Abstract
Signaling by Eph receptors and cell-surface ephrin ligands modulates adhesive cell properties and thereby coordinates cell movement and positioning in normal and oncogenic development. While cell contact–dependent Eph activation frequently leads to cell-cell repulsion, also the diametrically opposite response, cell-cell adhesion, is a probable outcome. However, the molecular principles regulating such disparate functions have remained controversial. We have examined cell-biologic mechanisms underlying this switch by analyzing ephrin-A5–induced cell-morphologic changes of EphA3-positive LK63 pre-B acute lymphoblastic leukemia cells. Their exposure to ephrin-A5 surfaces leads to a rapid conversion from a suspended/nonpolarized to an adherent/polarized cell type, a transition that relies on EphA3 functions operating in the absence of Eph-kinase signaling. Cell morphology change and adhesion of LK63 cells are effectively attenuated by endogenous protein tyrosine phosphatase (PTP) activity, whereby PTP inhibition and productive EphA3-phosphotyrosine signaling reverse the phenotype to nonadherent cells with a condensed cytoskeleton. Our findings suggest that Eph-associated PTP activities not only control receptor phosphorylation levels, but as a result switch the response to ephrin contact from repulsion to adhesion, which may play a role in the pathology of hematopoietic tumors.
Introduction
Eph receptors and their membrane-bound ephrin ligands are developmental cell guidance cues that direct cell migration and orchestrate patterning processes by modulating adhesive or repulsive cell properties.1 While their expression and function in normal adult tissues is limited, Eph and ephrin overexpression in human cancers often correlates with aggressive, invasive, and metastatic phenotypes.2
Upon Eph/ephrin contact, heterotetrameric complexes between neighboring cells3 assemble into large signaling clusters that are rapidly internalized.4,5 Tyrosine autophosphorylation within the juxtamembrane region and kinase activation loop is required to fully activate the Eph kinase6,7 and triggers signal pathways regulating cytoskeletal plasticity.1,8 As a default, Eph activation leads to cytoskeletal contraction, loss of focal adhesions, cell rounding, and cell-cell repulsion or detachment.8,9 Repulsion also requires the active Eph tyrosine kinase to mediate ephrin shedding by the ADAM10 metalloprotease, releasing the molecular tether connecting the Eph- and ephrin-expressing cells.10,11 An alternative mechanism involving kinase-dependent “transendocytosis” of the whole EphB and ephrin-B signaling clusters into either cell has been proposed.12 However, ephrin ligation of Ephs also results in cell-cell and cell-substrate adhesion13,14 : interaction between kinase-deficient EphA7 splice variants and ephrin-A5 on opposing cell layers facilitates neural tube closure during mouse development,15 and retinal axons respond to low ephrin-A2 density with adhesion.14 Analysis of these disparate outcomes of Eph/ephrin signaling suggests that the Eph receptor signaling strength may influence whether cells respond by repulsion or by adhesion.13,14,16
There is emerging evidence that Eph receptors are expressed and active in lymphocytes, in particular modulating the function of T cells.17-22 EphA3 was originally identified in pre-B and T-cell leukemic cell lines, and cloned from LK63 pre-B acute lymphoblastic leukemia (ALL) cells,23,24 expressing the receptor at highly elevated levels.25 We have now studied the role of EphA3 by comparing responses to ephrin-A5 of LK63 cells and adherent tumor cells; in particular, we explored the mechanisms that can trigger diametrically opposite responses to the same Eph/ephrin signaling proteins in either of these cell types. We observe dose-dependent detachment of epithelial cells, but tight adhesion and spreading of LK63 cells onto ephrin-A5 surfaces that is independent of conventional VCAM/ICAM-mediated lymphocyte docking mechanisms.26 In LK63 cells, endogenous EphA3-associated protein tyrosine phosphatase (PTP) activity efficiently dampens EphA3 kinase activation and phosphotyrosine (PY)–dependent signaling. Time-lapse microscopy suggests that cell attachment is followed within minutes by extension and subsequent retraction of lamellipodia and uropod-like cell protrusions, leaving behind a radiating array of retraction fibers,27 Eph clusters, and focal adhesion contacts. The persisting EphA3/ephrin-A5 clusters in these cells provide a stable tether with an ephrin-A5–bearing surface, and subsequently form sites for the assembly of adhesion complexes. Silencing of EphA3 expression or inhibition of PTP activity prevents the rapid transition of nonpolarized suspension (leukemia) cells into adherent and outstretched cells that characteristically feature extensive actin-rich cell processes, focal adhesion–like contacts, and a diffuse actin cytoskeleton.
We suggest that persisting Eph/ephrin clusters, lacking forward Eph kinase signaling activity, trigger cytoskeletal remodeling that leads to adhesion and spreading of previously nonadherent leukemia cells. This mechanism may play a role in the pathology of some hematopoietic tumors.
Methods
Expression constructs and reagents
Mammalian pEFBos expression vectors for full-length EphA328 and ephrin-A5,29 production of ephrin-A5 and EphA3-Fc fusion proteins and their Alexa546 labeling,4,28 and the properties of the anti-(α)–EphA3 monoclonal antibody (mAb, clone IIIA4) and of rabbit polyclonal antibodies23 have been described previously. Affinity-purified sheep antibody against the soluble EphA3 extracellular domain was used in most recent experiments. Rabbit α-PTP-PEST antibody was a gift from N. Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY); mAbs against human ICAM-1 and VCAM-1 were gifts from Ian Wicks (The Walter & Eliza Hall Institute, Melbourne, Australia). Other antibodies and reagents were from Zymed (rabbit α-PY antibody; South San Francisco, CA), Chemicon (rabbit α-phospho-EphA3, α-PTP-PEST mAb, clone AG25; Temecula, CA), Cell Signaling Technology (α-PTP-PEST mAb, clone Ag10; Beverly, MA), New England Biolabs (α-P-Tyr100; Beverly, MA), The Jackson Laboratory (α-human Fc; Bar Harbor, ME), Upstate (rabbit α-FAK; Lake Placid, NY), and BD Biosciences (α-paxillin mAb, clone 349; San Jose, CA). HRP-labeled secondary antibodies were from Jackson Laboratories (α-mouse) and BioRad (α-rabbit; Hercules, CA). Alexa-labeled secondary antibodies and phalloidin were from Molecular Probes (Eugene, OR). Reacti-Bind protein A–coated plates were from Pierce (Rockford, IL); lipopolysaccharide (LPS) was from Sigma-Aldrich (St Louis, MO).
Cell culture
LK63, A02, and LiBr melanoma lines were described previously.9,23 Human kidney epithelial 293 (HEK293T; ATCC, Manassas, VA) cells were maintained in DMEM, 10% FCS. Stable EphA3 (EphA3/HEK293)– and ephrin-A5 (ephrin-A5/HEK293)–expressing cell clones and EphA3-expressing AO2 melanoma clones4,5 were kept in DMEM, 10% FCS, 0.8 μg/mL puromycin (EphA3 expression), or 200 μg/mL G418 (ephrin-A5 expression). Human microvascular endothelial cells (HMVECs; Clonetics, Walkersville, MD) were cultured in endothelial cell basal medium (EBM; Clonetics), 5% FCS, glutamine, bovine brain extract (BBE), hydrocortisone, and GA-1000 (gentamicin, amphotericin B). Cell culture surfaces were coated for 1 hour at 22°C or overnight at 4°C with PBS containing 10 μg/mL fibronectin (“fibronectin surface”) or 10 μg/mL ephrin-A5 Fc, 2 μg/mL fibronectin (“ephrin-A5 surface”). For 3-dimensional cell culture, 8-well chamber glass slides were coated with 200 μL of 50% growth factor reduced Matrigel (BD Biosciences). Ephrin-A5/HEK293 cells were allowed to form a monolayer, and LK63 cells were seeded onto the monolayer. Prior to all experiments, cell viability was assessed by vital dye exclusion.
Transfections and gene knockdown by RNA interference
Lipofectamine 2000 (Invitrogen, Frederick, MD) was used for transient cell transfections. Transfection of LK63 cells with PTP-PEST siRNA (HP validated siRNA 1027400; Qiagen, Hilden, Germany) or control siRNA (human cyclophilin, NM 000942 [Dharmacon, Lafayette, CO], D-001820-01) was achieved in cells adhering to ephrin-A5 Fc-coated tissue culture surfaces. EphA3 silencing in LK63 cells was done using MISSION Lentiviral Transduction Particles (Sigma-Aldrich), according to the manufacturer's instructions. Four hairpin constructs, identified by “The RNAi Consortium” (TRC) clone numbers TRCN0000006409 (no. 9), TRCN00000064010 (no. 10), TRCN00000064011 (no. 11), TRCN00000064012 (no. 12), as well as control (pLKO.1-puro) lentivirus particles were used. Stable LK63 cell lines were maintained by selection with puromycin (0.2 μg/mL). Transfected LK63 cells with maximally reduced EphA3 levels (no. 9) were further purified by magnetic affinity cell sorting (MACS; Miltenyi Biotec, Auburn, CA), using Alexa647-IIIA4 mAb and anti–Alexa647-MACS MicroBeads to eliminate cells with residual EphA3 expression.
Cell manipulations, immunoprecipitation, and Western blotting
HMVECs were stimulated with LPS (1 μg/mL), or left untreated, prior to addition of LK63 cells. Stimulation with preclustered ephrin-A5 Fc or IIIA4 mAb was described previously.5 In some experiments, cells were treated with the PTP inhibitors sodium pervanadate or hydrogen peroxide as indicated in figures. Pervanadate was prepared from 50 μL of 0.5 M sodium vanadate and 1.7 μL hydrogen peroxide (30% vol/vol in 20 mM HEPES, pH 7.3) in 48.3 μL H2O. For inhibition of metalloproteases, cells were treated with the pan-specific inhibitor 1,10-O-phenanthroline for 4 hours as described.11 .
