Abstract
Differentiation of functional dendritic cells (DCs) critically depends on the microenvironment. DCs differentiate in hypoxic tumor sites and inflamed or damaged tissue. Because local concentrations of adenosine reach high physiologically relevant levels in these conditions, we assessed the expression of adenosine receptors and the effect of their activation on differentiation of human monocytes and mouse peritoneal macrophages and hematopoietic progenitor cells (HPCs) into myeloid DCs. Stimulation of adenosine receptors skews DC differentiation toward a distinct cell population characterized by expression of both DC and monocyte/macrophage cell surface markers. Pharmacologic analysis and experiments with cells from A2B adenosine receptor knockout mice identified A2B receptor as the mediator of adenosine effects on DCs. Unlike normal myeloid DCs, adenosine-differentiated DCs have impaired allostimulatory activity and express high levels of angiogenic, pro-inflammatory, immune suppressor, and tolerogenic factors, including VEGF, IL-8, IL-6, IL-10, COX-2, TGF-β, and IDO. They promoted tumor growth if injected into tumors implanted in mice. Using adenosine desaminase knockout animals, we showed that DCs with proangiogenic phenotype are highly abundant under conditions associated with elevated levels of extracellular adenosine in vivo. Adenosine signaling through A2B receptor is an important factor of aberrant DC differentiation and generation of tolerogenic, angiogenic, and proinflammatory cells.
Introduction
The endogenous adenine nucleotides and adenosine are normally present at low concentrations in the extracellular milieu. However, metabolically stressful conditions, including inflammation and hypoxia characteristic of asthma, solid tumors, and other pathologic conditions, result in dramatic increases in extracellular concentrations of adenosine.1-3 There are also mechanisms of nonlytic secretion of adenosine during hypoxic conditions.
There is growing evidence that adenosine can actively modulate differentiation and function of myeloid cells.4 Circulating cells of myeloid lineage, including monocytes and dendritic cell (DC) precursors, migrate to tissues where they differentiate into macrophages or DCs. DCs show impressive interaction with the adjacent microenvironment,5,6 which regulates formation of DC subtypes and their functional properties, including expression of cytokines and growth factors. Because of rapid growth, solid tumors routinely experience severe hypoxia and necrosis, which causes adenine nucleotide degradation and adenosine release. Therefore, high levels of extracellular adenosine contribute to the local tumor microenvironment and may greatly influence differentiation of DCs from monocyte/macrophages and DC precursors migrating into tumor tissue. Adenosine acts through 4 subtypes of adenosine receptors, A1, A2A, A2B, and A3, which are members of the G-protein–coupled family of receptors.7,8 A2A adenosine receptors are generally anti-inflammatory, whereas A2B and A3 receptors are implicated in proinflammatory action of adenosine. Adenosine receptors are expressed abundantly on monocytes, and through these receptors adenosine exerts substantial modulatory effects on monocyte function and further differentiation. A1 receptors were shown to stimulate formation of giant multinucleated cells from monocytes, whereas A2 receptors inhibited this process.9 A2B receptors were implicated in mediating the inhibitory effect of adenosine on macrophage proliferation induced by M-CSF.10 Exogenous adenosine can prevent monocytes from differentiating into macrophages, leading them to an intermediate differentiation stage between immature DCs and monocytes.11 Cyclic nucleotides, including cAMP, which intracellular level increases in response to stimulation of adenosine A2 receptors, regulate certain steps of monocyte differentiation and promote their differentiation toward a CD1alowCD14+/lowCD209+ intermediate cell but impair differentiation into functional DCs.12 Up-regulation of DC-specific ICAM-3–grabbing nonintegrin (CD209) was not affected by cyclic nucleotides,12 indicating that DC development was not blocked at the monocyte stage. The expression of all 4 adenosine receptor subtypes has been reported in human monocytes and myeloid DCs.9,13-15 However, the effects of adenosine on differentiation of myeloid DCs from monocytes, macrophages, and hematopoietic progenitor cells (HPCs) and the roles of specific adenosine receptor subtypes involved in this process have not been investigated.
Here, we show that adenosine is an important factor affecting differentiation of myeloid DCs. Signaling through A2B adenosine receptors diverts human blood monocytes, mouse bone marrow HPCs, and peritoneal monocyte/macrophages from normal differentiation into myeloid DCs toward the formation of a distinct DC population that produces high levels of angiogenic factors and Th2-type cytokines, phenotype associated with promotion of angiogenesis, tumor growth, immune suppression, and tolerance. We also show that these cells appear in vivo under conditions associated with elevated levels of extracellular adenosine and that they can enhance vascularization and growth of tumor. Generation of these adenosine-differentiated DCs would have an adverse effect in cancer, asthma, and inflammatory diseases through a process that could be prevented by A2B antagonists.
Methods
Mice
Male 6- to 8-week-old C57BL/6 mice were obtained from Harlan (Indianapolis, IN). Homozygous A2B adenosine receptor knockout mice were obtained from Deltagen (San Mateo, CA). These mice were back-crossed to C57BL/6 genetic background for more than 10 generations.
Adenosine desaminase (ADA) knockout mice were described previously.16 Homozygous ADA−/− knockout newborn mice received polyethylene glycol–modified ADA therapy after birth, until 6 weeks of age. Therapy was then discontinued, and mice were left untreated for an additional 15 days to allow the signs of respiratory distress to develop. Control mice were wild-type littermates. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (NIH). Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the Vanderbilt University.
Reagents
Adenosine receptor antagonists were from the following sources: A1 receptor antagonists N6-endonorboran-2-yl-9-methyladenine (N-0861) was a gift from Whitby Research (Richmond, VA); A2A receptor antagonist 5-amino-7-(phenylethyl)-2-(1-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH 58261) was a generous gift from Drs C. Zocchi and E. Ongini (Schering Plough Research Institute, Milan, Italy), A2B receptor antagonist 3-isobutyl-8-pyrrolidinoxanthine (IPDX) was synthesized as previously described17 ; A2B receptor antagonist CVT-6883 was provided by CV Therapeutics (Palo Alto, CA). A3 receptor antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate (MRS 1191) was purchased from Sigma/RBI (Natick, MA). Inhibitor of adenosine deaminase erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA), lipopolysaccharide (LPS; Escherichia coli 055:B5), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO). Final concentrations of DMSO did not exceed 0.1%, and the same concentrations were used in controls.
Cell purification
Monocytes were purified from peripheral blood mononuclear cells from consented healthy donors. Mononuclear cells were isolated from blood on Ficoll-Hypaque (GE Healthcare Bio-Sciences, Little Chalfont, United Kingdom) gradient. CD14+ monocytes were then immunomagnetically purified using CD14 monoclonal antibody–conjugated microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's protocol. Mouse HPCs were isolated from bone marrow of 6- to 8-week-old mice using immunomagnetic negative selection with biotinylated antibodies against a panel of lineage antigens CD5, CD45R (B220), CD11b, Gr-1, 7-4, and Ter-119 and anti–biotin microbeads (Miltenyi Biotec) according to the manufacturer's protocol. The purity of CD14+ or Lin− cells exceeded 95% by flow cytometric analysis. Mouse peritoneal macrophages were elicited by injecting 2.5 mL of 3% thioglycolate into the peritoneal cavities of mice. Four days later, peritoneal exudate cells were harvested by lavage, washed, and resuspended in culture medium (RPMI 1640 medium with 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 10% fetal bovine serum). Cells were seeded in 24-well culture plates at 106 cells/well in a final volume of 1 mL and cultured at 37°C for 90 minutes to allow macrophages to adhere. Nonadherent cells were removed by washing with warm Hanks balanced salt solution, and adherent cells were cultured in the above-mentioned medium.
