WT leukocytes, consistent with lower surface expression of ΔCD PSGL-1. Nevertheless, as on WT leukocytes, approximately 90% of the gold particles were concentrated on microvilli of ΔCD leukocytes, with the remainder scattered on the cell body (Figure 2A). These data demonstrate that PSGL-1 does not require its cytoplasmic domain to concentrate in microvilli.
ΔCD PSGL-1 distributes normally to microvilli, lipid rafts, and uropods of leukocytes. (A) PSGL-1 on surfaces of WT and ΔCD leukocytes was visualized by immunogold labeling and scanning electron microscopy. Shown are pseudo-colored images of backscattered electrons (gold detection), secondary electrons (cell surface detection), and an overlay. Arrowheads mark gold particles on microvilli. Bar represents 250 nm. Quantification of gold particles on microvilli and cell bodies of WT and ΔCD leukocytes is shown on the right. (B) WT or ΔCD leukocytes were incubated in control buffer or buffer containing methyl-β-cyclodextrin (MβCD) and then lysed in cold 1% Triton X-100. The lysate was centrifuged in a discontinuous OptiPrep gradient. Fractions collected from the top were analyzed by Western blotting with antibodies to the indicated proteins. Results are representative of 3 independent experiments. (C) WT or ΔCD neutrophils stimulated with fMLP were fixed, incubated with biotinylated rabbit anti–mouse PSGL-1 IgG, followed by streptavidin–Alexa 488. After washing, cells were incubated with goat anti–mouse CD43 IgG, followed by donkey anti–goat IgG–Alexa 546. Cells were visualized by fluorescence microscopy. The specificity of staining was confirmed with control incubations (“Immunofluorescence”). Images are representative of 40 polarized cells examined for each genotype. Bar represents 1 μm.
ΔCD PSGL-1 distributes normally to microvilli, lipid rafts, and uropods of leukocytes. (A) PSGL-1 on surfaces of WT and ΔCD leukocytes was visualized by immunogold labeling and scanning electron microscopy. Shown are pseudo-colored images of backscattered electrons (gold detection), secondary electrons (cell surface detection), and an overlay. Arrowheads mark gold particles on microvilli. Bar represents 250 nm. Quantification of gold particles on microvilli and cell bodies of WT and ΔCD leukocytes is shown on the right. (B) WT or ΔCD leukocytes were incubated in control buffer or buffer containing methyl-β-cyclodextrin (MβCD) and then lysed in cold 1% Triton X-100. The lysate was centrifuged in a discontinuous OptiPrep gradient. Fractions collected from the top were analyzed by Western blotting with antibodies to the indicated proteins. Results are representative of 3 independent experiments. (C) WT or ΔCD neutrophils stimulated with fMLP were fixed, incubated with biotinylated rabbit anti–mouse PSGL-1 IgG, followed by streptavidin–Alexa 488. After washing, cells were incubated with goat anti–mouse CD43 IgG, followed by donkey anti–goat IgG–Alexa 546. Cells were visualized by fluorescence microscopy. The specificity of staining was confirmed with control incubations (“Immunofluorescence”). Images are representative of 40 polarized cells examined for each genotype. Bar represents 1 μm.
ΔCD PSGL-1 associates normally with cholesterol-dependent membrane domains
PSGL-1 is enriched in cholesterol-dependent membrane domains of leukocytes.35 To determine whether the cytoplasmic domain partitions PSGL-1 in these domains, also called lipid rafts, we extracted WT or ΔCD leukocytes in cold 1% Triton X-100 and fractionated the extracts in an OptiPrep gradient. The most buoyant fractions at the top of the gradient are enriched in cholesterol-rich domains, whereas the less buoyant fractions contain nonraft components. Western blotting revealed that the raft constituent flotillin-17 was located in the buoyant fractions (Figure 2B). In contrast, L-selectin was mostly in the nonraft fractions, confirming previous observations.42 Both WT and ΔCD PSGL-1 were located primarily in the buoyant fractions (Figure 2B). Treatment of extracts with methyl-β-cyclodextrin, a cholesterol chelator that disrupts raft structures,7 moved both WT and ΔCD PSGL-1 to the less buoyant fractions. These data demonstrate that PSGL-1 does not require its cytoplasmic domain to associate with lipid rafts.