EphA3 was immunoprecipitated from cell lysates in 50 mM Tris, pH 7.4, 150 mM NaCl, 1% TritonX100, 0.2% SDS, 0.5% deoxycholate, 1 mM NaV04, 10 mM NaF, and Complete protease inhibitor cocktail (Roche, Indianapolis, IN) with IIIA4 mAb conjugated to Mini-Leak beads (KemEnTec, Copenhagen, Denmark) as previously described.30 Paxillin coprecipitation was performed with IIIA4 conjugated to protein A–coated Dynabeads (Invitrogen) from EphA3 cell-surface complexes isolated as described.31 Western blots were visualized using an enhanced chemiluminescence (ECL) substrate (Pierce).
Microscopy, adhesion, and kinase assays
LK63 adhesion to ephrin-A5-Fc–coated coverslips in Sykes Moore chambers was monitored on a Leica AF6000LX Live Cell Imaging workstation (Mannheim, Germany) equipped with a temperature and CO2-controlled climate chamber, using a 63×/1.3 NA glycerol lens. EphA3 stimulation with ephrin-A5–coated beads was analyzed by time-lapse confocal microscopy (Olympus FV1000; Mt Waverly, Australia) as described4 ; images were processed using analySIS (version 5, professional; Olympus) and Imarisx64 (version 6.0; Bitplane, Zurich, Switzerland) software and mounted into figures using CorelDraw (version X3; Corel, Ottawa, ON).
In adhesion assays, serum-starved LK63 (2 × 105cells/well) and LiBr cells (5 × 104cells/well) were seeded onto ephrin-A5-Fc–coated9,23 Reacti-Bind surfaces in the presence or absence of soluble monomeric ephrin-A5 as inhibitor at 100-fold molar excess. Following extensive washes (PBS), adherent cells were quantitated (A492 absorbance) by XTT assay (Roche). The adherent cell fraction was estimated from values in wells lacking ephrin and containing most adherent cells as reference points, and correcting for A492 absorbance in wells lacking cells. Means are plus or minus standard error (SE) and are representative of 3 independent experiments.
To assess EphA3 kinase activity in LK63 cells, EphA3 was isolated from whole cell lysates with α-EphA3 (IIIA4) beads. After successive washing steps in lysis buffer, 50 mM ethanolamine (pH 10) containing 0.1% Triton X-100,23 and kinase buffer (50 mM HEPES, pH 7.4, 10 mM MnCl2, 0.1% Triton X-100), beads were incubated with 0.2 mM ATP for 20 minutes at room temperature (RT). Following 3 additional washes with kinase buffer, EphA3 was eluted from the beads with SDS-sample buffer and analyzed by Western blotting.
Results
EphA3-positive cells respond to ephrin-A5 contact either with cell repulsion or adhesion.
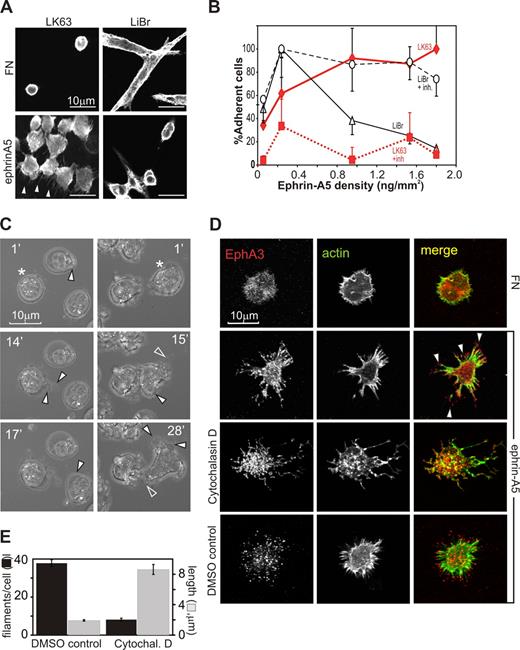
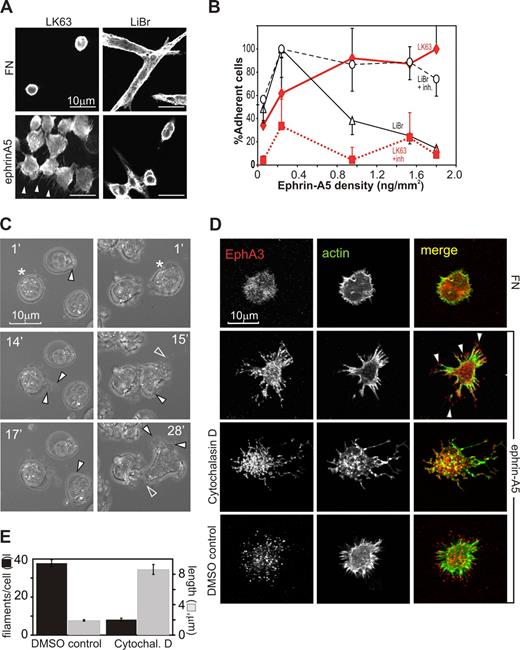
We initially compared ephrin-A5–dependent cell-morphologic changes of adherent or nonadherent EphA3-positive tumor cell lines: LK63 pre-B leukemia and LiBr melanoma cells express significant endogenous EphA3, and have been used for the initial isolation of EphA323 and to study ephrin-A5–triggered cell detachment,9 respectively. On fibronectin (FN)–coated surfaces (10 μg/mL FN), LiBr melanoma cells are adherent, spindle-shaped cells with a distinct actin cytoskeleton and dendritic cell processes (Figure 1A, FN, LiBr). LK63 leukemia cells are suspension cells with a cortical actin cytoskeleton that do not adhere to FN (Figure 1A, FN, LK63). Exposure to ephrin-A5 surfaces (10 μg/mL ephrin-A5 Fc, 2 μg/mL FN) affects the phenotypes of the 2 tumor cells in opposite directions.
Surface-bound ephrin-A5 triggers cytoskeletal reorganization, causing repulsion or adhesion of EphA3-positive tumor cells. (A) EphA3-positive LK63 human pre-B leukemia cells and LiBr melanoma cells were cultured on FN- or ephrin-A5-Fc–coated surfaces (ephrin-A5). For confocal microscopy, the cytoskeleton of fixed cells was stained with rhodamine-phalloidin. Scale bar represents 10 μm. (B) Dose-dependent adhesion of LK63 and de-adhesion of LiBr cells. LK63 leukemia and LiBr melanoma cells were seeded into wells of protein A–coated 96-well plates coupled with ephrin-A5-Fc at indicated densities. Soluble, monomeric ephrin-A5 was added as inhibitor (+inh) to parallel cultures at 100-fold molar excess. Adherent cells were quantitated by XTT assay (A492 absorbance). Cell attachment is expressed as a percentage (mean, SE from 3 independent assays) relative to maximal adherence; ♦ represents LK63 cells; ▵, LiBr cells; ■, LK63 cells with ephrin inhibition (+inh); and ○, LiBr cells with ephrin inhibition. (C) Adhesion of LK63 cells onto ephrin-A5-Fc–coated coverslips was documented by live-cell imaging, starting immediately after first contact of the cell with the tissue culture surface. Cells were imaged every minute and representative micrographs are shown. Corresponding videos for images in the left and right columns, Video S1 and Video S2, respectively, are available as data supplements. (D) LK63 cells on ephrin-A5-Fc (ephrin)– or fibronectin (FN)–coated coverslips (“Methods”) were fixed and stained with Alexa488-phalloidin and anti-EphA3/Alexa594 secondary antibodies for confocal microscopy. Some cells were treated with 5 μM cytochalasin D or solvent (DMSO), 30 minutes before and during plating as indicated. Micrographs of typical anti-EphA3 (red), actin (green) fluorescence images, and merged images are shown. (E) The number/cell (■) and length ( ) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.
) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.
Surface-bound ephrin-A5 triggers cytoskeletal reorganization, causing repulsion or adhesion of EphA3-positive tumor cells. (A) EphA3-positive LK63 human pre-B leukemia cells and LiBr melanoma cells were cultured on FN- or ephrin-A5-Fc–coated surfaces (ephrin-A5). For confocal microscopy, the cytoskeleton of fixed cells was stained with rhodamine-phalloidin. Scale bar represents 10 μm. (B) Dose-dependent adhesion of LK63 and de-adhesion of LiBr cells. LK63 leukemia and LiBr melanoma cells were seeded into wells of protein A–coated 96-well plates coupled with ephrin-A5-Fc at indicated densities. Soluble, monomeric ephrin-A5 was added as inhibitor (+inh) to parallel cultures at 100-fold molar excess. Adherent cells were quantitated by XTT assay (A492 absorbance). Cell attachment is expressed as a percentage (mean, SE from 3 independent assays) relative to maximal adherence; ♦ represents LK63 cells; ▵, LiBr cells; ■, LK63 cells with ephrin inhibition (+inh); and ○, LiBr cells with ephrin inhibition. (C) Adhesion of LK63 cells onto ephrin-A5-Fc–coated coverslips was documented by live-cell imaging, starting immediately after first contact of the cell with the tissue culture surface. Cells were imaged every minute and representative micrographs are shown. Corresponding videos for images in the left and right columns, Video S1 and Video S2, respectively, are available as data supplements. (D) LK63 cells on ephrin-A5-Fc (ephrin)– or fibronectin (FN)–coated coverslips (“Methods”) were fixed and stained with Alexa488-phalloidin and anti-EphA3/Alexa594 secondary antibodies for confocal microscopy. Some cells were treated with 5 μM cytochalasin D or solvent (DMSO), 30 minutes before and during plating as indicated. Micrographs of typical anti-EphA3 (red), actin (green) fluorescence images, and merged images are shown. (E) The number/cell (■) and length ( ) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.
) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.
While low ephrin-A5 coating densities promote adhesion in both cell types, with increasing ephrin-A5 surface concentration, LiBr melanoma cells are repelled in a dose-dependent manner, as previously described9 (Figure 1A, ephrin, LiBr), and the remaining adherent cells feature contracted cortical actin and little substratum contact.