Murine lung DCs were purified as described previously.18 Briefly, mice were humanely killed, and the pulmonary cavities were opened. The blood circulatory system in the lungs was cleared by perfusion through the pulmonary artery with 3 mL of saline containing 50 U/mL of heparin (Sigma-Aldrich). Lungs were aseptically removed and cut into small pieces in cold RPMI medium. The dissected tissue was then incubated in RPMI medium containing collagenase XI (0.7 mg/mL; Sigma-Aldrich) and type IV bovine pancreatic DNase (30 μg/mL; Sigma-Aldrich) for 30 to 45 minutes at 37°C. After that, 10 mL of cold RPMI was added, and digested lungs were further disrupted by gently pushing the tissue through a nylon screen. The single-cell suspension was washed and centrifuged at 200g. To lyse red blood cells, the cell pellet was incubated for 5 minutes at room temperature with 5 mL of ACK Lysing Buffer (BioSource International, Camarillo, CA) and washed with PBS containing 0.5% fetal bovine serum (FBS). Cells were then incubated with fluorochrome-conjugated monoclonal antibodies CD11b-PE, CD11c-FITC, and CD45-PerCP or APC; washed; and analyzed or sorted using FACSCalibur (fluorescence-activated cell sorting; FACS) flow cytometer.
For separation of human adenosine-differentiated DCs into CD1a−CD14+ and CD1a+CD14− fractions, cells were incubated with fluorochrome-conjugated monoclonal antibodies CD1a-PE and CD14-FITC, washed, and flow sorted.
DC differentiation
Purified human monocytes, mouse HPCs, or peritoneal monocytes/macrophages were cultured in RPMI 1640 medium containing 10% FBS (Invitrogen, Carlsbad, CA) at concentration of 0.5 × 106/mL in the presence of 20 ng/mL GM-CSF and 10 ng/mL IL-4 (R&D Systems, Minneapolis, MN) in 24-well plate. Monocytes and mouse cells were cultured for 5 and 7 days, respectively; human or mouse cytokines were used as appropriate. For maturation of DCs, 20 ng/mL TNF-α or 1 μg/mL LPS were added for an additional 24 to 48 hours of culture. Half of the medium was replenished with fresh medium with cytokines every 3 days. Concentrated solutions of adenosine receptor agonists and antagonists were prepared in DMSO (vehicle) and added to cells at indicated concentrations. The maximal concentration of DMSO in culture did not exceed 0.1% and was found to have no significant effect.
Flow cytometry
Human and mouse DCs were characterized by flow cytometry according to the manufacturer's recommendation (Becton Dickinson, Franklin Lakes, NJ). Aliquots of cells (105 cells in 100 μL buffer) were incubated with the respective fluorochrome-conjugated monoclonal antibodies (CD1a-PE, CD11b-PE or PerCP, CD11c-FITC or PE, CD14-FITC, CD45-PerCP or APC, CD83-FITC, CD86-FITC, CD205-FITC, CD209-FITC, MHCII-FITC or PE; all from BD PharMingen, San Diego, CA) for 30 minutes on ice, washed, and analyzed using a FACSCalibur flow cytometer; data were collected by analyzing 10 to 50 000 events using CellQuest software (Becton Dickinson).
Mixed lymphocyte reaction
For allogeneic mixed lymphocyte reaction (MLR), human T cells were purified from peripheral blood mononuclear cells from consented healthy donors by negative selection using immunomagnetic Pan T-cell Isolation Kit II with a cocktail of biotinylated antibodies CD14, CD16, CD19, CD56, CD36, CD123, and CD235a (Miltenyi Biotec) according to the manufacturer's protocol. MLR was set up by culturing 50 000 T cells/well for 4 days with varying concentrations of irradiated DCs in 96-well plates. T-cell proliferation was assessed after the addition of 1 μCi (0.037 MBq)/well of [3H]-thymidine (GE Healthcare) for the final 18 hours. All measurements were performed in triplicate and presented as mean CPM plus or minus SE.
Real-time polymerase chain reaction protocol and primers
Total RNA was isolated from cells using RNeasy Mini kit (Qiagen, Valencia, CA). Real-time reverse transcription–polymerase chain reaction (RT-PCR) was performed on ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Primer pairs for adenosine receptors (A1, A2A, A2B, A3) were obtained from Applied Biosystems (catalog nos. Hs00181231-m1, Hs00169123-m1, Hs00386497-m1, and Hs00181232-m1, respectively). The following pairs of primers were used for quantification of mRNA transcripts: human VEGF, forward primer 5′-GGGCAGAATCATCACGAAGTG-3′ and reverse primer 5′-ATTGGATGGCAGTAGCTGCG-3′; IL-8, forward primer 5′-TGCCAAGGAGTGCTAAAG-3′ and reverse primer 5′-TCCACAACCCTCTGCAC-3′; COX-2, forward primer 5′-TGCATTCTTTGCCCAGCACT-3′ and reverse primer 5′-AAAGGCGCAGTTTACGCTGT-3′; arginase 2, forward primer 5′-TCGGTACCATTAGTGGCCATG-3′ and reverse primer 5′-CTGTCCATGGAGATTTCCTGATG-3′; IL-10, forward primer 5′-GGTGATGCCCCAAGCTGA-3′ and reverse primer 5′-TCCCCCAGGGAGTTCACA-3′; IL-6, forward primer 5′-CACAGACAGCCACTCACCTC-3′ and reverse primer 5′-TTTTCTGCCAGTGCCTCTTT-3′; IDO, forward primer 5′-AGTCCGTGAGTTTGTCCTTTCAA-3′ and reverse primer 5′-TTTCACACAGGCGTCATAAGCT-3′; β-actin, forward primer 5′-CGCCCCAGGCACCAGGGC-3′ and reverse primer 5′-GGCTGGGGTGTTGAAGGT-3′. RT-PCR was performed using 1 μg of DNase-treated total RNA under conditions recommended by the manufacturer.
Measurements of secreted factors
Mouse VEGF, IL-6, and CXCL1 and human IL-6, IL-8, IL-10, VEGF, and IFN-γ secretion were quantified using the DuoSet ELISA (enzyme-linked immunoabsorbent assay) Development Systems (R&D Systems) according to the manufacturer's instructions.