ΔCD PSGL-1 redistributes normally to the uropods of polarized leukocytes
On activation, leukocytes polarize and redistribute CD43, PSGL-1, and other membrane proteins to uropods.17,43 In parallel, actin polymerizes and ERM proteins concentrate in uropods. Because ERM proteins link cytoplasmic domains of some membrane proteins to actin, they could cause such proteins to redistribute to uropods.43,44 Consistent with this notion, mutation of the ERM-binding site in the cytoplasmic domain decreases PSGL-1 in uropods of transfected cells.18 However, confocal microscopy revealed that, like CD43, both WT and ΔCD PSGL-1 redistributed normally to the uropods of neutrophils stimulated with the bacterial peptide fMLP (Figure 2C) or the chemokine CXCL1 (data not shown). These results demonstrate that PSGL-1 does not require its cytoplasmic domain to move to uropods of polarized, primary leukocytes.
ΔCD leukocytes roll on P-selectin
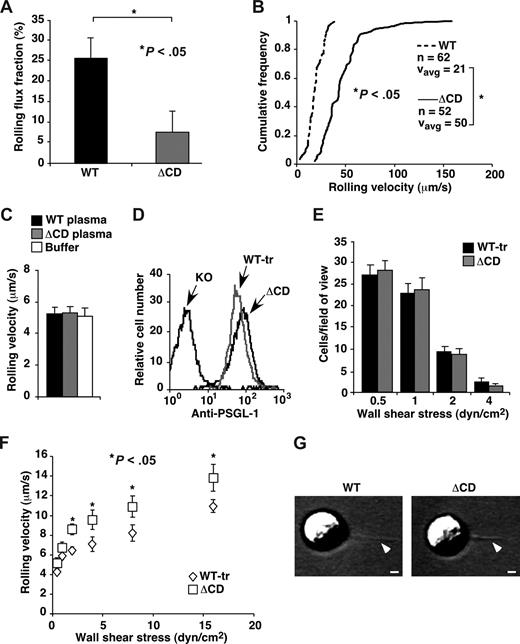
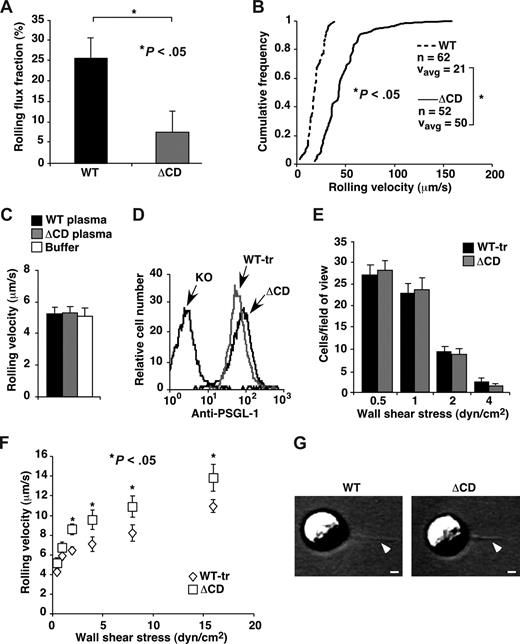
Truncating the cytoplasmic domain of PSGL-1 was reported to prevent rolling of transfected cells on P-selectin in vitro.19 To determine whether primary leukocytes require the cytoplasmic domain of PSGL-1 to roll on P-selectin, we used intravital microscopy to visualize rolling of neutrophils on postcapillary venules of the cremaster muscle, which express P-selectin on the endothelial cell surface after mild trauma.14 Despite the 10-fold reduction in surface expression of PSGL-1, ΔCD leukocytes rolled on P-selectin, although in fewer numbers (Figure 3A) and at greater velocities (Figure 3B) than WT leukocytes. In contrast, virtually no PSGL-1–deficient neutrophils rolled on venules in this setting.14 We also studied rolling of isolated bone marrow leukocytes in vitro. In these experiments, more than 90% of the cells that rolled on P-selectin were Gr-1–positive myeloid cells, most of which were neutrophils. WT leukocytes suspended in buffer or in plasma from WT or ΔCD mice rolled with identical velocities on P-selectin, demonstrating that the low levels of plasma PSGL-1 did not affect rolling (Figure 3C). ΔCD leukocytes rolled faster than WT leukocytes on the same density of P-selectin. We titrated the concentration of a blocking mAb to PSGL-1 to occupy approximately 90% of the PSGL-1 molecules on WT leukocytes. Under these conditions, ΔCD leukocytes rolled slightly faster than the mAb-blocked WT leukocytes (data not shown). To more accurately match the densities of PSGL-1 on WT and ΔCD leukocytes, we subjected WT