In contrast, LK63 leukemia cells strongly adhere and spread onto the ephrin-A5 surface, and confocal microscopy reveals prominent actin-rich protrusions extending from the adherent, irregularly shaped cells toward the ephrin-A5 surface (Figure 1A arrowheads, ephrin, LK63). Both responses are dose dependent with increasing ephrin-A5-Fc coating densities: adhesion of LK63 cells reaches an apparent maximum at approximately 1 ng ephrin-A5-Fc/mm2 (Figure 1B, LK63). The presence of competing, nonclustered ephrin-A5 inhibits LK63 attachment and LiBr repulsion, suggesting that both responses rely on ephrin-modulated EphA receptor function (Figure 1B, LiBr + inh, LK63 + inh). Clones of EphA3-negative (AO2) melanoma cells9 stably expressing w/t or signaling defect EphA3 confirmed that Eph PY signaling is essential for cell rounding13 : preclustered ephrin-A5-Fc triggers contraction of the actin cytoskeleton, cell rounding, and detachment only in signaling-competent, w/t EphA3-expressing cells (Figure S1A,B, available on the Blood website; see the Supplemental Materials link at the top of the online article), while cells with cytoplasmic truncated (Y570stop) or tyrosine-mutated EphA3 (3YF EphA3) retain the spread phenotype of parental, EphA3-negative cells.
We examined ephrin-dependent LK63 cell adhesion by imaging the binding to ephrin-A5–coated surfaces in real time. A frame-by-frame inspection of typical experiments (Videos S1,S2) suggests that within 15 minutes of attachment leukemia cells undergo extensive morphologic changes, including the development of lamellipodia or uropod-like protrusions (Figure 1C filled arrowheads) and retraction fibers27 around the cell perimeter (Figure 1C open arrowheads). Confocal microscopy of the LK63 cells adhering to ephrin-A5 reveals a footprint of EphA3 clusters and actin fibers radiating out from the cell center (Figure 1D). To test whether actin reassembly is involved in the formation of these cell processes and EphA3 clusters, we inhibited actin polymerization using the cell-permeable toxin cytochalasin D. Treated LK63 cells had an overall vacillating appearance of their cytoskeletal architecture (Figure 1D, cytochalasin D). They also had significantly fewer filamentous-actin protrusions, which however were significantly longer than those of untreated cells (Figure 1E).
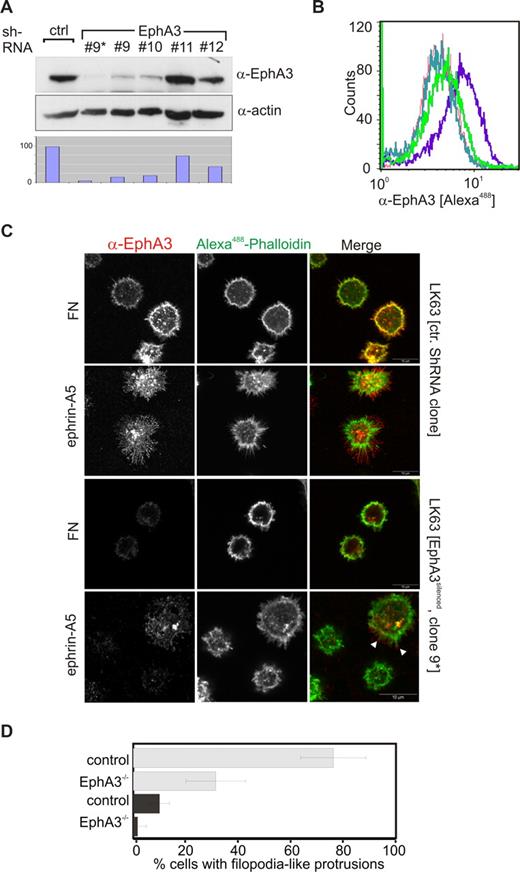
We thought to assess the role of EphA3 in facilitating ephrin-A5–mediated LK63 leukemia cell adhesion. Quantitative (Q)–PCR of the Eph/ephrin mRNA and flow cytometric analysis of LK63 cells confirmed EphA3 overexpression, whereas cell-surface levels of other potential ephrin-A5–binding Ephs were undetectable. It is of note that cDNA and protein levels of ephrin family members were also low or undetectable (Figure S2A,B), suggesting EphA3 as principle ephrin-A5–binding partner in LK63 cells. This prompted us to silence its expression using lentivirus-delivered shRNA: One of the derived clones (no. 9) with a significantly reduced EphA3 level, further purified by magnetic affinity cell sorting (MACS), was used for subsequent experiments (Figure 2A,B, no. 9*). Confocal analysis revealed that these EphA3silenced LK63 cells were notably less outstretched on ephrin-A5–coated surfaces, and were largely devoid of the distinct protrusions typical of the parental cells (Figure 2C,D). Cells with residual EphA3 expression displayed short, actin-rich cell extensions (Figure 2C arrowheads), supporting the notion that this feature depends on the presence of EphA3 in these cells. Interestingly, we noticed that EphA3 silencing also reduced the fraction of cells displaying a spread phenotype on the FN surface (Figure 2D).
EphA3 silencing prevents spreading of LK63 cells on ephrin-A5 surfaces. (A) Silencing of EphA3 in LK63 cells by lentivirus-shRNA knockdown was assessed by Western blot analysis of cell lysates. Four different TCR constructs, nos. 9 to 12, were used for the experiment. Cells derived from transfection with TCR no. 9 were further enriched for EphA3silenced cells by MACS (no. 9*). Relative EphA3 levels in cell lysates were compared by densitometry (bottom panel) using α-actin Western blot as reference. (B) Comparison of EphA3 cell-surface expression in parental and EphA3 knockdown LK63 cells by flow cytometry using Alexa488 IIIA4 α-EphA3 mAb. The profile of clone no. 9* transfected cells (light green) is compared with parental LK63 cells (purple) and with HEK293T cells (green) with known, low EphA3 expression.25 The profiles of LK63 cells stained with a nonrelevant, isotype-matched control antibody (dark green), and in the absence of IIIA4 (red), are illustrated as controls. (C) The ability of EphA3silenced LK63 cells (clone no. 9*), or control lentivirus-transfected cells, to adhere and spread onto ephrin-A5-Fc– or fibronectin-coated surfaces (as indicated) was examined by confocal microscopy. Fixed cells were stained with Alexa488-phalloidin and anti-EphA3 antibodies/Alexa546 secondary antibodies. White arrowheads indicate the presence of EphA3 clusters on the tips of filopodia-like cell processes in a cell with residual EphA3 expression. Scale bar represents 10 μm. (D) The fraction of adherent LK63 cells on ephrin-A5-Fc–coated (light gray) or fibronectin-coated (dark gray) surfaces characterized by a spread-out phenotype with actin-rich extensions (as illustrated in panel C) was estimated by a blinded observer counting phalloidin-stained cells. Mean and SE, estimated from 10 microscopic fields per group containing 20 to 40 cells each, are shown.
EphA3 silencing prevents spreading of LK63 cells on ephrin-A5 surfaces. (A) Silencing of EphA3 in LK63 cells by lentivirus-shRNA knockdown was assessed by Western blot analysis of cell lysates. Four different TCR constructs, nos. 9 to 12, were used for the experiment. Cells derived from transfection with TCR no. 9 were further enriched for EphA3silenced cells by MACS (no. 9*). Relative EphA3 levels in cell lysates were compared by densitometry (bottom panel) using α-actin Western blot as reference. (B) Comparison of EphA3 cell-surface expression in parental and EphA3 knockdown LK63 cells by flow cytometry using Alexa488 IIIA4 α-EphA3 mAb. The profile of clone no. 9* transfected cells (light green) is compared with parental LK63 cells (purple) and with HEK293T cells (green) with known, low EphA3 expression.25 The profiles of LK63 cells stained with a nonrelevant, isotype-matched control antibody (dark green), and in the absence of IIIA4 (red), are illustrated as controls. (C) The ability of EphA3silenced LK63 cells (clone no. 9*), or control lentivirus-transfected cells, to adhere and spread onto ephrin-A5-Fc– or fibronectin-coated surfaces (as indicated) was examined by confocal microscopy. Fixed cells were stained with Alexa488-phalloidin and anti-EphA3 antibodies/Alexa546 secondary antibodies. White arrowheads indicate the presence of EphA3 clusters on the tips of filopodia-like cell processes in a cell with residual EphA3 expression. Scale bar represents 10 μm. (D) The fraction of adherent LK63 cells on ephrin-A5-Fc–coated (light gray) or fibronectin-coated (dark gray) surfaces characterized by a spread-out phenotype with actin-rich extensions (as illustrated in panel C) was estimated by a blinded observer counting phalloidin-stained cells. Mean and SE, estimated from 10 microscopic fields per group containing 20 to 40 cells each, are shown.
Exposure to ephrin-A5 surfaces triggers cell polarization and focal contact assembly
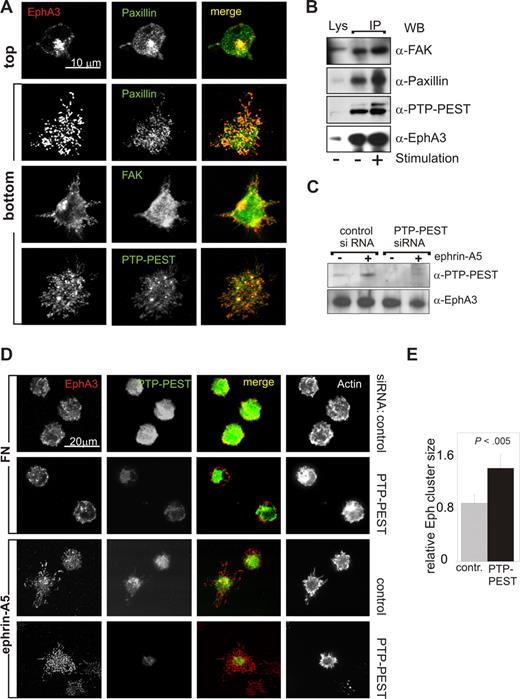
The spread-out phenotype of adherent LK63 cells and the resemblance of the EphA3 clusters with focal contacts prompted us to examine the distribution of EphA3 and various key focal adhesion components by confocal microscopy. Vertical optical (confocal) sectioning of fixed, adherent cells, which had been stained with antibodies against EphA3, FAK, paxillin, and PTP-PEST, revealed accumulation of these proteins in optical sections in proximity of the ephrin-coated surface (Figure 3A “bottom”; Figure S3A). We next assessed association of these focal adhesion proteins with EphA3, by probing anti-EphA3 IPs from whole cell lysates by immunoblot analysis: While moderate, apparently constitutive FAK association did not notably change during a 1-hour time course (Figure 3B; Figure S3B), the association with paxillin and PTP-PEST appeared to increase in agonist-exposed LK63 cells (Figure 3B). We also tested association of EphA3 with β1-integrins, but despite their expression in LK63 cells (not shown), we found no evidence for the presence α4- and α5- β1-integrins in Eph/ephrin-signaling complexes.