Mouse tumor model experiments
C57BL/6 mice were injected subcutaneously with 0.5 × 106 of Lewis lung carcinoma cells (LLC) from ATCC (Manassas, VA). Two weeks later when tumors reached approximately 7 mm in diameter, mice were randomly divided into 3 groups with 5 animals in each. Immature DCs generated from HPCs in the presence or absence of 100 μM NECA were washed and suspended in PBS. Groups of mice received intratumoral injections of 0.25 × 106 of normal or adenosine-differentiated DCs in 50 μL PBS or 50 μM PBS as a control. The number of injected DCs was equal to the average number of tumor-infiltrating CD11c+ cells, which accumulate in 2-week-old LLC tumors of the same size in C57BL/6 mice, as we determined in our preliminary experiments. Seven days later mice received an intravenous injection of FITC-labeled dextran (molecular weight, 2 000 000; Sigma-Aldrich) for visualization of blood vessels in tissues.19,20 After 15 minutes, mice were killed; tumors were extracted, weighed, fixed in 10% formalin, embedded in paraffin, and sectioned (5 μm). Fluorescent images were taken from tumor sections, and the number of tumor blood vessels identified by dextran-FITC fluorescence was counted using Axiophot fluorescent microscope (Carl Zeiss, Thornwood, NY) with a 63×/1.4 NA Plan Apochromat oil objective and with a MicroMax CCD camera for low-light, quantitative fluorescence (Princeton Instruments, Trenton, NJ). Tissue slides were also stained with peroxidase-labeled mouse monoclonal antibodies to the endothelial surface marker CD34 (Santa Cruz Biotechnology, Santa Cruz, CA) or von Willebrand factor (Dako North America, Carpinteria, CA), and the number of tumor blood vessels identified by peroxidase substrate staining was counted using a Nikon Eclipse E600 with a 40×/1.0 NA Plan Apochromat oil objective (Nikon Instruments, Tokyo, Japan) and with Olympus DP-11 digital camera (Olympus America, Melville, NY). Images were processed using MetaMorph 5.0.7 (Universal Imaging, West Chester, PA) and ImageJ (National Institutes of Health, Bethesda, MD) software.
Statistical analysis
Statistical analyses were performed using Excel software (Microsoft, Redmond, WA). Differences between mean values were assessed using the Student t test. Statistical significance was set at P less than .05.
Results
Adenosine receptors on human monocytes, DCs, and adenosine-differentiated DCs
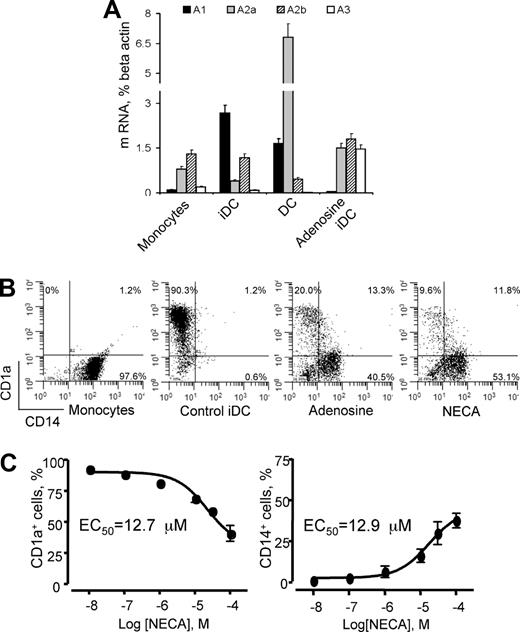
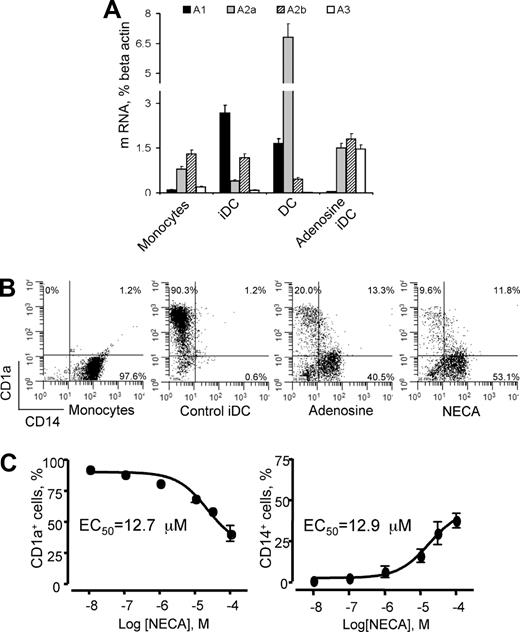
We have assessed the expression of adenosine receptors on human CD14+ monocytes as well as on immature and mature DCs and on adenosine-differentiated cells by real-time PCR and have found various levels of all 4 receptor mRNA transcripts (Figure 1A). A2A and A2B receptors were most abundant on monocytes and adenosine-differentiated cells. Differentiation of immature DCs was associated with a remarkable increase in A1 expression, whereas A2A expression was down-regulated. On further maturation of DCs in the presence of LPS, the receptor profile changed again and showed dramatic up-regulation of A2A receptors. Overall, the adenosine receptor profiles for monocytes and adenosine-differentiated DCs were remarkably similar and were characterized by high and almost equal levels of A2A and A2B receptors and lack or low levels of A1. The major difference was a much higher expression of A3 receptor in adenosine-differentiated cells. We believe that such a profile of intermediate cells reflects their immature state and suggests that in terms of differentiation they are closer to monocytes than to DCs yet different from both of those.
Expression of adenosine receptors on human monocytes, immature, mature, and adenosine-differentiated DCs and the effect of adenosine on DC differentiation. (A) CD14+ monocytes were purified from peripheral blood; immature DCs (iDCs) were generated from monocytes cultured in the presence of GM-CSF and IL-4 for 5 days without (Control iDC) or with NECA (Adenosine iDC); for maturation 1 μg/mL LPS was added to immature DCs for 2 additional days (DC). Expression of adenosine receptor mRNA transcripts was assessed by real-time PCR as described in “Methods.” Average values from 4 different experiments are shown. Error bars denote SE. (B) Addition of adenosine or its stable analog NECA skews DC differentiation from monocytes toward a CD1alowCD14+ cell population. Monocytes were differentiated into immature DCs as above in the absence (Control iDC) or in the presence of 100 μM adenosine plus 10 μM EHNA (Adenosine iDC) or 100 μM NECA (NECA iDC). Combination of adenosine and the adenosine deaminase inhibitor EHNA was used to decelerate adenosine catabolism in cell culture. Expression of CD1a+ and CD14+ markers was assessed by flow cytometry; results are representative of 7 experiments. (C) Concentration-dependence curves of the generation of CD1a+ and CD1alowCD14+ cells in the presence of NECA. Concentrations of NECA corresponding to 50% of maximal effects are shown. Average values from 3 different experiments are shown. Error bars denote SE.