leukocytes to a limited digestion with OSGE, which cleaves proteins bearing clustered, sialylated O-glycans, such as PSGL-1.16 In this manner, we reduced the density of PSGL-1 on treated WT leukocytes (WT-tr) to the level of PSGL-1 on ΔCD leukocytes, as measured by binding of a mAb to an N-terminal epitope that overlaps the P-selectin binding site (Figure 3D). The OSGE treatment did not alter the densities of CD44, L-selectin, or integrins αLβ2 and αMβ2, based on binding of mAbs to epitopes near the N-termini of these proteins (data not shown). Equivalent numbers of WT-tr and ΔCD leukocytes tethered to and rolled on P-selectin (Figure 3E), although the ΔCD leukocytes rolled slightly faster at higher shear stresses (Figure 3F). Thus, when PSGL-1 densities were matched, ΔCD leukocytes rolled on P-selectin nearly as well as WT leukocytes.
Rolling of WT and ΔCD leukocytes on P-selectin in vivo and in vitro. (A) Leukocyte rolling flux fractions in venules of cremaster muscle from WT and ΔCD mice subjected to trauma to mobilize P-selectin to the endothelial cell surface. (B) Leukocyte rolling velocity distributions in venules of cremaster muscle from WT and ΔCD mice subjected to trauma. The cumulative histograms of rolling velocities of the indicated number of leukocytes are depicted. The mean rolling velocities (Vavg) are also shown. (C) Velocities of WT neutrophils suspended in buffer, WT plasma, or ΔCD plasma rolling on P-selectin at a wall shear stress of 1 dyne/cm2. (D) Flow cytometric analysis of PSGL-1 expression on ΔCD leukocytes and on WT leukocytes treated with OSGE (WT-tr). Leukocytes were incubated with PE-conjugated anti–PSGL-1 2PH1, which recognizes an epitope within the N-terminal P-selectin–binding region. (E) Number of WT-tr or ΔCD neutrophils rolling on P-selectin (235 sites/μm2) captured on the floor of a flow chamber at the indicated wall shear stress. (F) Velocities of WT-tr or ΔCD neutrophils rolling on P-selectin at the indicated wall shear stress. (G) Representative membrane tethers, marked by arrowheads, extending from the trailing edge of a WT or ΔCD neutrophil rolling on P-selectin at a wall shear stress of 1 dyne/cm2. Flow is from right to left. Bar represents 1 μm. In panels A and B, injection of 30 μg anti–P-selectin mAb RB40.34 detached all rolling leukocytes, confirming the P-selectin–dependency of rolling. In panels C and in E-G, rolling was eliminated by anti–PSGL-1 mAb 4RA10 or anti–P-selectin mAb RB40.34. The data represent the mean plus or minus SEM from at least 3 experiments.