Ephrin-A5–facilitated cell adhesion is accompanied by recruitment of focal adhesion components. (A) The distribution of EphA3 and of the focal adhesion proteins FAK, PTP-PEST, and paxillin was analyzed in LK63 cells adhering onto FN- or ephrin-A5-Fc–coated glass coverslips by optical sectioning on a confocal microscope. Alexa546 secondary antibody was used to detect α-EphA3 antibody; Alexa488 secondary antibodies were used to detect antibodies against all other proteins. Optical sections at the top of an LK63 cell and at the cell surface facing the bottom of the coverslip are illustrated for the paxillin/EphA3 costained sample. All other micrographs illustrate optical sections at the cell surface facing the coverslip. Scale bar represents 10 μm. (B) The recruitment of focal adhesion proteins to EphA3 was analyzed in α-EphA3 immunoprecipitates from LK63 cells adhering to FN-coated (−) or ephrin-A5-Fc–coated (+) surfaces, using antibodies against FAK, paxillin, and PTP-PEST, as indicated. The levels of each of the tested proteins in parallel cell lysates, as well as EphA3 levels in the IPs, are shown for comparison. (C) The expression of PTP-PEST was silenced by treating LK63 cells with PTP-PEST–specific siRNA; parallel cell cultures were transfected with cyclophilin control-siRNA for 48 hours. Whole cell lysates of stimulated (+) and control (−) cells were probed with antibodies as indicated. (D) To assess a potential role of PTP-PEST in LK63 adhesion, cells with silenced PTP-PEST expression and control siRNA-transfected cells were seeded onto FN- or ephrin-A5–coated glass coverslips; for confocal microscopic analysis, fixed cells were stained with antibodies/secondary antibodies against EphA3 (Alexa546, red) and PTP-PEST (Alexa488, green), and with Alexa647-phalloidin to mark filamentous actin. Representative images are shown. (E) The relative area of Eph clusters/cell, defined as ratio between the cellular footprints of EphA3 and of actin staining, was estimated from image raw data files using analySIS software. Mean and SE are shown, using data from 5 separate fields of view, each containing approximately 20 cells.
Ephrin-A5–facilitated cell adhesion is accompanied by recruitment of focal adhesion components. (A) The distribution of EphA3 and of the focal adhesion proteins FAK, PTP-PEST, and paxillin was analyzed in LK63 cells adhering onto FN- or ephrin-A5-Fc–coated glass coverslips by optical sectioning on a confocal microscope. Alexa546 secondary antibody was used to detect α-EphA3 antibody; Alexa488 secondary antibodies were used to detect antibodies against all other proteins. Optical sections at the top of an LK63 cell and at the cell surface facing the bottom of the coverslip are illustrated for the paxillin/EphA3 costained sample. All other micrographs illustrate optical sections at the cell surface facing the coverslip. Scale bar represents 10 μm. (B) The recruitment of focal adhesion proteins to EphA3 was analyzed in α-EphA3 immunoprecipitates from LK63 cells adhering to FN-coated (−) or ephrin-A5-Fc–coated (+) surfaces, using antibodies against FAK, paxillin, and PTP-PEST, as indicated. The levels of each of the tested proteins in parallel cell lysates, as well as EphA3 levels in the IPs, are shown for comparison. (C) The expression of PTP-PEST was silenced by treating LK63 cells with PTP-PEST–specific siRNA; parallel cell cultures were transfected with cyclophilin control-siRNA for 48 hours. Whole cell lysates of stimulated (+) and control (−) cells were probed with antibodies as indicated. (D) To assess a potential role of PTP-PEST in LK63 adhesion, cells with silenced PTP-PEST expression and control siRNA-transfected cells were seeded onto FN- or ephrin-A5–coated glass coverslips; for confocal microscopic analysis, fixed cells were stained with antibodies/secondary antibodies against EphA3 (Alexa546, red) and PTP-PEST (Alexa488, green), and with Alexa647-phalloidin to mark filamentous actin. Representative images are shown. (E) The relative area of Eph clusters/cell, defined as ratio between the cellular footprints of EphA3 and of actin staining, was estimated from image raw data files using analySIS software. Mean and SE are shown, using data from 5 separate fields of view, each containing approximately 20 cells.
PTP-PEST is known to regulate focal adhesion proteins, and recent studies in PTP-PEST−/− mouse embryonic fibroblasts indicate that its inhibition of spreading relies on direct interaction with paxillin.32 To assess the potential role of PTP-PEST in modulating Eph/ephrin-induced LK63 spreading, we reduced its protein expression level using PTP-PEST–specific siRNA (Figure 3C,D). While this loss of PTP-PEST did not noticeably reduce LK63 cell adhesion onto ephrin-A5 surfaces, we noticed a more spread-out appearance of these cells. Fluorescence confocal microscopy revealed that in the ephrin-facing membrane-proximal part of PTP-PESTsilenced cells, the spread of EphA3 clusters is significantly increased compared with w/t LK63 cells (Figure 3D,E). Considering that PTP-PEST and paxillin are recruited to ephrin-induced Eph clusters (Figure 3A), this finding is consistent with their role in regulating lamellipodia formation and cell spreading during EphA3/ephrin-A5–mediated cell adhesion.
Eph/ephrin-mediated cell-cell adhesion
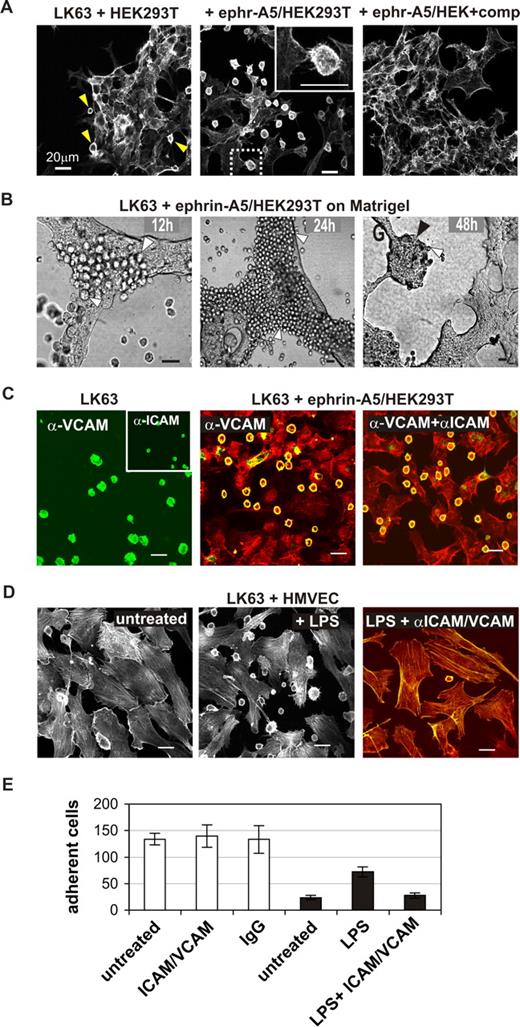
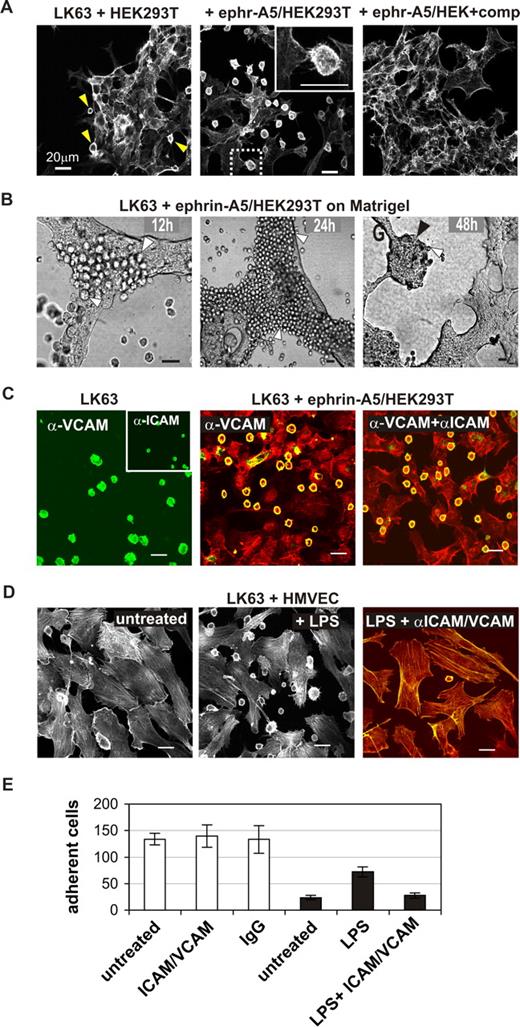
We thought to recapitulate EphA3/ephrin-A5–facilitated cell adhesion by coculturing LK63 cells on monolayers of HEK293T cells, engineered to stably express ephrin-A5.11 Confocal microscopy of phalloidin-stained cells confirmed tight contacts between the LK63 cells and ephrin-A5/HEK293T cells (Figure 4A). Similar to the previous experiments, adherent LK63 cells feature flat, irregular morphology and filopodia-like extensions toward the underlying cell monolayer (Figure 4A inset). In control experiments, parental HEK293T cells with marginal ephrin-A5 expression bound only very few LK63 cells (Figure 4A arrowheads), and adhesion to ephrin-A5/HEK293T cells was blocked effectively in the presence of soluble ephrin-A5-Fc (Figure 4A right panel). Importantly, we found that the adhesion between LK63 and ephrin-A5-HEK293T cells is persistent (Figure 4B): Analysis of cocultures, maintained for 2 days on 3-dimensional Matrigel, revealed tumor-like LK63 cell colonies and suggested that proliferating LK63 daughter cells remain attached to the ephrin-A5/HEK293 monolayer (Figure 4B).