Expression of adenosine receptors on human monocytes, immature, mature, and adenosine-differentiated DCs and the effect of adenosine on DC differentiation. (A) CD14+ monocytes were purified from peripheral blood; immature DCs (iDCs) were generated from monocytes cultured in the presence of GM-CSF and IL-4 for 5 days without (Control iDC) or with NECA (Adenosine iDC); for maturation 1 μg/mL LPS was added to immature DCs for 2 additional days (DC). Expression of adenosine receptor mRNA transcripts was assessed by real-time PCR as described in “Methods.” Average values from 4 different experiments are shown. Error bars denote SE. (B) Addition of adenosine or its stable analog NECA skews DC differentiation from monocytes toward a CD1alowCD14+ cell population. Monocytes were differentiated into immature DCs as above in the absence (Control iDC) or in the presence of 100 μM adenosine plus 10 μM EHNA (Adenosine iDC) or 100 μM NECA (NECA iDC). Combination of adenosine and the adenosine deaminase inhibitor EHNA was used to decelerate adenosine catabolism in cell culture. Expression of CD1a+ and CD14+ markers was assessed by flow cytometry; results are representative of 7 experiments. (C) Concentration-dependence curves of the generation of CD1a+ and CD1alowCD14+ cells in the presence of NECA. Concentrations of NECA corresponding to 50% of maximal effects are shown. Average values from 3 different experiments are shown. Error bars denote SE.
Presence of adenosine leads to formation of phenotypically and functionally distinct DC population
Myeloid DCs can develop from infiltrating tissue monocyte/macrophages and bone marrow–derived DC precursors. We assessed the effect of adenosine on DC differentiation from human blood monocytes and from mouse HPCs and peritoneal macrophages.
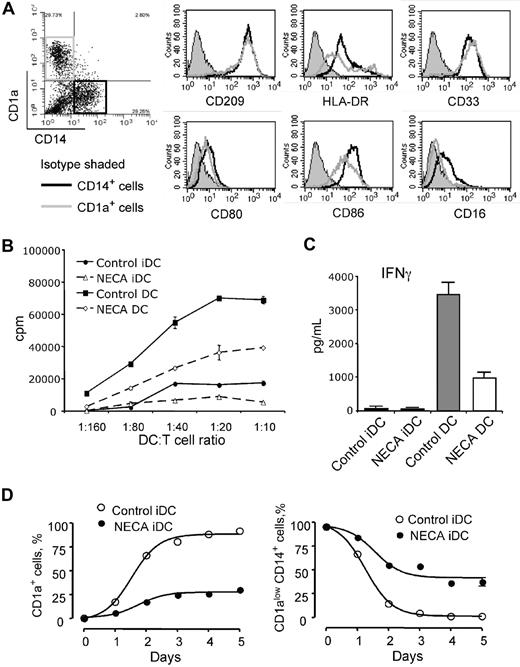
When cultured in vitro in the presence of GM-CSF and IL-4, human monocytes differentiate into immature DCs. They acquire specific morphologic features and markers of immature DCs, including CD1a and CD209, but lose the marker of monocyte/macrophages (CD14). Subsequent treatment with LPS or TNF-α induces maturation of DCs associated with up-regulation of MHC class II and costimulatory molecules and increased T-cell stimulatory activity. We differentiated monocytes into DCs in the absence or presence of adenosine and monitored phenotypical changes using CD1a and CD14 cell surface markers. Monocytes express high levels of CD14 but lack the expression of CD1a, whereas DCs express a high level of CD1a and virtually no CD14 (Figure 1B). Adenosine, added to cell culture, affects DC differentiation from monocytes in the presence of GM-CSF and IL-4 and dramatically decreases production of CD1a+ DCs. Differentiation of monocytes in the presence of a stable cell-impermeable adenosine analog NECA has a similar effect on DC differentiation as adenosine and is exerted in a concentration-dependent manner (Figure 1B,C). The population of adenosine-differentiated DCs produced from human monocytes in the presence of NECA resembles classic DCs morphologically but is phenotypically distinct from either monocytes or conventional immature DCs (iDC). These cells are characterized by the expression of CD209, a DC-specific marker (Figure 2A), and low or no expression of DC marker CD1a, and they fail to lose the monocytic marker CD14 (Figure 1B).
Phenotype and functional activity of human adenosine-differentiated DCs and time course of their differentiation. (A) Immature DCs were generated from human monocytes in the presence of 30 μM NECA to produce about equal proportions of CD1a+ and CD1alowCD14+ cells; these cells were separated by flow sorting into CD1a+CD14− and CD1a−CD14+ cell populations (indicated on a dot plot) and expression of myeloid and DC cell surface markers was assessed by flow cytometry (histograms). Results are representative of 3 experiments. (B,C) Adenosine-differentiated DCs have impaired ability to stimulate proliferation and IFN-γ production by allogeneic T cells. Immature (iDC) and mature (DC) DCs were generated from human monocytes with or without 100 μM NECA and used as stimulators in MLR with allogeneic donor's T cells. MLR was performed as described in the “Methods.” IFN-γ concentrations were measured in MLR supernatants by ELISA. Results are representative of 3 experiments. (C) Average values from 3 different experiments are shown. Error bars denote SE. (D) Time course of generation of CD1a+ and CD1alowCD14+ DCs from human monocytes in the presence of 100 μM NECA. NECA was added to monocytes at day 0, and the proportions of CD1a+ and CD1alowCD14+ DCs were assessed daily by flow cytometry. Average values from 3 different experiments are shown. Error bars denote SE.
Phenotype and functional activity of human adenosine-differentiated DCs and time course of their differentiation. (A) Immature DCs were generated from human monocytes in the presence of 30 μM NECA to produce about equal proportions of CD1a+ and CD1alowCD14+ cells; these cells were separated by flow sorting into CD1a+CD14− and CD1a−CD14+ cell populations (indicated on a dot plot) and expression of myeloid and DC cell surface markers was assessed by flow cytometry (histograms). Results are representative of 3 experiments. (B,C) Adenosine-differentiated DCs have impaired ability to stimulate proliferation and IFN-γ production by allogeneic T cells. Immature (iDC) and mature (DC) DCs were generated from human monocytes with or without 100 μM NECA and used as stimulators in MLR with allogeneic donor's T cells. MLR was performed as described in the “Methods.” IFN-γ concentrations were measured in MLR supernatants by ELISA. Results are representative of 3 experiments. (C) Average values from 3 different experiments are shown. Error bars denote SE. (D) Time course of generation of CD1a+ and CD1alowCD14+ DCs from human monocytes in the presence of 100 μM NECA. NECA was added to monocytes at day 0, and the proportions of CD1a+ and CD1alowCD14+ DCs were assessed daily by flow cytometry. Average values from 3 different experiments are shown. Error bars denote SE.
At 30 μM of NECA about equal proportions of CD1a+ and CD1alowCD14+ cells are produced from human monocytes. We assessed the expression of myeloid and DC-specific cell surface markers on CD1a+ and CD1alowCD14+ cells after separating them by flow sorting. The CD1alowCD14+ cell fraction of adenosine-differentiated DCs expressed CD40, CD80, and CD209 markers at levels similar to those of CD1a+ cells (Figure 2A). However, in contrast to normal CD1a+ DCs, they expressed significantly higher levels of MHC class II and CD86 molecules and bear low levels of CD16, a marker present on macrophages. The acquisition of DC-specific marker CD209 not present on monocyte/macrophages by CD1alowCD14+ cells indicates that these cells are a phenotypically distinct DC population rather than underdifferentiated monocytes.