Rolling of WT and ΔCD leukocytes on P-selectin in vivo and in vitro. (A) Leukocyte rolling flux fractions in venules of cremaster muscle from WT and ΔCD mice subjected to trauma to mobilize P-selectin to the endothelial cell surface. (B) Leukocyte rolling velocity distributions in venules of cremaster muscle from WT and ΔCD mice subjected to trauma. The cumulative histograms of rolling velocities of the indicated number of leukocytes are depicted. The mean rolling velocities (Vavg) are also shown. (C) Velocities of WT neutrophils suspended in buffer, WT plasma, or ΔCD plasma rolling on P-selectin at a wall shear stress of 1 dyne/cm2. (D) Flow cytometric analysis of PSGL-1 expression on ΔCD leukocytes and on WT leukocytes treated with OSGE (WT-tr). Leukocytes were incubated with PE-conjugated anti–PSGL-1 2PH1, which recognizes an epitope within the N-terminal P-selectin–binding region. (E) Number of WT-tr or ΔCD neutrophils rolling on P-selectin (235 sites/μm2) captured on the floor of a flow chamber at the indicated wall shear stress. (F) Velocities of WT-tr or ΔCD neutrophils rolling on P-selectin at the indicated wall shear stress. (G) Representative membrane tethers, marked by arrowheads, extending from the trailing edge of a WT or ΔCD neutrophil rolling on P-selectin at a wall shear stress of 1 dyne/cm2. Flow is from right to left. Bar represents 1 μm. In panels A and B, injection of 30 μg anti–P-selectin mAb RB40.34 detached all rolling leukocytes, confirming the P-selectin–dependency of rolling. In panels C and in E-G, rolling was eliminated by anti–PSGL-1 mAb 4RA10 or anti–P-selectin mAb RB40.34. The data represent the mean plus or minus SEM from at least 3 experiments.
Neutrophils rolling on P-selectin rapidly extend and retract thin membrane tethers, which stabilize rolling by reducing force on adhesive bonds.40 WT and ΔCD neutrophils rolling on P-selectin densities adjusted to match rolling velocities formed tethers of similar dimensions at similar rates (Figure 3F and data not shown). These results demonstrate that tether extension and retraction do not require linkage of the cytoplasmic domain of PSGL-1 to the cytoskeleton.
Transfected cells expressing matched densities of WT or ΔCD PSGL-1 roll equivalently on P-selectin
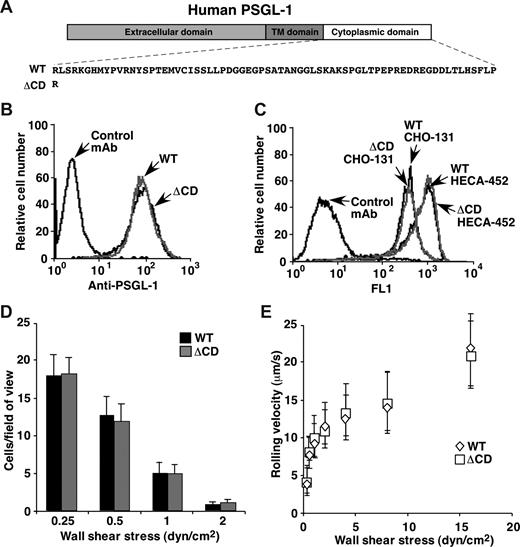
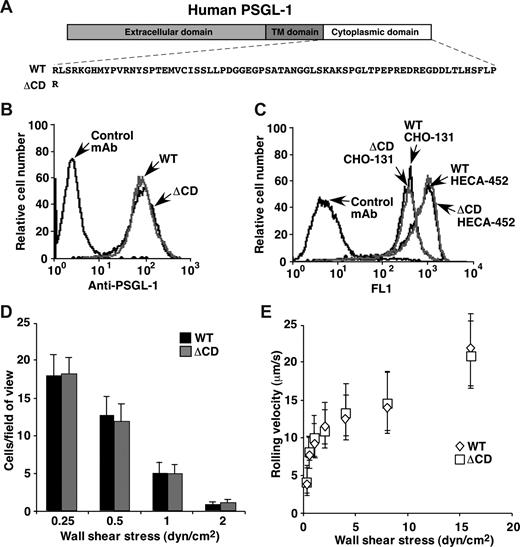
The ability of ΔCD leukocytes to roll on P-selectin was unexpected because the cytoplasmic domain of human PSGL-1 was reported to be essential for transfected cells to roll on P-selectin.19 We therefore studied rolling of transfected CHO cells expressing WT or ΔCD human PSGL-1 (Figure 4A) at matched densities (Figure 4B). The CHO cells were stably transfected with expression vectors for core 2 β1-6-N-acetylglucosaminyltransferase-I and α1-3-fucosyltransferase FucT-VII, which construct core 2 O-glycans capped with sialyl Lewis x (NeuAcα2-3Galβ1-4[Fucα1–3]GlcNAcβ1-R) that PSGL-1 requires to bind P-selectin.33 The antisialyl Lewis x mAb HECA-452 and mAb CHO-131, which identifies core 2 O-glycans capped with sialyl Lewis x,32 bound similarly to CHO cells expressing WT or ΔCD PSGL-1, confirming equivalent surface glycosylation of the cells (Figure 4C). A similar number of transfected cells expressing WT or ΔCD PSGL-1 tethered to and rolled on P-selectin (Figure 4D), and they rolled with indistinguishable velocities (Figure 4E). These data demonstrate that the cytoplasmic domain of human PSGL-1 is not required to support rolling of transfected cells on P-selectin.