EphA3-mediated LK63 cell adhesion is independent of VCAM and ICAM functions. (A) LK63 cells were cocultured with parental HEK293T cells or ephrin-A5–expressing HEK293T clones (ephr-A5/HEK293T) as indicated; the inset is a magnification of the boxed section in the middle panel. Soluble monomeric ephrin-A5 was added to one of the cultures at 100-fold molar excess as competitive inhibitor of cell-surface ephrin-A5. (B) LK63 cells were cocultured on a layer of ephrin-A5/HEK293T cells in Matrigel. After 12, 24, and 48 hours, cells were imaged by bright-field microscopy. Individual LK63 cells (white arrowheads) remain bound to the ephrin-expressing cells to form large coherent colonies (black arrowhead) after 48 hours of culture. (C) Adhesion of LK63 cells to ephrin-A5/HEK293T cells was monitored in the presence of α-VCAM and α-ICAM antibodies. VCAM and ICAM expression on adherent LK63 cells was assessed by staining with α-VCAM and α-ICAM (inset) antibodies and Alexa488-conjugated secondary antibodies (left). Following 60-minute coculture in the presence of α-VCAM antibodies alone (middle), or together with α-ICAM antibodies (right), the cytoskeleton of fixed cells was stained with rhodamine-phalloidin. α-VCAM and α-ICAM were detected with Alexa488 secondary antibodies; the merged images are shown. (D) LK63 cells were cocultured on a monolayer of untreated (left) or LPS-treated (middle) HMVECS. To affect LPS-induced cell adhesion, HMVECS were incubated with function-blocking α-VCAM and α-ICAM antibodies (right) as indicated. Merged microscopic images (Alexa488, green; rhodamine-phalloidin, red) are shown. Scale bars represent 20 μm. (E) Cell-cell adhesion was quantified by counting LK63 cells remaining attached to untreated, α-ICAM-1/α-VCAM–treated or control IgG-treated ephrin-A5/293 cells (□), or untreated, LPS or LPS and α-ICAM-1/α-VCAM treated HMVECs (■) in a minimum of 4 representative microscopic sections at 10× magnification. Mean cell number and SE are shown.
EphA3-mediated LK63 cell adhesion is independent of VCAM and ICAM functions. (A) LK63 cells were cocultured with parental HEK293T cells or ephrin-A5–expressing HEK293T clones (ephr-A5/HEK293T) as indicated; the inset is a magnification of the boxed section in the middle panel. Soluble monomeric ephrin-A5 was added to one of the cultures at 100-fold molar excess as competitive inhibitor of cell-surface ephrin-A5. (B) LK63 cells were cocultured on a layer of ephrin-A5/HEK293T cells in Matrigel. After 12, 24, and 48 hours, cells were imaged by bright-field microscopy. Individual LK63 cells (white arrowheads) remain bound to the ephrin-expressing cells to form large coherent colonies (black arrowhead) after 48 hours of culture. (C) Adhesion of LK63 cells to ephrin-A5/HEK293T cells was monitored in the presence of α-VCAM and α-ICAM antibodies. VCAM and ICAM expression on adherent LK63 cells was assessed by staining with α-VCAM and α-ICAM (inset) antibodies and Alexa488-conjugated secondary antibodies (left). Following 60-minute coculture in the presence of α-VCAM antibodies alone (middle), or together with α-ICAM antibodies (right), the cytoskeleton of fixed cells was stained with rhodamine-phalloidin. α-VCAM and α-ICAM were detected with Alexa488 secondary antibodies; the merged images are shown. (D) LK63 cells were cocultured on a monolayer of untreated (left) or LPS-treated (middle) HMVECS. To affect LPS-induced cell adhesion, HMVECS were incubated with function-blocking α-VCAM and α-ICAM antibodies (right) as indicated. Merged microscopic images (Alexa488, green; rhodamine-phalloidin, red) are shown. Scale bars represent 20 μm. (E) Cell-cell adhesion was quantified by counting LK63 cells remaining attached to untreated, α-ICAM-1/α-VCAM–treated or control IgG-treated ephrin-A5/293 cells (□), or untreated, LPS or LPS and α-ICAM-1/α-VCAM treated HMVECs (■) in a minimum of 4 representative microscopic sections at 10× magnification. Mean cell number and SE are shown.
To elucidate a potential role of the classic intercellular cell adhesion molecules (ICAM) and vascular cell adhesion molecules (VCAM) in Eph/ephrin–mediated cell-cell adhesion, we examined the effect of function-blocking anti-ICAM and anti-VCAM antibodies on the attachment of LK63 cells to cell-surface ephrin-A5. Immunocytochemical analysis confirmed endogenous expression of VCAM-1 and ICAM-1 (Figure 4C left panel), adhesion molecules that are essential for adhesion and migration of normal lymphocytes and leukemia cells.26 Accordingly, LK63 cell adhesion to lipopolysaccharide (LPS)–treated, activated human microvascular endothelial cells was effectively inhibited by function-blocking anti-ICAM and anti-VCAM antibodies (Figure 4D,E). By contrast, the anti–VCAM-1/anti–ICAM-1 antibodies (Figure 4C,E) did not affect LK63 adhesion to ephrin-A5/HEK293T cells, suggesting that here the EphA3/ephrin-A5 contact provides the principle tether to initiate cell-cell adhesion.
Binding of surface-tethered ephrin-A5 to EphA3 on LK63 cells does not lead to ADAM10-mediated cleavage and internalization
Because contact-dependent cell-cell repulsion between A-type Eph- and ephrin-expressing cells relies on cleavage of cell-surface ephrin by the ADAM10 metalloprotease,10,11 we assessed if Eph/ephrin-mediated LK63 cell adhesion coincided with the absence of ephrin shedding. Cleavage and internalization of ephrin-A5 can be monitored by exposing EphA3-expressing cells to Alexa564ephrin-A5-Fc coated beads11 : Confocal time-lapse microscopy revealed rapid binding of these beads to EphA3/HEK293 and to LK63 cells (Figure 5A,C). In addition, ephrin-A5 shedding in EphA3/HEK293 cells,11 resulting in dispersion of ephrin-associated fluorescence across the cell surface and internalization into cytosolic vesicles (Figure 5A; Video S3), was blocked with the pan-specific metalloprotease inhibitor 1,10-O-phenanthroline, in agreement with the role of ADAM10 as ephrin-A5 sheddase.11 By contrast, ephrin-A5 cleavage or internalization into LK63 cells was not evident (Figure 5C), although the ephrin-A5–loaded beads remained tightly cell-bound during the experiment (Video S4). As this lack of ephrin shedding could potentially be explained by absence of ADAM10 in LK63 cells, we assessed its expression and recruitment to EphA3. Reverse-transcription–polymerase chain reaction (RT-PCR) analysis confirmed normal ADAM10 expression (data not shown) and immunoblots of LK63 or EphA3/HEK293 cell lysates revealed comparable levels of ADAM10 coprecipitating with ephrin-ligated EphA3 from both cells (Figure 5D). Together these experiments suggest that it is not the absence of EphA3-associated ADAM10 that is responsible for the persisting LK63 adhesion to cell-surface ephrin-A5.
Eph/ephrin-mediated cell-cell repulsion, but not adhesion, leads to ephrin-A5 cleavage by a metalloprotease. Alexa546ephrin-A5-Fc–coated beads were added to cultures of EphA3/HEK293 cells (A), EphA3/HEK293 cells treated (4 hours) with the metalloprotease-inhibitor 1,10-O-phenanthroline (B), or LK63 cells (C). Cells were imaged by time-lapse confocal fluorescence microscopy to illustrate cleavage and internalization of Alexa546ephrin-A5. Representative images at indicated times (minutes) after addition of ephrin-coated beads are shown. In panel A, cleaved Alexa546ephrin-A5 outlines the cell perimeter, and at later time points also labels cytosolic compartments. Phenanthroline-treated EphA3/HEK293 (B) and LK63 cells (C), lacking cleaved Alexa546ephrin-A5, are not visible by fluorescence microscopy, and merged bright-field (gray) and fluorescence (red pseudocolor) images are shown to illustrate ephrin-A5-Fc–coated beads in relation to the cells. Corresponding videos for EphA3/293T and LK63 cells are provided as Video S3 and Video S4, respectively. Scale bars represent 10 μm. (D) Anti-EphA3 immunoprecipitates from nonstimulated (−) or ephrin-A5-Fc–stimulated (+) LK63 or EphA3/293T cells were analyzed by Western blot with anti-ADAM10 and anti-EphA3 antibodies as indicated. p and m indicate the pro (unprocessed) and mature (processed) forms of ADAM10.
Eph/ephrin-mediated cell-cell repulsion, but not adhesion, leads to ephrin-A5 cleavage by a metalloprotease. Alexa546ephrin-A5-Fc–coated beads were added to cultures of EphA3/HEK293 cells (A), EphA3/HEK293 cells treated (4 hours) with the metalloprotease-inhibitor 1,10-O-phenanthroline (B), or LK63 cells (C). Cells were imaged by time-lapse confocal fluorescence microscopy to illustrate cleavage and internalization of Alexa546ephrin-A5. Representative images at indicated times (minutes) after addition of ephrin-coated beads are shown. In panel A, cleaved Alexa546ephrin-A5 outlines the cell perimeter, and at later time points also labels cytosolic compartments. Phenanthroline-treated EphA3/HEK293 (B) and LK63 cells (C), lacking cleaved Alexa546ephrin-A5, are not visible by fluorescence microscopy, and merged bright-field (gray) and fluorescence (red pseudocolor) images are shown to illustrate ephrin-A5-Fc–coated beads in relation to the cells. Corresponding videos for EphA3/293T and LK63 cells are provided as Video S3 and Video S4, respectively. Scale bars represent 10 μm. (D) Anti-EphA3 immunoprecipitates from nonstimulated (−) or ephrin-A5-Fc–stimulated (+) LK63 or EphA3/293T cells were analyzed by Western blot with anti-ADAM10 and anti-EphA3 antibodies as indicated. p and m indicate the pro (unprocessed) and mature (processed) forms of ADAM10.
LK63 cells feature marginal, ephrin-A5–triggered EphA3 phosphorylation despite expression of an intact EphA3
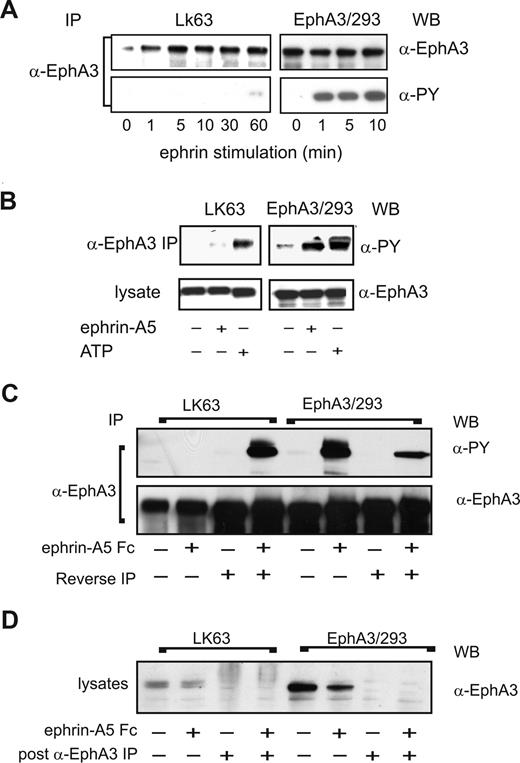
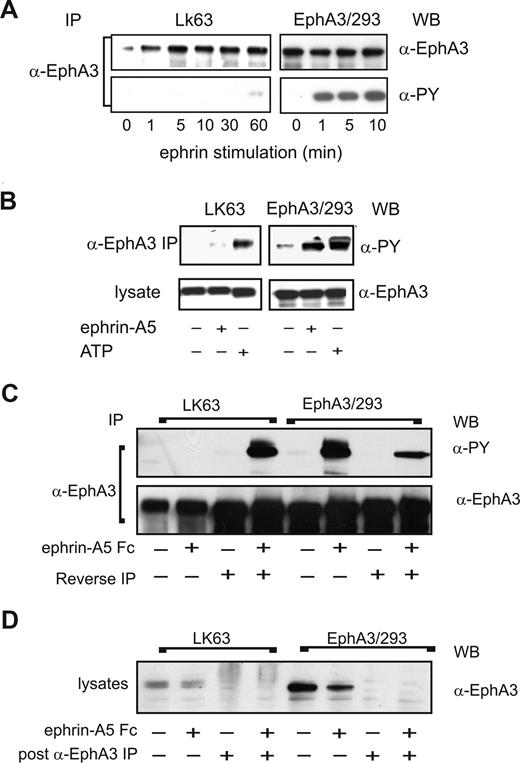
We next sought to examine potential differences in EphA3 signaling during EphA3-mediated cell adhesion or cell detachment/repulsion. Immunoprecipitation analysis of ephrin-A5–stimulated, EphA3-expressing cells revealed striking differences in the EphA3 tyrosine phosphorylation levels of the 2 cell types (Figure 6A): In agreement with published data,9 ephrin-A5 stimulation of EphA3/HEK293 cells triggers rapid EphA3 phosphorylation within 10 minutes. By contrast, EphA3 is only marginally phosphorylated in LK63 cells, even after prolonged (60 minutes) stimulation. We thus examined whether the EphA3 kinase is functional in LK63 cells. Incidentally, EphA3 was originally cloned from LK63 cells, with no evidence for mutations, rearrangements or chromosomal translocation.24,33 Furthermore, nested RT-PCR of the LK63 cell EphA3 mRNA to detect potential somatic mutations within the ephrin-binding or kinase domains34 revealed only a single prominent transcript encoding wild-type EphA3; smaller, low-abundance RT-PCR products with frame shift mutations notionally encoded a small 21-residue N-terminal EphA3 fragment (data not shown).
EphA3 kinase in LK63 cells is inhibited by strong PTP activity. (A) EphA3 tyrosine phosphorylation in α-EphA3 immunoprecipitates (IPs) of whole cell lysates from LK63 or EphA3/HEK293T cells stimulated for indicated times with preclustered ephrin-A5-Fc was examined with α-phosphotyrosine (PY) and α-EphA3 antibodies. (B) In vitro kinase activity was assessed in α-EphA3 IPs from nonstimulated (−) cells, subjected to stringent washes prior to incubation with exogenous ATP (+) and α-PY Western blot analysis. IPs from lysates of nonstimulated (−) or ephrin-A5-Fc–stimulated (+) cells are analyzed for comparison. (C) EphA3 was depleted by immunoprecipitation from lysates of ephrin-A5-Fc–stimulated cells; α-EphA3 IPs from LK63 cells were incubated (20 minutes) in EphA3-depleted cytosolic fractions of EphA3/HEK293 cells (reverse IP) and vice versa. The tyrosine phosphorylated EphA3 from these in vitro kinase assays was compared with the in vivo phosphorylation levels in the corresponding IPs from both cell types. α-EphA3 Western blots of parallel samples indicate that similar quantities of EphA3 were analyzed in all samples. (D) EphA3 levels in the cytosolic fractions used in the kinase assays were determined by Western blot analysis before and after immunodepletion.
EphA3 kinase in LK63 cells is inhibited by strong PTP activity. (A) EphA3 tyrosine phosphorylation in α-EphA3 immunoprecipitates (IPs) of whole cell lysates from LK63 or EphA3/HEK293T cells stimulated for indicated times with preclustered ephrin-A5-Fc was examined with α-phosphotyrosine (PY) and α-EphA3 antibodies. (B) In vitro kinase activity was assessed in α-EphA3 IPs from nonstimulated (−) cells, subjected to stringent washes prior to incubation with exogenous ATP (+) and α-PY Western blot analysis. IPs from lysates of nonstimulated (−) or ephrin-A5-Fc–stimulated (+) cells are analyzed for comparison. (C) EphA3 was depleted by immunoprecipitation from lysates of ephrin-A5-Fc–stimulated cells; α-EphA3 IPs from LK63 cells were incubated (20 minutes) in EphA3-depleted cytosolic fractions of EphA3/HEK293 cells (reverse IP) and vice versa. The tyrosine phosphorylated EphA3 from these in vitro kinase assays was compared with the in vivo phosphorylation levels in the corresponding IPs from both cell types. α-EphA3 Western blots of parallel samples indicate that similar quantities of EphA3 were analyzed in all samples. (D) EphA3 levels in the cytosolic fractions used in the kinase assays were determined by Western blot analysis before and after immunodepletion.
To confirm our conclusion from these experiments that LK63 cells contain a functional EphA3 kinase, we analyzed in vitro phosphorylation profiles of EphA3 IPs, prepared from ephrin-A5–stimulated or untreated EphA3/HEK293 cells or LK63 cells. We compared these with IPs that had been incubated with exogenous ATP prior to Western blot analysis (Figure 6B). Rigorous washing steps, previously used for the isolation of EphA3,23 ensured that phosphorylation in these samples was due to EphA3 kinase and not due to an associated enzyme activity. Indeed, incubation with ATP yielded robust EphA3 phosphorylation in LK63-derived IPs, similar to those of ephrin-stimulated EphA3/HEK293 cells (Figure 6B), suggesting that the lack of notable EphA3 phosphorylation in LK63 cells may be due to endogenous down-regulation of kinase activity.
We tested this hypothesis by isolating full-length EphA3 from ephrin-stimulated or from nonstimulated LK63 or EphA3/HEK293T cells by immunoprecipitation with anti-EphA3 mAb Sepharose. We then exposed the extensively washed, LK63-derived anti-EphA3 IPs to EphA3/HEK293T cell lysates that had been depleted of all endogenous EphA3 (Figure 6D). In the same manner, EphA3 from EphA3/HEK293T cells was exposed to EphA3-depleted LK63 lysates (Figure 6C, reverse IP). Strikingly, incubation of EphA3 from ephrin-stimulated EphA3/293T cells in LK63 lysate (reverse IP) reduced the EphA3 phosphorylation level substantially (Figure 6C, EphA3/293). On the other hand, exposure of LK63-derived EphA3 to EphA3/HEK293T lysate yielded dramatically increased EphA3 phosphorylation (Figure 6C, LK63). Together these experiments indicate that strong endogenous PTP activity down-regulates the EphA3 kinase in LK63 cells.