DCs generated in the presence of adenosine are functionally impaired
DCs generated from human monocytes in the presence of NECA have impaired ability to induce T-cell proliferation, as assessed by allogeneic mixed lymphocyte reaction (MLR; Figure 2B). Adenosine-differentiated DCs respond to maturation stimuli by LPS or TNF-α by gaining allostimulatory activity, but it is much less than in normal DCs. The impaired ability of adenosine-differentiated DCs to induce proliferation of allogeneic T cells coincides with significantly decreased production of IFN-γ by stimulated T cells (Figure 2C).
Time course of DC generation in the presence of adenosine
Kinetics of DC generation from human monocytes in the presence of NECA follows the same pattern as control cells, but the number of CD1a+ DCs remains lower at all times and is associated with increased proportion of CD14+ cells (Figure 2D). Similar data were obtained for DC differentiation from mouse HPCs.
Adenosine-differentiated DCs are proangiogenic
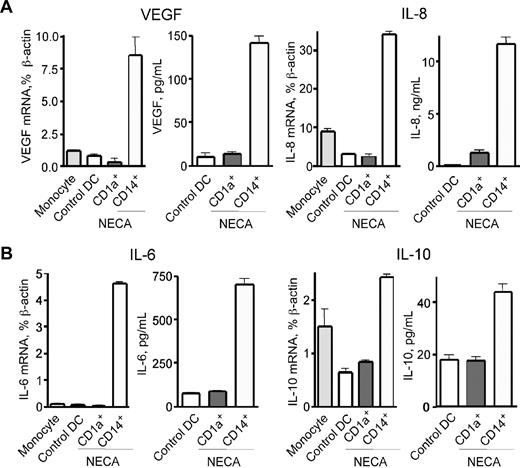
Skewed differentiation of immune cells in pathologic micro-environment may induce them to release a variety of factors that further augment local inflammation and angiogenesis.21 We assessed the inflammatory and angiogenic properties of adenosine-differentiated DCs and specifically those of CD1alowCD14+ cells generated from human monocytes. We found high levels of mRNA transcripts and secreted proteins of key angiogenic factors such as vascular endothelial growth factor (VEGF) and IL-8 in CD1alowCD14+ DCs, whereas CD1a+CD14− cells barely had any of those (Figure 3A).
Adenosine-differentiated CD1alowCD14+ DCs express VEGF, IL-8, IL-6, and IL-10, a combination of angiogenic, proinflammatory, and immune suppressive factors. (A,B) Immature DCs were generated from human monocytes in the presence of 30 μM NECA, and CD1a+ and CD1a−CD14+ cells were separated by immunomagnetic technique. These cells, as well as freshly purified monocytes as control, were cultured under the same conditions with the addition of 20 ng/mL TNF-α. Cells were harvested after 6 hours for mRNA quantification; culture supernatants for measurements of secreted cytokines were collected from duplicate samples after 24 hours. Specific mRNA transcripts were quantified by real-time PCR, and concentrations of secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
Adenosine-differentiated CD1alowCD14+ DCs express VEGF, IL-8, IL-6, and IL-10, a combination of angiogenic, proinflammatory, and immune suppressive factors. (A,B) Immature DCs were generated from human monocytes in the presence of 30 μM NECA, and CD1a+ and CD1a−CD14+ cells were separated by immunomagnetic technique. These cells, as well as freshly purified monocytes as control, were cultured under the same conditions with the addition of 20 ng/mL TNF-α. Cells were harvested after 6 hours for mRNA quantification; culture supernatants for measurements of secreted cytokines were collected from duplicate samples after 24 hours. Specific mRNA transcripts were quantified by real-time PCR, and concentrations of secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
DCs generated in the presence of adenosine express Th2-type immune response cytokines, proinflammatory, immune suppressor, and tolerogenic factors
Adenosine-differentiated DCs have profoundly altered cytokine expression profile compared with classic myeloid DCs. It is characterized by a mix of proinflammatory and anti-inflammatory cytokines and up-regulation of immune suppressor and tolerogenic factors. Using real-time PCR and ELISA we quantified expression of mRNA transcripts and secretion of soluble factors by isolated CD1alowCD14+ cells generated from human monocytes. These cells secret high levels of IL-6 and IL-10 (Figure 3B), the cytokines that polarize naive CD4+ T cells toward a Th2-type immune response.22 In addition to secretion of IL-6 associated with acute phase of inflammation, mature adenosine-differentiated DCs also showed a more than 10-fold increase in expression of proinflammatory COX-2 mRNA (Figure 4A). Significant secretion of IL-10 suggests that adenosine-differentiated DCs are immune suppressive, consistent with their impaired ability to stimulate proliferation and IFN-γ production by allogeneic T cells (Figure 2B,C), and can contribute to the induction of immune tolerance. DCs obtained in the presence of adenosine have also markedly increased expression of TGF-β, another factor promoting generation of regulatory T cells (Figure 4B).
Adenosine-differentiated DCs have up-regulated expression of pro-inflammatory COX-2, tolerogenic TGF-β and IDO, and tumor growth–promoting ARG2. (A-D) Immature or mature DCs were generated from human monocytes without or with 100 μM NECA, and total RNA was purified from cells as described in the “Methods.” Specific mRNA transcripts for indicated proteins were quantified by real-time PCR. Average values from 3 different experiments are shown. Error bars denote SE.
Adenosine-differentiated DCs have up-regulated expression of pro-inflammatory COX-2, tolerogenic TGF-β and IDO, and tumor growth–promoting ARG2. (A-D) Immature or mature DCs were generated from human monocytes without or with 100 μM NECA, and total RNA was purified from cells as described in the “Methods.” Specific mRNA transcripts for indicated proteins were quantified by real-time PCR. Average values from 3 different experiments are shown. Error bars denote SE.
A subset of DCs expressing indoleamine 2,3-dioxygenase (IDO) has been identified both in humans and mice. These cells suppress proliferation of CD4+ and CD8+ T cells, render the suppressed cells anergic, and are able to induce apoptosis ofT cells.23-25 These activities are mediated by increased production of tryptophane catabolites, quinolinic acid, and 3-hydroxyanthranilic acid. IDO and tryptophanyl-tRNA-synthetase (TTS) are the enzymes catalyzing degradation and synthesis of tryptophane, respectively. Our data show that adenosine-differentiated DCs from monocytes have markedly increased expression of IDO (Figure 4C), whereas TTS expression level does not change (not shown). This indicates that adenosine-differentiated DCs might be an important factor of immune tolerance via production of IDO.
DCs generated in the presence of adenosine produce tumor growth–promoting factor
DCs generated from human monocytes in the presence of NECA had significantly up-regulated levels of arginase 2, an enzyme that catalyses conversion of l-arginine into l-ornithine26,27 and thus provides a substrate for synthesis of polyamines (putrescine, spermidine, spermine), which are essential nutrients used for proliferation, neoplastic transformation of mammalian cells, and tumor growth (Figure 4D). This observation indicates that in addition to generation of angiogenic and proinflammatory factors adenosine-differentiated DCs also produce metabolites promoting neoplasia and tumor growth.
A2B adenosine receptors mediate the effect of adenosine on DC differentiation
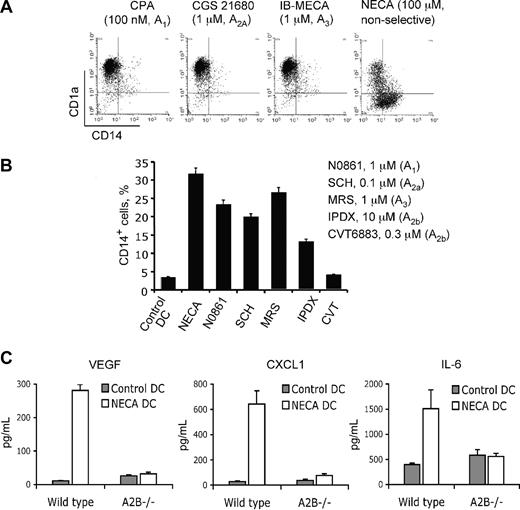
We have several lines of evidence identifying A2B adenosine receptor as a mediator of adenosine effects on DC differentiation. (1) The generation of adenosine-differentiated cells by NECA from human monocytes follows a concentration-dependent curve with an estimated EC50 value of 12.7 μM, suggestive of the involvement of low-affinity A2B receptors (Figure 1C). (2) Receptor-specific agonists are currently available for A1, A2A, and A3 adenosine receptors but not for the A2B receptor. None of these selective agonists had any effect on DC differentiation from human monocytes (Figure 5A). Lack of effect of A1, A2A, and A3 receptor-specific agonists, coupled with a profound effect of the nonselective agonist NECA, indicates the involvement of A2B receptor. (3) The A2B receptor–selective antagonists CVT 6883 and IPDX, but not A1-, A2A-, or A3-specific antagonists, were able to efficiently block the effect of NECA on DC differentiation from both human monocytes (Figure 5B) and mouse peritoneal macrophages (not shown). (4) We assessed the effect of NECA on DC differentiation from HPCs and peritoneal macrophages obtained from wild-type and A2B knockout mice. We found that, although NECA had minimal effect on DC phenotype, it dramatically influenced cytokine expression. Mature DCs generated from mouse HPCs or peritoneal macrophages (not shown) in the presence of NECA showed a cytokine expression profile similar to that of human DCs produced from monocytes (Figure 5C). They secrete high amounts of VEGF and CXCL1, a mouse ortholog of IL-8. They also significantly up-regulate secretion of proinflammatory IL-6. These effects are lost in cells generated from A2B receptor knockout animals, consistent with the role of this receptor in altered differentiation of DCs.
A2B adenosine receptor mediates effects of adenosine on DC differentiation. (A) Selective agonists to A1, A2A, and A3 adenosine receptors do not affect DC differentiation. Immature DCs were generated from human monocytes in the presence of the indicated concentrations of receptor-selective agonists or NECA. Agonist concentrations were 10-fold higher than their Kd for the respective receptor. Results are representative of 3 experiments. (B) Selective antagonists to A2B receptor but not to A1, A2A, or A3 adenosine receptors reverse NECA-induced alteration of DC differentiation. Immature DCs were generated from human monocytes in the presence of 100 μM NECA and selective antagonists at indicated concentrations. Antagonist concentrations were 10-fold higher than their Ki for the respective receptor. The proportion of CD14lowCD14+ cells was measured by flow cytometry. Average values from 3 different experiments are shown. Error bars denote SE. (C) Effect of NECA on cytokine secretion by DCs from mouse HPCs is not reproduced in cells from A2B−/− knockout animals. Mature DCs were generated from wild-type or A2B−/− knockout mouse HPCs with or without 100 μM NECA with 20 ng/mL TNF-α added for 24 hours for maturation. Culture supernatants from the maturation step were collected, and secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
A2B adenosine receptor mediates effects of adenosine on DC differentiation. (A) Selective agonists to A1, A2A, and A3 adenosine receptors do not affect DC differentiation. Immature DCs were generated from human monocytes in the presence of the indicated concentrations of receptor-selective agonists or NECA. Agonist concentrations were 10-fold higher than their Kd for the respective receptor. Results are representative of 3 experiments. (B) Selective antagonists to A2B receptor but not to A1, A2A, or A3 adenosine receptors reverse NECA-induced alteration of DC differentiation. Immature DCs were generated from human monocytes in the presence of 100 μM NECA and selective antagonists at indicated concentrations. Antagonist concentrations were 10-fold higher than their Ki for the respective receptor. The proportion of CD14lowCD14+ cells was measured by flow cytometry. Average values from 3 different experiments are shown. Error bars denote SE. (C) Effect of NECA on cytokine secretion by DCs from mouse HPCs is not reproduced in cells from A2B−/− knockout animals. Mature DCs were generated from wild-type or A2B−/− knockout mouse HPCs with or without 100 μM NECA with 20 ng/mL TNF-α added for 24 hours for maturation. Culture supernatants from the maturation step were collected, and secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
Adenosine-differentiated DCs present in vivo under conditions associated with elevated levels of extracellular adenosine
We showed that high physiologically relevant levels of adenosine lead to the generation of proangiogenic DCs in vivo using ADA−/− knockout mice. ADA is a ubiquitous and essential enzyme of purine catabolism that is responsible for hydrolytic deamination of adenosine to inosine and maintaining low concentrations of adenosine in the extracellular milieu. Mice deficient in ADA develop pulmonary inflammation and damage in association with adenosine elevations, making them a useful model for assessing the role of adenosine signaling under pathologic conditions in the lung.16
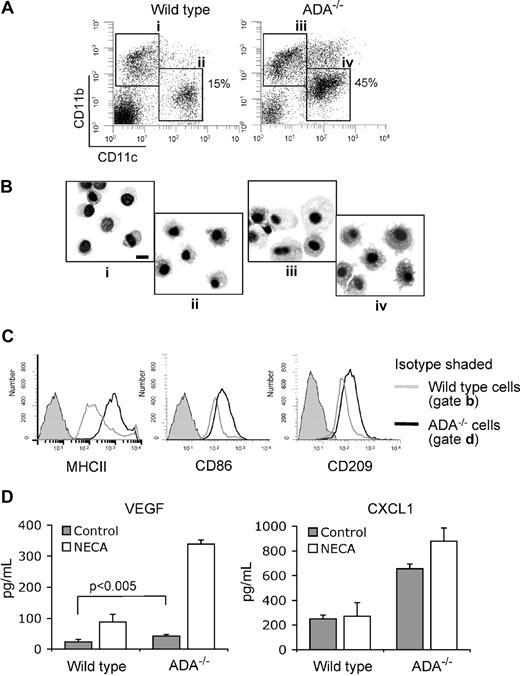
We characterized CD11blowCD11c+CD45+ cells in the lungs of wild-type and ADA−/− knockout mice. These cells are not lung macrophages, which also express CD11c marker. When flow sorted as indicated in Figure 6A and stained with Diff-Quick, they showed typical DC morphology with characteristic long projections (Figure 6Bii,iv). These cells express lower levels of CD11b but have a high expression of MHCII, CD86, and CD209 markers, consistent with the phenotype of DCs (Figure 6A,C). The total numbers of hematopoietic CD45+ cells in the lungs in both types of animals were equal, but there were 3-fold more of CD11blowCD11c+ cells in ADA−/− knockouts than in wild-type mice, and they had a higher expression of MHCII (Figure 6C) and were bigger in size (Figure 6B). We assessed the secretion of VEGF and CXCL1 by these cells without or in the presence of NECA (Figure 6D). Lung DCs from ADA−/− knockout mice had higher basal secretion levels of VEGF and CXCL1 and responded to NECA by a dramatic increase in VEGF production (Figure 6D). These data directly show that elevated adenosine affects differentiation of DCs in vivo and render them a proangiogenic phenotype.