Transfected CHO cells expressing matched densities of WT or ΔCD human PSGL-1 roll equivalently on P-selectin. (A) Schematic of WT and ΔCD human PSGL-1 constructs. (B) Surface expression of WT and ΔCD PSGL-1 on transfected CHO cells measured by binding of anti–human PSGL-1 mAb PL1. (C) Surface expression of core 2 O-glycans capped with sialyl Lewis x on transfected CHO cells measured by binding of mAb CHO-131. (D) Number of cells rolling on P-selectin at the indicated wall shear stress. (E) Velocities of cells rolling on P-selectin at the indicated wall shear stress. The data represent the mean plus or minus SD from 3 experiments. In panels D and E, rolling was eliminated by anti–human P-selectin mAb G1 or anti–human PSGL-1 mAb PL1.
Transfected CHO cells expressing matched densities of WT or ΔCD human PSGL-1 roll equivalently on P-selectin. (A) Schematic of WT and ΔCD human PSGL-1 constructs. (B) Surface expression of WT and ΔCD PSGL-1 on transfected CHO cells measured by binding of anti–human PSGL-1 mAb PL1. (C) Surface expression of core 2 O-glycans capped with sialyl Lewis x on transfected CHO cells measured by binding of mAb CHO-131. (D) Number of cells rolling on P-selectin at the indicated wall shear stress. (E) Velocities of cells rolling on P-selectin at the indicated wall shear stress. The data represent the mean plus or minus SD from 3 experiments. In panels D and E, rolling was eliminated by anti–human P-selectin mAb G1 or anti–human PSGL-1 mAb PL1.
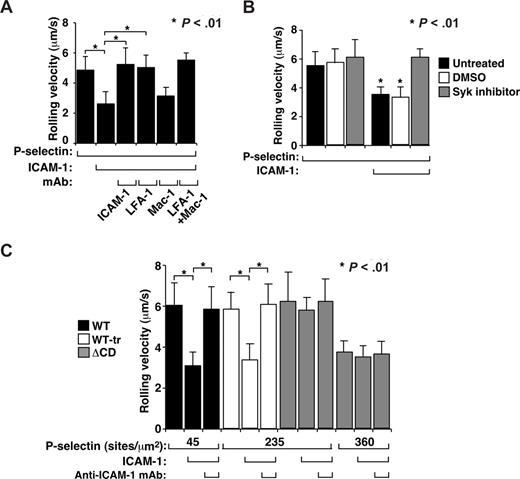
ΔCD neutrophils rolling on P-selectin do not activate β2 integrins to slow rolling on ICAM-1
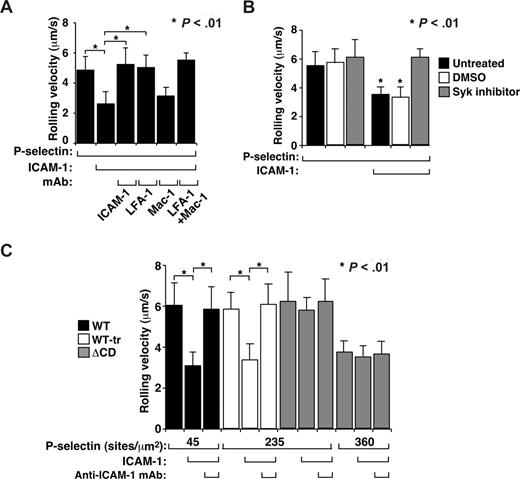
In a whole-blood autoperfusion system, murine neutrophils rolling on P-selectin or E-selectin activate integrin LFA-1 to a conformation that causes the cells to roll more slowly but not to arrest on coimmobilized ICAM-1.29 We confirmed these findings using bone marrow leukocytes. Neutrophils rolled more slowly on coimmobilized P-selectin and ICAM-1 than on P-selectin alone (Figure 5A). A mAb to ICAM-1 or to LFA-1, but not to integrin Mac-1, increased the velocity to the level of rolling on P-selectin alone, confirming that slower rolling was mediated by interactions of LFA-1 with ICAM-1 (Figure 5A). Treating neutrophils with the Syk inhibitor piceatannol prevented slow rolling on P-selectin plus ICAM-1 (Figure 5B), extending previous observations that slow rolling of neutrophils on E-selectin plus ICAM-1 requires Syk.29 To compare Syk-dependent integrin activation in WT and ΔCD neutrophils, we perfused ΔCD leukocytes over a 5-fold higher P-selectin density (235 sites/μm2) to increase the number of P-selectin/PSGL-1 bonds per unit time45 and therefore reduce the mean rolling velocity to match that of WT neutrophils rolling on P-selectin at 45 sites/μm2 (Figure 5C). Alternatively, ΔCD cells were perfused over an 8-fold higher P-selectin density (360 sites/μm2) so that ΔCD neutrophils formed even more P-selectin/PSGL-1 bonds per unit time and therefore rolled slower than WT cells. In a complementary approach, we treated WT cells with OSGE to reduce the PSGL-1 density to that of ΔCD cells, yielding similar rolling velocities of WT-tr and ΔCD neutrophils on P-selectin (235 sites/μm2) in the absence of ICAM-1. In all these conditions, ΔCD neutrophils failed to roll more slowly on coimmobilized ICAM-1 (Figure 5C). These data demonstrate that neutrophils rolling on P-selectin require the PSGL-1 cytoplasmic domain to trigger Syk-dependent β2 integrin activation that slows rolling on ICAM-1.
ΔCD neutrophils rolling on P-selectin do not activate β2 integrins to slow rolling on ICAM-1. (A) Velocities of WT neutrophils rolling on P-selectin (45 sites/μm2) without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of blocking mAb to the indicated protein. (B) Velocities of WT neutrophils rolling on P-selectin (45 sites/μm2) without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of the Syk inhibitor piceatannol or the solvent control DMSO. (C) Velocities of WT, WT-tr, or ΔCD neutrophils rolling on the indicated density of P-selectin without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of blocking mAb to ICAM-1. The wall shear stress in all experiments was 1 dyne/cm2. The data represent the mean plus or minus SEM from 3 experiments.
ΔCD neutrophils rolling on P-selectin do not activate β2 integrins to slow rolling on ICAM-1. (A) Velocities of WT neutrophils rolling on P-selectin (45 sites/μm2) without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of blocking mAb to the indicated protein. (B) Velocities of WT neutrophils rolling on P-selectin (45 sites/μm2) without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of the Syk inhibitor piceatannol or the solvent control DMSO. (C) Velocities of WT, WT-tr, or ΔCD neutrophils rolling on the indicated density of P-selectin without or with coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of blocking mAb to ICAM-1. The wall shear stress in all experiments was 1 dyne/cm2. The data represent the mean plus or minus SEM from 3 experiments.