PTPs control EphA3 phosphorylation and the cell-morphologic responses to ephrin-A5 exposure
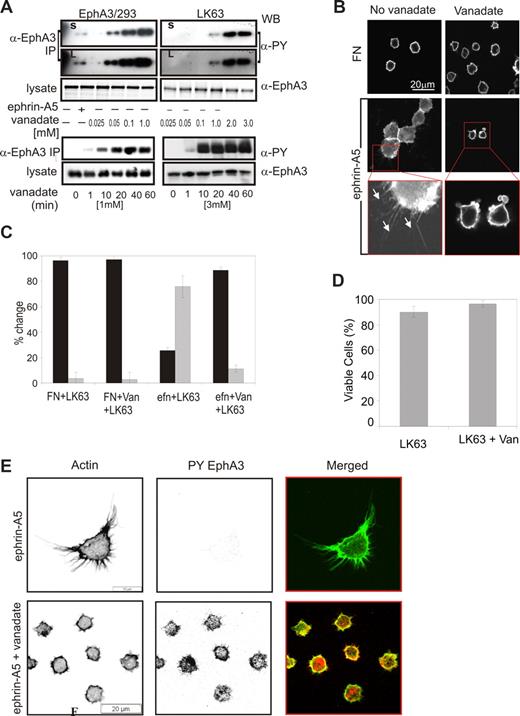
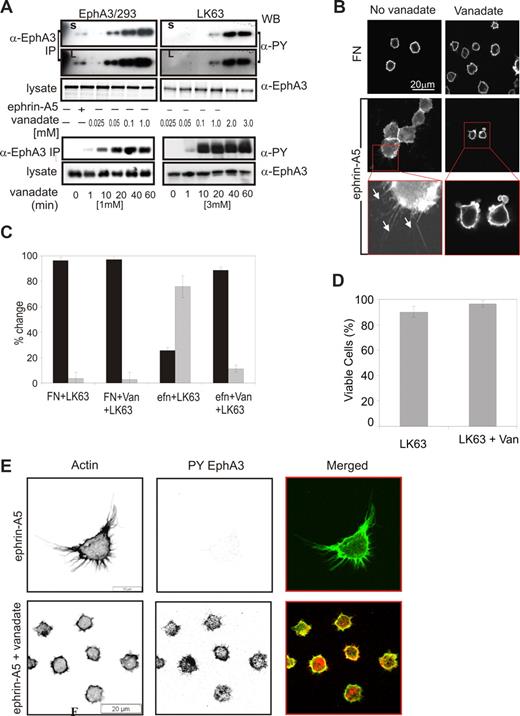
The observed cell type–specific differences in EphA3 phosphorylation levels suggested that PTP regulation of Eph kinase activity may be critical in switching between EphA3-triggered cell-cell repulsion and adhesion. We previously demonstrated that sodium pervanadate treatment35 of EphA3-GFP–expressing HEK293T cells leads to dose-dependent EphA3 phosphorylation in the absence of ephrin-A5 stimulation.4 In agreement, treatment of EphA3/293T cells, but also of LK63 leukemia cells with cell-permeable sodium pervanadate (Figure 7A), resulted in dose-dependent increases in EphA3 phosphorylation. However, the concentration of pervanadate required to achieve comparable EphA3 phosphorylation levels in the 2 cell types varied significantly: 0.05 mM pervanadate was sufficient to cause notable EphA3 phosphorylation in EphA3/293T cells (Figure 7A top panel), whereas a 20- to 40-fold higher (1-2 mM) concentration was required in LK63 cells (Figure 7A, LK63). Pervanadate-induced EphA3 phosphorylation in both cell lines rapidly increased to an apparent maximum after 20 minutes (Figure 7A bottom panels). We confirmed this apparent difference in the sensitivity to global PTP inhibition by incubating cells with increasing concentrations of hydrogen peroxide. Apparent concentrations between 0.5 to 4 mM H2O2 triggered notable EphA3 phosphorylation in EphA3/293T cells and EphA3/AO2 melanoma cells, whereas an approximately 40-fold higher concentration (50 mM) was required to induce similar phosphorylation levels in LK63 cells (Figure S4). We examined PTPs implemented in Eph signaling, including LMW-PTP, SHP2,36-39 and PTP-PEST (Figure 3), for their potential role in regulating EphA3 phosphorylation. Coprecipitation analysis confirmed that in LK63 and EphA3/293T cells PTP-PEST was the PTP recruited most notably to ephrin-stimulated EphA3 (Figure S5a). However, its silencing or overexpression did not affect EphA3 phosphorylation levels directly (Figure S5c), arguing for a role in downstream signaling rather than regulation of EphA3 phosphorylation.
Ephrin-A5–induced LK63 cell adhesion and spreading result from PTP–down-modulated EphA3 kinase activity. (A) The dose response (top panels) and time dependence (bottom panels) of sodium pervanadate (vanadate) induced EphA3 phosphorylation in EphA3/HEK293T and LK63 cells were analyzed in α-PY Western blots of EphA3 IPs. For comparison, the ephrin-A5-Fc–induced phosphorylation in EphA3/HEK293 cells is shown. Short (S) and long (L) exposures of the dose-response panels are shown to emphasize significantly different phosphorylation levels. (B) Adhesion and spreading of LK63 cells on ephrin-A5 surfaces is abrogated in the presence of 1 mM sodium pervanadate: The actin cytoskeleton of LK63 cells adhering to ephrin-A5 surfaces was imaged by confocal microscopy. A 2.7-fold magnification reveals filopodia-like cell processes in the adherent LK63 cells (white arrows), and membrane blebbing in the pervanadate-treated cells exposed to the ephrin-A5 surface. Scale bar represent 20 μm. (C) The number of cells with round (contracted) morphology (■) and of spread cells with filopodia-like extensions ( ) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.
) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.
Ephrin-A5–induced LK63 cell adhesion and spreading result from PTP–down-modulated EphA3 kinase activity. (A) The dose response (top panels) and time dependence (bottom panels) of sodium pervanadate (vanadate) induced EphA3 phosphorylation in EphA3/HEK293T and LK63 cells were analyzed in α-PY Western blots of EphA3 IPs. For comparison, the ephrin-A5-Fc–induced phosphorylation in EphA3/HEK293 cells is shown. Short (S) and long (L) exposures of the dose-response panels are shown to emphasize significantly different phosphorylation levels. (B) Adhesion and spreading of LK63 cells on ephrin-A5 surfaces is abrogated in the presence of 1 mM sodium pervanadate: The actin cytoskeleton of LK63 cells adhering to ephrin-A5 surfaces was imaged by confocal microscopy. A 2.7-fold magnification reveals filopodia-like cell processes in the adherent LK63 cells (white arrows), and membrane blebbing in the pervanadate-treated cells exposed to the ephrin-A5 surface. Scale bar represent 20 μm. (C) The number of cells with round (contracted) morphology (■) and of spread cells with filopodia-like extensions ( ) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.
) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.
We assessed whether down-modulation of EphA3 kinase activity by PTPs controls ephrin-mediated cell adhesion, by monitoring the cell morphology and degree of adhesion of LK63 cells that had been treated with pervanadate. In untreated samples, most cells tightly attached to the ephrin-coated surface (Figure 7B) and only approximately 25% of the cells remained spherical (Figure 7C). By comparison, on a FN-coated surface 90% to 96% of cells retained the round morphology of suspension cells. Pervanadate treatment reversed cell adhesion to ephrin, and more than 88% of cells on the ephrin-coated surface featured contracted cell morphology and condensed cortical actin cytoskeleton (Figure 7B,C Efn + LK63 + van). A vital dye exclusion assay demonstrated that the pervanadate-induced change in cell morphology is not due to any loss in cell viability (Figure 7D). Furthermore, staining with anti–EphA3-PY antibodies and Alexa488-phalloidin revealed pronounced EphA3 phosphorylation and concurrent contraction of the cytoskeleton (Figure 7E bottom panels) only after LK63 cells on ephrin-A5–coated surfaces had been treated with sodium pervanadate, while no phosphorylated EphA3 was detected in untreated cells (Figure 7E top panels). Together our data suggest that ephrin-A5–dependent adhesion and spreading of the leukemia cells can be reversed by PTP inactivation and concurrent activation of EphA3 kinase signaling.
Discussion
Considerable evidence has established cell-cell repulsion as paradigm and default mechanism of Eph function,8 but equally well documented, Eph/ephrin-facilitated adhesive cell-cell contacts are critical during normal and oncogenic development.1,13 Such opposite outcomes pose an apparent paradox and suggest a molecular switch controlling the direction of cellular responses to Eph ligation.40 We now demonstrate in typically nonadherent pre-B ALL cells with high endogenous EphA3 expression levels that a PTP-regulated balance between the active and inactive EphA3 kinase directs the response to ephrin exposure to cause sustained, integrin-independent cell adhesion.
In previous studies, we found that HEK293T and melanoma cells respond to ephrin-A5 stimulation with marked Eph phos-phorylation, Rho-mediated cytoskeletal contraction, and dose-dependent cell repulsion.9 Signaling components that leads to Eph-triggered repulsion, include Rho-GTPases, PI3-kinase, RAS/ERK/MAP kinase pathways, and crosstalk with integrin pathways to down-modulate focal adhesion complexes.1,41 In agreement, we confirm here that adherent, EphA3-negative AO2 melanoma cells9 can be manipulated to detach upon ephrin-A5 exposure by stably expressing w/t EphA3, but not mutants lacking the cytoplasmic domain, kinase activity, or consensus SH2-domain docking sites.
It is currently less clear how the same Eph/ephrin interaction can trigger the opposite response and lead to increased cell-cell or cell-matrix adhesion,1 even involving similar signaling components.39,42 It has been suggested that attenuation of cell contraction signals through coexpression of signaling-compromised receptor splice variants15 and low ephrin surface concentrations16,43 may result in integrin-dependent39,43-45 or -independent adhesion.42 In addition, down-modulation of EphA signaling by interaction with ephrins on the same cell surface has been suggested,46 although in contradiction, earlier studies elegantly demonstrated that Eph and ephrin signaling clusters are well segregated when they are coexpressed on the same cell membrane.47
To some extent, the difficulty of dissecting the underlying mechanisms experimentally may lie in the use of adherent cell lines, which usually deploy several adhesion mechanisms for cell-cell and cell-substratum contacts. In this regard, our study with LK63 cells, typically growing in suspension and adhering via ICAM and VCAM interactions only after cytokine-mediated integrin activation,48 provided a simpler system to examine the adhesion to cell-surface ephrin. Emerging literature provides evidence for a role of Eph signaling in leukocyte biology, such as development49 and migration22,50 of T cells, and integrin-mediated adhesion of T-cell lymphomas51 and dendritic cells.52 However, despite the expression of ICAM1, VCAM1 (Figure 4C), and of α4- and α5-integrin on LK63 cells, we did not detect β1-integrins in EphA3 signaling complexes (not shown) and found no evidence for an involvement of these classical adhesion molecules in sustained Eph/ephrin-mediated adhesion.
