To the editor:
Patients with HIV on highly active antiretroviral therapy (HAART) have protection against the development of non-Hodgkin lymphoma (NHL) compared with HIV-positive patients not on HAART, and most studies have suggested that there may be improved outcomes when adding HAART to chemotherapy for the treatment of HIV-associated NHL.1 Although HIV-related Kaposi sarcoma, extranodal mucosal-associated lymphoid tissue (MALT) lymphoma, and primary effusion lymphomas have all been reported to respond to HAART in the absence of chemotherapy,2-4 these diseases have clear infectious etiologies. Despite the report of a mixed large and small cell lymphoma regressing to zidovudine alone,5 the response of HIV-related NHLs to HAART alone is largely unknown. Here we report a case of diffuse large B-cell lymphoma (DLBCL) that responded solely to initiation of HAART.
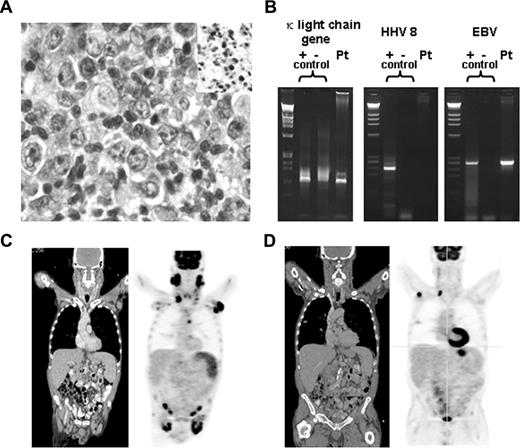
A 62-year-old man presented with large neck, axillary, and inguinal masses of 2 months' duration. Past medical history included thrush, anemia, and zoster. He denied fevers or night sweats, but reported an unintentional 25-pound weight loss. Exam revealed large, deforming, bilateral anterior and posterior cervical, axillary, and inguinal lymphadenopathy. Laboratory data revealed a positive HIV test with CD4 count of 185 mm3 and viral load > 100 000 copies per milliliter, mild anemia, and normal lactate dehydrogenase (LDH). Right cervical lymph node biopsy demonstrated an Epstein-Barr (EBV)–positive CD20+, CD10−, CD 5−, DLBCL with monoclonality demonstrated by Ig polymerase chain reaction (PCR; Figure 1A,B). Bone marrow was negative for disease. Fluorodeoxyglucose–positron emission tomography/compound tomography (FDG-PET/CT) scan confirmed extensive lymphadenopathy throughout the neck, chest, abdomen, and pelvis and splenomegaly with marked hypermetabolic activity. Standard uptake values (SUVs) ranged from 4 to 16. Of note, the biopsy-proven lymphoma site had an SUV of 16.2 (Figure 1C). Thus, the patient was diagnosed with stage IIIB DLBCL with a low- to intermediate-risk international prognostic indicator score.
Pathologic and radiologic data. (A) High-power magnification of right cervical lymph node biopsy. Micrographs were acquired with an Olympus BX41 microscope (Tokyo, Japan) using a 40×/0.75 objective on cells stained with hematoxylin and eosin and in situ EBV staining (insert). Images were captured with a color mosaic camera (model no. 11.2) using Advanced Spot software, version 4.0.9 (Diagnostic Instruments, Sterling Heights, MI). (B) PCR for κ light chain gene rearrangement, HHV8 and EBV; positive controls are on the left, negative controls in the middle, and patient sample on the right for each set of PCRs. (C) Coronal sections of CT (left) and PET (right) scan before initiation of HAART. (D) Coronal sections of CT (left) and PET (right) scan after initiation of HAART and before chemotherapy.
Pathologic and radiologic data. (A) High-power magnification of right cervical lymph node biopsy. Micrographs were acquired with an Olympus BX41 microscope (Tokyo, Japan) using a 40×/0.75 objective on cells stained with hematoxylin and eosin and in situ EBV staining (insert). Images were captured with a color mosaic camera (model no. 11.2) using Advanced Spot software, version 4.0.9 (Diagnostic Instruments, Sterling Heights, MI). (B) PCR for κ light chain gene rearrangement, HHV8 and EBV; positive controls are on the left, negative controls in the middle, and patient sample on the right for each set of PCRs. (C) Coronal sections of CT (left) and PET (right) scan before initiation of HAART. (D) Coronal sections of CT (left) and PET (right) scan after initiation of HAART and before chemotherapy.
The patient was started on Atripla (efavirenz, emtricitabine, tenofovir; Bristol-Myers Squibb/Gilead Sciences), Bactrim (trimethoprim and sulfamethoxazole; Roche), and fluconazole, and was scheduled to start immuno/chemotherapy immediately. The patient deferred therapy for approximately 6 weeks, during which time his CD4 count rose to 264 mm3, his viral load fell to 2880 copies/mL, and complete clinical resolution of his lymphadenopathy occurred. Repeat PET/CT scan confirmed the dramatic resolution of his adenopathy; for example, an axillary lymph node measuring 5 cm with SUV of 15 measured 3.6 cm with SUV of 4.7 after HAART. The biopsy proven right cervical lymph node decreased from 3.7 cm to 1.2 cm with a decrease in SUV from 16 to 6 (Figure 1D). The patient was scheduled for chemotherapy as initially planned.
We have thus observed a dramatic clinical improvement of DLBCL to 6 weeks of HAART alone. Although PET scans may be positive in patients with benign HIV-related lymphadenopathy, typically there is minimal uptake in the absence of infections. There was no evidence that our patient had any infectious diseases that caused increased uptake. Although we cannot confirm that all PET-positive sites contained lymphoma, the site of biopsy-documented disease also demonstrated dramatic clinical and PET improvement after initiation of HAART.
The role that HIV plays in DLBCL is unclear. In contrast to case reports demonstrating improvement with HAART alone in MALT and effusion lymphoma, there are no data that suggest DLBCL is driven by infectious antigenic stimuli. The high incidence of DLBCL in immunosuppressed states, as well as data from allogeneic transplantation, suggest a role for immunosurveillance in this disease. This is the likely etiology of our patient's response to HAART, despite only a modest rise in CD4 count. More than 25% of HIV-associated DLBCLs are EBV-positive; we cannot rule out the possibility that immune reconstitution, including reconstitution of responses targeting EBV proteins, may have played a role in the improvement of his lymphoma and/or PET/CT examination. Our observation stresses the importance of HAART in patients with HIV-related lymphoma and suggests that active HIV infection may play a role in the pathogenesis of HIV-related DLBCL.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence B. Gardner, New York University School of Medicine, Tisch 334, 550 1st Ave, NY 10016; e-mail: lawrence.gardner@med.nyu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal