To the editor:
Hepatitis C virus (HCV) infection can lead to B-cell malignancy via direct infection and transformation of B lymphocytes, or via indirect transformation by chronic antigen-driven stimulation.1-3 Both mechanisms may occur simultaneously, as we previously reported in a case of HCV infection followed by plasma-cell leukemia (PCL), where blasts were infected with HCV and the monoclonal immunoglobulin (Ig) they produced was directed against the core protein of the virus.4 Approximately 10% of HCV-positive patients responding to viral infection with poly- or oligoclonal Ig develop a monoclonal Ig, the specificity of which is usually unknown.5,6 The present study aimed at evaluating the link between chronic HCV-antigen–driven stimulation and plasma-cell transformation by determining the specificity of monoclonal Ig developed in the context of HCV infection.
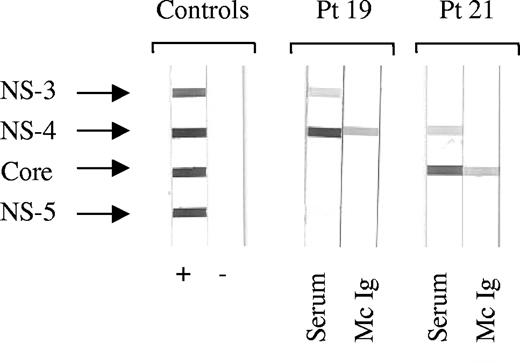
Over a period of 13 months beginning in January 2002, all sera from patients consulting or hospitalized at the Centre Hospitalier Universitaire of Nantes that the Biochemistry Laboratory declared positive for monoclonal Ig were systematically tested for the presence of HCV RNA and anti-HCV Ig. Among the 700 patients studied, 10 (1.4%) were found positive for HCV; 2 of these 10 patients were also positive for human immunodeficiency virus. Only 3 of 10 patients were infected with HCV genotype 1, the predominant genotype in western France; 7 of 10 patients were infected with genotypes 2 (5 patients), 3, or 5 (Table 1), suggesting contamination from blood products before 1980. Purification of the monoclonal Ig was achieved for 7 of 10 patients. Using immunoblotting, the purified monoclonal Ig (2 IgG, 1 IgA, 1 IgM) of 4 patients, all with genotype 2, recognized the C22-3 fragment of HCV-core protein; 2 (IgG) recognized NS-4 and 1 did not recognize HCV (Table 1, Figure 1). Among the 4 patients with anti-HCV core monoclonal Ig, 2 presented with mixed (type II) cryoglobulinemia (patients 12 and 20) and one was diagnosed with multiple myeloma (patient 21). Anti-HCV treatment resulted in the disappearance of the monoclonal Ig (patients 8, 9, and 10).
Determination of HCV protein specificity by recombinant immunoblot assay. For each patient (Pt), serum (S) and purified monoclonal Ig (Mc Ig) were incubated with blots carrying recombinant HCV nonstructural (NS) and core proteins. Serum and Mc Ig protein concentration was 4 μg/blot. Depending on the patient's Mc Ig, the horseradish peroxidase conjugates used were anti-γ, anti-α, or anti-μ chains. Typical results are shown for Mc Ig specific for HCV NS-4 protein (Pt 19) and Mc Ig specific for HCV core protein (Pt 21).
Determination of HCV protein specificity by recombinant immunoblot assay. For each patient (Pt), serum (S) and purified monoclonal Ig (Mc Ig) were incubated with blots carrying recombinant HCV nonstructural (NS) and core proteins. Serum and Mc Ig protein concentration was 4 μg/blot. Depending on the patient's Mc Ig, the horseradish peroxidase conjugates used were anti-γ, anti-α, or anti-μ chains. Typical results are shown for Mc Ig specific for HCV NS-4 protein (Pt 19) and Mc Ig specific for HCV core protein (Pt 21).
Altogether, for all but one patient presenting with monoclonal Ig in the context of HCV infection, the monoclonal Ig was directed against the virus. Taking into account the first reported case,4 2 of 5 patients who responded to HCV infection with anti-HCV core monoclonal Ig developed multiple myeloma or PCL, implying that a monoclonal Ig response directed against HCV core may distinguish patients with increased risk of plasma-cell malignancy. Efforts should be made to identify such patients, as associated antiviral therapy should help eradicate the malignant, HCV-driven plasma-cell clone.7
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edith Bigot-Corbel, Laboratoire de Biochimie Générale, Institut de Biologie, Centre Hospitalier Universitaire (CHU) de Nantes, 9 quai Moncousu, 44093 Nantes cedex, France; e-mail: edith.bigot@chu-nantes.fr.
This work was supported by a grant from the Direction à la Recherche Clinique (DRC) from the Centre Hospitalier Universitaire de Nantes, France (O.D. and S.H.).