Discussion
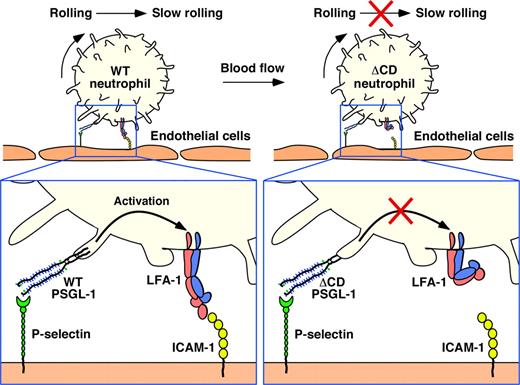
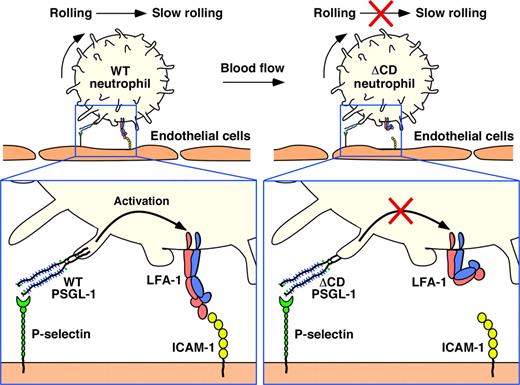
We found that PSGL-1 in primary leukocytes did not require its cytoplasmic domain to concentrate in microvilli, redistribute to uropods, associate with lipid rafts, or support rolling on P-selectin. However, neutrophils rolling on P-selectin did require the cytoplasmic domain of PSGL-1 to activate β2 integrins to slow rolling on ICAM-1 (Figure 6).
Separable requirements for the cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling. The cytoplasmic domain is not required to localize PSGL-1 in microvilli or to support neutrophil rolling on P-selectin. However, it is essential to transmit signals that activate LFA-1 to slow rolling on ICAM-1 under flow.
Separable requirements for the cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling. The cytoplasmic domain is not required to localize PSGL-1 in microvilli or to support neutrophil rolling on P-selectin. However, it is essential to transmit signals that activate LFA-1 to slow rolling on ICAM-1 under flow.
Interactions of ERM proteins with the cytoplasmic domains of some membrane proteins are thought to be important for maintaining microvillus architecture and for redistributing these membrane proteins to the uropods of cells as they polarize.46 Substituting residues in the putative ERM-binding region of the cytoplasmic domain reduces the distribution of PSGL-1 to uropods of polarized myeloma cells.18 However, ΔCD PSGL-1 was localized normally in both microvilli and uropods of primary leukocytes, indicating that PSGL-1 does not require direct linkage of its cytoplasmic domain to the cytoskeleton to distribute to microvilli or to uropods. Instead, PSGL-1 might interact indirectly with the cytoskeleton. One mechanism may be through lipid rafts. Although some membrane proteins use palmitoylated cysteines in their cytoplasmic domains to partition into rafts, ΔCD PSGL-1 was still able to associate with these lipid domains. The cytoplasmic domains of other raft proteins may bind directly to the cytoskeleton. Furthermore, rafts are enriched in phosphatidylinositol-4,5-bisphosphate, which recruits adaptor proteins that link to actin.47 Rafts are very heterogeneous,48 and different raft subsets migrate to the lamellipodia and uropods of polarized leukocytes.49 PSGL-1 might have affinity for a particular type of raft domain and/or interact with another membrane protein that partitions into a raft subset. Testing these hypotheses will require determining how PSGL-1 associates with rafts.