Proangiogenic DCs are generated in vivo under conditions associated with elevated levels of extracellular adenosine. (A) Single-cell suspensions from the lungs of wild-type or ADA−/− knockout mice were analyzed by multicolor flow cytometry. CD45+ cells were gated and assessed for expression of CD11b and CD11c markers. (B) Cells gated and sorted as shown in (Ai-iv) were spun on glass slides and stained with Diff-Quick to assess their morphology. Microscopy was performed with a Nikon Eclipse E600 (Nikon Instruments) using a Nikon 100×/1.4 NA oil objective. Images were captured with Olympus DP-11 digital camera system (Olympus America). Note DCs with projections in (ii) and (iv) and that cells in (iv) are bigger. Bar, 10 μm. (C) CD11blowCD11c+ cells were gated as shown in (Aii,iv) and assessed for expression of MHCII, CD86, or CD209 markers. (D) These cells were cultured for 18 hours in RPMI with 10% FBS without or with 100 μM NECA. Culture supernatants were collected, and secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
Proangiogenic DCs are generated in vivo under conditions associated with elevated levels of extracellular adenosine. (A) Single-cell suspensions from the lungs of wild-type or ADA−/− knockout mice were analyzed by multicolor flow cytometry. CD45+ cells were gated and assessed for expression of CD11b and CD11c markers. (B) Cells gated and sorted as shown in (Ai-iv) were spun on glass slides and stained with Diff-Quick to assess their morphology. Microscopy was performed with a Nikon Eclipse E600 (Nikon Instruments) using a Nikon 100×/1.4 NA oil objective. Images were captured with Olympus DP-11 digital camera system (Olympus America). Note DCs with projections in (ii) and (iv) and that cells in (iv) are bigger. Bar, 10 μm. (C) CD11blowCD11c+ cells were gated as shown in (Aii,iv) and assessed for expression of MHCII, CD86, or CD209 markers. (D) These cells were cultured for 18 hours in RPMI with 10% FBS without or with 100 μM NECA. Culture supernatants were collected, and secreted cytokines were measured by ELISA. Average values from 3 different experiments are shown. Error bars denote SE.
Adenosine-differentiated DCs act as angiogenic and tumor growth-promoting factor in vivo
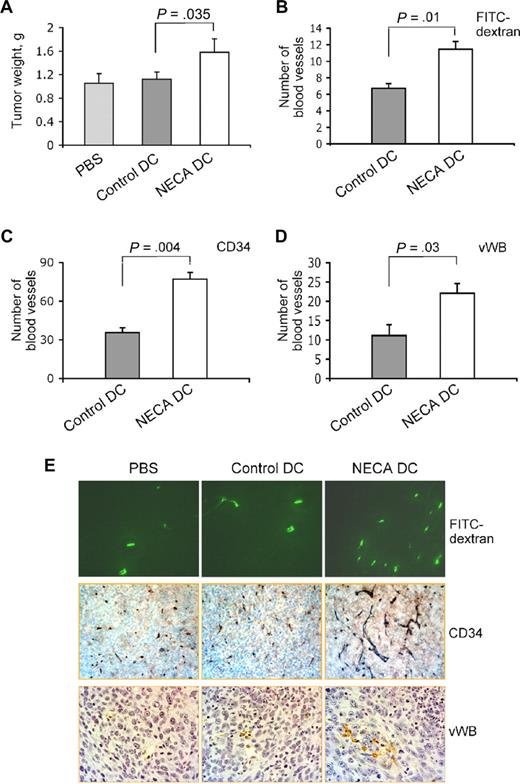
To determine whether our in vitro findings are relevant to the regulation of tumor angiogenesis and tumor growth in vivo, we conducted mouse tumor model studies with the injection of normal or adenosine-differentiated DCs produced from HPCs into subcutaneous tumor. The experiments directly showed that the presence of adenosine-differentiated cells in the tumor could significantly increase its vascularization and promote growth. To determine the tumor microvessel density, we used the FITC-dextran method19,20 and immunostaining with anti-CD34 and anti–von Willebrand factor antibodies, which allow visualization of both microcapillaries and larger more mature vessels.28,29 The tumor weight and the number of blood vessels in tumor tissue sections were significantly increased in the group of mice that received adenosine-differentiated immature DCs compared with mice that received PBS or normal immature DCs (Figure 7A-D). The fact that there is no difference in tumor growth between PBS and control DC groups indicates that immune reactions have not fully developed during a period of 7 days in mice that received DCs. It is conceivable that the observed differences in tumor growth and vascularization between control DC and NECA DC mice are due to the production of angiogenic and tumor growth-promoting factors by adenosine-differentiated DCs.
Adenosine-differentiated DCs act as angiogenic and tumor growth–promoting factor in vivo. Immature DCs were generated with or without 100 μM NECA from mouse HPCs and injected into subcutaneous LLC tumors in mice. Seven days later tumors were isolated and weighted (A), and tumor vascularization was assessed using FITC-dextran (B) or immunostaining with antibodies to CD34 (C) or von Willebrand factor (D) as described in “Mouse tumor model experiments.” Images were taken from tumor sections, and the number of tumor blood vessels identified by FITC-dextran fluorescence (green) or immunostaining (brown) was counted. Images of representative tumor sections show a higher number of blood vessels in tumors that received injection of adenosine-differentiated DCs (E). Five animals per group. Fifteen fields on 2 nonadjacent sections were counted for each sample.
Adenosine-differentiated DCs act as angiogenic and tumor growth–promoting factor in vivo. Immature DCs were generated with or without 100 μM NECA from mouse HPCs and injected into subcutaneous LLC tumors in mice. Seven days later tumors were isolated and weighted (A), and tumor vascularization was assessed using FITC-dextran (B) or immunostaining with antibodies to CD34 (C) or von Willebrand factor (D) as described in “Mouse tumor model experiments.” Images were taken from tumor sections, and the number of tumor blood vessels identified by FITC-dextran fluorescence (green) or immunostaining (brown) was counted. Images of representative tumor sections show a higher number of blood vessels in tumors that received injection of adenosine-differentiated DCs (E). Five animals per group. Fifteen fields on 2 nonadjacent sections were counted for each sample.
Discussion
Recent studies show a remarkable ability of DC precursors to differentiate into DC subpopulations with various functional features. A set of differentiation/maturation signals determines the properties of resultant DCs. DCs generated under certain conditions may have an adverse effect in the pathogenesis of a variety of diseases. Limited effectiveness of DC-based cancer vaccines in controlling tumor growth in multiple clinical trials reflects the ability of the pathologic microenvironment to significantly affect DC differentiation and function. This underscores the necessity for further identification of pathologic mechanisms that alter DC functionality, converting them from a protective to a pathologic factor.
In the present study, we have identified an important mechanism of regulation of DC differentiation and properties by adenosine. Our data show that elevated levels of extracellular adenosine could lead DCs to acquire a specific phenotype associated with proangiogenic and proinflammatory properties, immune suppression, immune tolerance, and polarization of immune response toward a Th2 type. Both in vitro and in vivo experiments indicate that adenosine induces generation of a phenotypically and functionally distinct subset of DCs rather than blockage of monocyte/macrophage differentiation or modification of their activation. The acquisition of DC-specific cell surface marker (CD209) by adenosine-differentiated DCs suggest a role for adenosine in DC differentiation as well as in modulation of their activation.
Adenosine actions are mediated by a P1 family of 7-transmembrane G-protein–coupled cell surface receptors.30-33 Of the 4 adenosine receptor subtypes, adenosine binds with high affinity to the human A1 and A3 receptors, with a dissociation constant (Kd) of 0.3 μM and to the A2A receptors with a Kd of 0.7 μM. Affinity of A2B receptors to adenosine is considerably lower (Kd of 24 μM).34 Because of this unique feature, A2B receptors are likely to remain silent under normal physiologic conditions and to become important only during conditions of ischemia or inflammation when interstitial concentrations of adenosine increase. Although all 4 adenosine receptors are present on human peripheral blood monocytes and immature and mature DCs, results from our experiments convincingly identify the A2B receptor as a mediator of adenosine effects on DCs. Thus, it is conceivable that adenosine becomes a factor affecting DC differentiation in pathologic but not in normal tissue. Because NECA used in our experiments is cell-impermeable, the observed effects are due to activation of A2B receptor rather than to a metabolic effect of adenosine. We suggest that these effects are mediated by activation of adenylate cyclase and increased levels of intracellular cAMP, because cyclic nucleotides were previously shown to produce similar phenotypical changes during DC differentiation.12 However, the exact signaling mechanism is a matter of further investigation. In contrast to other factors of aberrant DC differentiation (IL-10, M-CSF, VEGF, gangliosides), adenosine is a local rather than a systemic factor, which exerts its effect on cells infiltrating inflamed/injured tissue, including monocytes, macrophages, or DC precursors generated from HPCs. Note that in macrophages adenosine strongly up-regulates the expression of VEGF in an A2A receptor–dependent manner.35 The expression of the A2A receptor increases during DC differentiation. From our experiments, the possibility remains that the mechanism of regulation of VEGF expression in adenosine-differentiated DCs is similar to that in macrophages and involves A2A receptor signaling, whereas A2B receptor regulates the differentiation of DCs.
During immunologic reactions, proinflammatory and anti-inflammatory mechanisms and polarization of T-cell responses are regulated in a finely tuned manner. Expression of proinflammatory and Th1 immune response-promoting cytokines (TNF-α, IL-1, IL-6) are followed by signals associated with type 2–polarized response (IL-4, IL-10, IL-13). Adenosine seems to induce the generation of mixed cytokine signals from DCs. Expression of high levels of proinflammatory IL-6 and COX-2 associates with the initiation phase of the immune reaction. In combination with VEGF and IL-8 (CXCL1 in mice), this might work to mobilize cells from blood. However, by up-regulating IL-10 and TGF-β adenosine-differentiated DCs would affect Th1-mediated immune reactions, induce the generation of regulatory T cells, and polarize the immune response toward Th2 type. This conclusion is in agreement with published data showing the ability of adenosine to induce the Th2-polarizing capacity of monocyte-derived DCs.36
The finding that adenosine can drastically alter DC functionality has enormous implications to our understanding of the pathology of diseases such as cancer and asthma. Tumors are known to produce factors skewing normal differentiation of DCs and rendering them incapable of inducing tumor-specific immune responses. Solid tumors have extended areas of necrosis and tissue damage with high local concentrations of adenosine.1 It is likely, therefore, that tumor-associated monocyte/macrophages, immature DCs, and DC precursors are exposed to high concentrations of adenosine. We propose that, under such conditions, DCs differentiate toward cells that not only are unable to elicit potent Th1-type immune response but, on the contrary, induce immune suppression and tolerance by secreting IL-10 and TGF-β. Another mechanism of immune suppression is likely engaged through the significant up-regulation of IDO by adenosine-derived DCs. Abnormal numbers of IDO-expressing cells were seen in tumor-bearing animals and in sentinel lymph nodes of patients with cancer and were associated with a significantly worse prognosis.23,25 To make the picture worse, adenosine-differentiated DCs can significantly contribute to tumor angiogenesis and growth by producing high levels of angiogenic factors and by providing nutrients for its growth. Thus, exposure of DCs to adenosine might be an important mechanism of tumor escape from the immune system and resistance to immune therapies based on DC activation/mobilization. Our data are the first evidence showing the regulation of IDO expression in DCs by adenosine. They identify activation of A2B receptor by tumor-derived adenosine as a novel mechanism of the tumor-associated increase of IDO-producing DCs and induction of immune tolerance.
Adenosine is also known to be highly elevated in the asthmatic lung.37-39 There is growing evidence that DCs play a critical role not only in allergic sensitization but also in the maintenance of chronic airway inflammation by promoting Th2 responses within the sites of inflammation.40 Our data suggest the existence of a pathologic loop in asthmatic lungs in which lung damage and inflammation cause adenosine release with subsequent generation of DCs secreting proinflammatory and proangiogenic factors, thus inducing tissue remodeling and further augmenting the disease.
The results of this work show that the A2B adenosine receptor could be a valuable therapeutic target. Given the profound effect that adenosine signaling through A2B receptors has on the course of DC differentiation and the generation of the proangiogenic/proinflammatory and immune suppressive DC population, it appears likely that targeting the A2B adenosine receptor might have enormous potential for therapeutic interventions in cancer and immune and inflammatory diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Luiz Belardinelli, Dewan Zeng, and Hongyan Zhong (CV Therapeutics) for scientific discussion and for providing CVT6883 compound for this study. We also thank the Vanderbilt Imaging Core for the assistance with the fluorescent microscopy and the Vanderbilt Immunohistochemistry Core for immunostaining of tissue slides (Vanderbilt-Ingram Cancer Center Support grant 5P30 CA068485).
This work was supported by National Institutes of Health grants CA100562 (M.M.D.), HL76306 (I.F.), HL-70952 (M.R.B), and CA76321 (D.P.C.) and by a research grant from Histiocytosis Association of America (Pitman, NJ; M.M.D.).
National Institutes of Health
Authorship
Contribution: S.V.N. and S.R. designed and performed the research and analyzed the data; R.Z. and A.E.G. performed some of the research; Y.H. and O.Y.T. discussed the results, analyzed the data, and provided helpful suggestions; M.R.B., I.B., and D.P.C. discussed the results and provided helpful suggestions; I.F. and M.M.D. designed the experiments, supervised their conduct, and analyzed the data; and M.M.D. wrote the paper.
Conflict-of-interest disclosure: The authors acknowledge financial support from CV Therapeutics Inc.
Correspondence: Mikhail M. Dikov, Vanderbilt University, 2200 Pierce Avenue, 648 PRB, Nashville, TN 37232; e-mail: mikhail.dikov@vanderbilt.edu.