First, LK63 cell binding to ephrin-A5–decorated but not to FN-coated tissue culture surfaces results in polarization, flattening, and spreading. LK63 adhesion and spreading on ephrin-A5 surfaces is inhibited with soluble ephrin-A5 and largely diminished by EphA3 silencing, respectively, together demonstrating that ephrin-A5/EphA3 interactions provide the essential tether for this cell adhesion.
Second, integrin-mediated LK63 adhesion to ephrin-A5/HEK293T cells is unaffected by function-neutralizing anti–ICAM-1 and anti–VCAM-1 antibodies, indicating an integrin-independent mechanism, similar to that reported previously for adhesion and spreading of EphA2-overexpressing NIH3T3 fibroblasts to ephrin-A1–coated surfaces.42
Third, confocal microscopy of adherent LK633 cells reveals a distinct pattern of Eph clusters that anchor the cell to the ephrin-A5 surface: colocalization and association with FAK, paxillin, and PTP-PEST suggests their productive association with the cell cytoskeleton. In agreement, real-time imaging reveals attached cells undergoing dramatic morphologic changes and generating radiating patterns of Eph clusters and actin retraction fibers facing the ephrin surface. While a significant reduction of their numbers by cytochalasin D treatment would argue for the involvement of actin remodeling, the remaining fibers are significantly longer, suggesting contribution also of an actin-independent process. Intriguingly, the lamellipodia on adherent LK63 cells appear reminiscent of those developing during transient spreading of mature B cells that accompanies formation of the immunologic synapse for cell-surface antigens53 ; however, LK63 cell spreading is persistent and leads to colonies of adherent pre-B cells.
Finally, the conceptually obvious requirement, of cleaving the Eph/ephrin tether to achieve repulsion,10,11 infers that lack of cleavage promotes cell-cell attachment. Indeed, our experiments demonstrate that while EphA3/HEK293T cells promote shedding of ephrin-A5 from ephrin-coated beads, no cleavage or internalization is seen with LK63 cells, which remain attached to ephrin-coated beads. We demonstrated recently that ephrin cleavage in trans relies on the interaction of the high-affinity Eph/ephrin complex with ADAM10.11 Surprisingly, our present study suggests increased ADAM10/EphA3 association after ephrin-A5 binding also in LK63 cells. However, this interaction is insufficient to trigger ephrin cleavage. In this context, it is noteworthy that ADAM10 shedding of substrates, including ephrin-A2,10 is regulated by tyrosine kinase activity,54 inferring that in LK63 cells the inactive EphA3 kinase may prevent productive ADAM10 association and ephrin-A5 cleavage.
So what causes this switch from repulsive, kinase-dependent to adhesive, kinase-independent EphA3 signaling? While EphA3 mutations potentially effecting kinase activity have been identified in colon cancer cells,34 we did not find evidence for mutant EphA3 mRNA in LK63 cells. However, our experiments demonstrate that an unusually strong EphA3-specific PTP activity prevents tyrosine phosphorylation, even after ephrin-mediated receptor clustering. Accordingly, we find that in LK63 cells compared with cell lines with productive EphA3 kinase activity, several-fold increased concentrations of global PTP inhibitors are required to achieve ephrin-independent EphA3 autophosphorylation.
Several PTPs have been implicated in Eph signaling, including LMW-PTP,36,38,55 SHP2,39 and “PTP receptor type O,” which is involved in axon guidance.56 Of particular interest for our study, overexpression of SHP2 was found to correlate with hyperproliferative capacity and decreased differentiation of primary leukemia cells and leukemia cell lines,57 while somatic SHP2 gain-of-function mutations contribute to leukemogenesis in B-ALL patients.58 However, our analysis of LK63 cells did not indicate elevated SHP2 mRNA levels or the presence of somatic SHP2 mutations.
We also considered LMW-PTP as a potential regulator of EphA3 activity, as it was reported to regulate EphA255 and EphA838 phosphorylation in vitro and to be required for ephrin-induced cell adhesion of EphB1-positive endothelial cells.36 Interestingly, in EphA3 the critical tyrosine-Y929 required for the LMW-PTP interaction and conserved in all other Eph family members is replaced by a cysteine; accordingly, we did not observe LMW-PTP recruitment to EphA3 clusters.
On the other hand, we observed marked recruitment of PTP-PEST to EphA3 in ephrin-A5–exposed LK63 (Figure 3) and EphA3/HEK293T cells (Figure S5A). PTP-PEST has a prominent role as phosphatase and scaffolding protein during cytoskeletal reorganization,59 but to our knowledge this is the first time it has been implicated with Eph signaling. Interestingly, in B cells PTP-PEST is constitutively associated with focal adhesion components including paxillin, and is responsible for the dephosphorylation of Pyk2, FAK, and p130cas.60 We did not find convincing evidence for a role in regulating Eph kinase activity, but our experiments clearly demonstrate its role in EphA3-induced cytoskeletal reorganization, a finding that warrants further investigation: LK63 cells with siRNA-reduced PTP-PEST levels display a more extensive spread of Eph clusters, which in accordance with a role of PTP-PEST in focal adhesion disassembly32 suggests a reduced capacity of these cells to retract lamellipodia.
The notion that Eph/ephrin signaling can modulate cell-cell adhesion in opposite directions by activating PY-dependent and -independent signaling pathways has been appreciated for some time,40 but underlying signaling mechanisms have remained controversial.14,15,39,42,43,46,47 We now have demonstrated that in tumor cells the balance between the Eph receptor tyrosine kinase and the corresponding tyrosine phosphatase activity provides a molecular switch that dynamically shifts the response to ephrin ligation from cell-cell repulsion to adhesion. Variable expression levels, even in different clones of the same tumor cell, will affect this balance and may explain some of the seemingly contradictory responses to ephrin stimulation that have been reported. It is likely that identification of the tyrosine phosphatases regulating Eph receptors will provide critical clues for the understanding of Eph function in oncogenesis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank N. Tonks (Cold Spring Harbor, NY) for providing α-PTP-PEST mAb, I. Wicks (The Walter and Eliza Hall Institute of Medical Research [WEHI], Melbourne, Australia) for α-ICAM and α-VCAM antibodies, and P. Chiarugi for α-LMW-PTP antibodies. We gratefully acknowledge the help of Ian Harper and Stephen Firth (Monash University Micro Imaging, Victoria, Australia) with confocal microscopy.
This work was supported by National Health & Medical Research Grant 234707. S.H.W.-K. is a CJ Martin Fellow, and M.L. is a Senior Research Fellow of the National Health and Medical Research Council (NH&MRC).
Authorship
Contribution: S.H.W.-K. and E.N. designed and performed the majority of the research, prepared figures, and contributed to writing the paper; K.G. and S.A. performed the analysis of paxillin and FAK association/colocalization; M.M. analyzed PTP-PEST association; T.Y. performed EphA3 expression analysis; A.W.B. provided vital reagents and contributed to data analysis; N.R.P. analyzed the EphA3 mRNA transcript in LK63 cells; M.L. provided the concept, supervised and evaluated all of the research, and wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martin Lackmann, Department of Biochemistry & Molecular Biology, PO Box 13D, Monash University, Clayton, Victoria 3800, Australia; e-mail: martin.lackmann@med.monash.edu.au.
References
Author notes
*S.H.W.-K. and E.N. are joint first authors.



 ) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.
) of filamentous protrusions of LK63 cells, treated with cytochalasin D (cytochal D) or solvent (DMSO control), was quantitated in 10 confocal microscopic field (100× lens) using IMARIS Filament Tracer software. Mean and SE estimates from n = 27 (DMSO) and n = 48 (control) cells are shown. Statistical analysis suggests significant differences (P < .001) in number and length of filamentous protrusions between treated and untreated cells.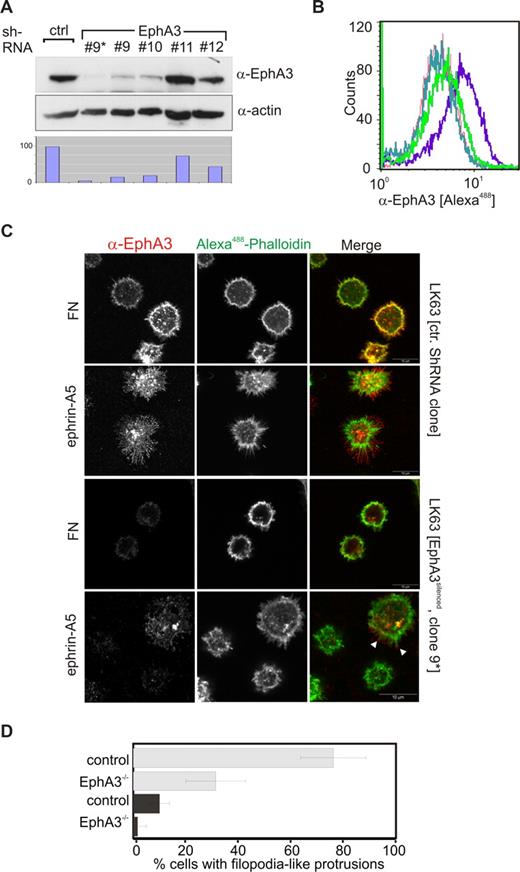


 ) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.
) was counted by a blinded observer in a minimum of 3 microscopic fields. Mean and SE were estimated from data representative of 2 independent experiments. (D) The effect of the sodium pervanadate treatment on LK63 cell viability was assessed by trypan-blue exclusion. (E) Confocal microscopic images of LK63 cells seeded onto ephrin-A5/FN–coated surfaces and probed with Alexa488-phalloidin and anti–PY-EphA3/Alexa546 secondary antibodies. Cells in the lower panels were treated with 1 mM sodium pervanadate as indicated. Scale bars represent 20 μm.