PSGL-1 did not require direct linkage of the cytoplasmic domain to the cytoskeleton to mediate neutrophil rolling on P-selectin. Therefore, loss of the cytoplasmic domain probably did not make PSGL-1 significantly more susceptible to forcible extraction from the membrane, although ΔCD plasma did have slightly more PSGL-1–containing microparticles. Like WT neutrophils, ΔCD neutrophils rolling on P-selectin extended and retracted membrane tethers. When force is applied to PSGL-1, cytoskeletal linkages with the membrane must be disrupted for tethers to extend.50 Because ΔCD PSGL-1 does not interact directly with the cytoskeleton, ΔCD neutrophils extending tethers probably disrupt cytoskeletal linkages with membrane proteins around the PSGL-1 molecule(s) to which force is applied. Despite the 10-fold reduction in PSGL-1 surface density, ΔCD neutrophils rolled on P-selectin in vivo and in vitro. Furthermore, ΔCD neutrophils rolled only slightly faster than OSGE-treated WT neutrophils with matched PSGL-1 density, suggesting that the cytoplasmic domain makes at most a minor contribution to rolling stability. Finally, transfected CHO cells expressing matched levels of WT and ΔCD human PSGL-1 rolled equivalent on P-selectin. Our data do not support a claim that the cytoplasmic domain of PSGL-1 is essential for leukocyte rolling on P-selectin.19 Instead, the impaired rolling of transfected cells expressing PSGL-1 with a truncated cytoplasmic domain may have resulted from a decline in surface expression19 or inadequate glycosylation51 of truncated PSGL-1 in the cells. The ability to localize in membrane domains and support leukocyte adhesion independently of the cytoplasmic domain distinguishes PSGL-1 from selectins6,7,10,11 and integrins.1,5
Signals that activate integrins are transmitted during the rapidly reversible interactions of PSGL-1 with P-selectin and E-selectin as cells roll under flow.29 Our data establish a requirement for the cytoplasmic domain of PSGL-1 to propagate these signals as neutrophils roll on P-selectin (Figure 6). PSGL-1–dependent signals cause LFA-1 to adopt an extended conformation without altering the low-affinity conformation of the ligand-binding site on the α-I domain4,29 (Figure 6). This extended LFA-1 conformation slows rolling velocities but does not cause cells to arrest. By contrast, chemokine receptor signals cause LFA-1 to extend and to convert its ligand-binding site to a high-affinity conformation.52 This extended conformation enables the cells to arrest. Under flow, forces applied to LFA-1/ICAM-1 bonds stabilize the high-affinity interaction induced by chemokine receptor signaling but do not convert the low-affinity interaction induced by PSGL-1 signaling to a high-affinity interaction.4,29,52 It will be important to understand how signals initiated through PSGL-1 or chemokine receptors differentially affect integrin conformation and function in the circulation.3
Extensive cross-linking of PSGL-1 recruits moesin or ezrin to the PSGL-1 cytoplasmic domain, which positions a cryptic ITAM in these proteins to recruit Syk.23 Src family kinases might phosphorylate this ITAM, allowing Syk to dock and in turn be activated. Although this is an attractive mechanism to activate Syk, it remains to be determined whether it operates as leukocytes roll on P-selectin. Alternatively, classical ITAM-bearing adaptors might recruit Syk.53 Cross-linking PSGL-1 recruits phosphoinositide-3-OH kinase δ to the cytoplasmic domain, where it is activated by Src family kinases and in turn activates integrins.25 However, T helper–1 cells rolling on P-selectin activate LFA-1 in the presence of phosphoinositide-3-OH kinase inhibitors.24 Further studies are required to clarify relationships among signaling effectors downstream of PSGL-1 as leukocytes roll under flow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Preston Larson for assistance with scanning electron microscopy; Richard Cummings, Bruce Walcheck, Geert Raes, and Dietmar Vestweber for antibodies; and Mark Coggeshall, William Rodgers, Paul Kincade, and Cheng Zhu for critically reading the manuscript.
This work was supported by the National Institutes of Health (grants HL 34363, HL 085607, and RR 018758).
National Institutes of Health
Authorship
Contribution: J.J.M., L.X., T.Y., J.K., Z.L., A.G.K., B.S., J.M.M., H.S., and D.W.S. designed, performed, and analyzed experiments; J.J.M., L.X., and R.P.M. contributed to writing the paper; and R.P.M. initiated the study, designed and supervised the research project, and contributed to data analysis and interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rodger P. McEver, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 Northeast 13th Street, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.
References
Author notes
*J.J.M. and L.X. contributed equally to this work.