Abstract
Currently, PRP is used clinically as a topical application to augment healing of both surgical and chronic, non-healing wounds. PRP exerts its efficacy by releasing growth factors that enhance clot formation and vasculogenesis. We conducted in vitro functional analyses comparing PRP and/or UCB-derived monocytes including cytokine production, cell migration, and HUVEC tubule formation in standard matrigel assays to test the hypothesis whether topical concurrent application of PRP and UCB-derived monocytes may serve to augment wound healing beyond the ability of topical PRP alone.
UCB was obtained according to institutional guidelines and collected into bags with citrate dextrose (Allegiance). MNC were separated on a Histopaque-1077 (Sigma) density gradient. UCB CD14+ monocytes were isolated using AutoMACS magnetic cell sorter (Miltenyi), and cultured in RPMI with 1% HSA. PRP was isolated from adult peripheral blood by centrifugation. To determine if the addition of UCB monocytes may improve the wound healing effects of PRP alone, VEGF, bFGF, and PDGF secreted by monocytes alone, PRP alone, and monocytes supplemented with 3% PRP, were measured by ELISA (RayBiotech) daily over 4 days.
PRP alone elicited no measurable secretion of VEGF. UCB-derived monocytes alone showed a low, constant production of VEGF over the four days of 0.868ng/ml. PRP supplemented with UCB-derived monocytes secreted VEGF at a 7.6-fold increase over either PRP or UCB monocytes alone, with a peak production at day three of 6.638ng/ml. PRP alone produced no measurable secretion of bFGF over the four day time course. UCB monocytes alone secreted bFGF in an increasing manner during the same time course. During days one to four, bFGF secreted by UCB monocytes was 33.8, 27.9, 115.4, and 452.1pg/ml, respectively. The presence of PRP suppressed this secretion, as PRP combined with UCB monocytes constantly secreted bFGF at an average of 39.9pg/ml throughout days one to four. Finally, secretion of PDGF was highest in conditions including PRP combined with UCB monocytes. PRP alone constantly produced PDGF at an average of 3,144pg/ml over a 4 day time course. Monocytes alone secreted PDGF constantly at a lower average of 597pg/ml. PRP supplemented with UCB monocytes secreted PDGF at a concentration 5.9-fold higher than PRP alone, producing an average of 18,534pg/ml over four days.
To determine whether UCB-derived monocytes respond to cytokines elicited by injured vascular endothelial cells, we measured UCB-derived monocyte chemotaxis to HUVEC conditioned media in hypoxic conditions (5% O2). Migration experiments were conducted using Transwell plates with 8.0 μm pores. Monocytes were cultured in RPMI with 5% FBS at a concentration of 5×106/ml and were allowed to migrate for four hours to either: media alone, PRP, HUVEC-conditioned media, or HUVEC-conditioned media supplemented with PRP. We observed a 3.3 fold increase in the migration of the monocytes to HUVECconditioned media over that of basal media. Experiments with PRP alone showed no significant difference in monocyte migration compared to basal medium.
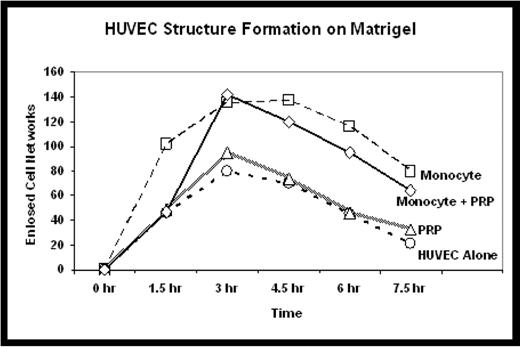
To determine whether UCB-derived monocytes may serve to augment endothelial cell function beyond that elicited by PRP alone, matrigel experiments were conducted by adding HUVEC in endothelial cell basal medium. HUVEC tubule formation in matrigel in basal media was compared in three conditions including media conditioned with: 1) PRP alone, 2) UCB monocytes alone, or 3) a combination of PRP + UCB monocytes. We compared the kinetics and stability of enclosed endothelial cell networks formed by HUVEC. No significant benefit was seen with addition of PRP conditioned media. The number of enclosed endothelial cell networks reached a higher maximum with the addition of monocyte conditioned media (137 networks) as well as PRP + monocyte conditioned media (142 networks), compared to non-conditioned media (80 networks). UCB monocyte and PRP + UCB monocyte conditioned media also improved the stability of the enclosed cell networks in culture as structures persisted beyond 24h, while none were present in the PRP-conditioned or non-conditioned media matrigel cultures.
In summary, these in vitro analyses support the hypothesis that UCB-derived monocytes significantly improve efficacy of PRP alone in augmentation of vasculogenesis and cell migration to vascular endothelial injury, thereby supporting potential concurrent topical application of UCB-derived monocytes to PRP in wound healing.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author