Abstract
Numerous chronic graft-versus-host disease (cGVHD) biomarkers have been identified in limited, single-institution studies without validation. We hypothesized that plasma-derived biomarkers could diagnose, classify, and evaluate response in children with cGVHD. We performed a concomitant analysis of a number of known and predicted peripheral blood cGVHD biomarkers from a Children's Oncology Group (COG) phase 3 cGVHD therapeutic trial. A total of 52 newly diagnosed patients with extensive cGVHD were compared for time of onset after blood and marrow transplantation (BMT) (early, 3-8 months; late, ≥ 9 months) with 28 time-matched controls with no cGVHD (early, 6 months after BMT; late, 12 months after BMT). Soluble B-cell activation factor (sBAFF), anti-dsDNA antibody, soluble IL-2 receptor alpha (sIL-2Rα), and soluble CD13 (sCD13) were elevated in patients with early-onset cGVHD compared with controls. sBAFF and anti-dsDNA were elevated in patients with late-onset cGVHD. Some of the biomarkers correlated with specific organ involvement and with therapeutic response. These 4 biomarkers had high specificity with higher sensitivity in combination. Changes in biomarker concentrations with immune reconstitution after transplantation significantly affected interpretation of results. The identified biomarkers have the potential for improved classification, early response evaluation, and direction of cGVHD treatment, but require validation in larger studies. This study is registered at www.cancer.gov/clinicaltrials as no. COG-ASCT0031.
Introduction
Chronic graft-versus-host disease (cGVHD) is a multisystem, alloimmune, and autoimmune disorder occurring in 40% to 70% of patients following allogeneic blood and marrow transplantation (allo-BMT).1-6 cGVHD negatively affects quality of life and is the major cause of late transplantation-related mortality (TRM) in allo-BMT survivors.5,7 Treatment of cGVHD is limited by a number of factors, including late diagnosis, an inability to predict outcome or response to therapy, and a poor understanding of the immune targets important for optimal therapy. Well-characterized biomarkers could be used to address each of these issues and be a critical component in the performance of clinical trials.
Recently, the National Institutes of Health (NIH)–supported cGVHD Biomarker Consensus Group made a number of recommendations regarding needed areas of focus in identifying cGVHD biomarkers.8 Biomarkers were defined as any biological product that could be used to predict cGVHD development and aid in establishing the diagnosis, classification, prognosis, or the therapeutic response to cGVHD treatment. Currently, cGVHD biomarkers are not well characterized and are limited to relatively small, single-center studies. The poor understanding of the biology of cGVHD has further hampered progress in the area.
Diverse clinical manifestations and response to therapy suggest the biologic basis of cGVHD is complex. Although alloreactive donor T cells are important in the pathophysiology of cGVHD, other cell populations appear to be important.9 Recent data support that B cells are an important part of the immune response in cGVHD because of their ability to produce autoantibodies and present antigens.10-12 Although our group has identified a cellular biomarker, Toll-like receptor 9 (TLR9) high-expressing CpG-responsive B cells,13 the role of other cellular targets, such as regulatory T cells, is not clear. A number of inflammatory cytokines are implicated in GVHD, including monocyte chemoattractant protein-1 (MCP-1),14,15 IL-6,16,17 transforming growth factor-beta (TGF-β),18 and interferon-alpha (IFN-α).19 As a marker of B-cell activation, soluble B-cell activation factor (sBAFF) appears to correlate with cGVHD20 and autoimmune disorders such as lupus and rheumatoid arthritis.21,22 As a marker of activated T cells, soluble IL-2 receptor alpha (sIL-2Rα) correlates with the severity of acute GVHD,23-25 cGVHD,26,27 and other autoimmune diseases.28
Larger, multicenter trials evaluating the relative importance of cGVHD biomarkers are required. The Children's Oncology Group (COG) trial ASCT0031 (“Phase III trial of hydroxychloroquine plus standard therapy for chronic graft-versus-host disease”) was designed to prospectively ask about both a therapeutic aim and secondary biological aims. Due to poor accrual, the study did not achieve the therapeutic endpoint. It did evaluate many of the biological endpoints focused on chronic GVHD biomarkers, including a number of plasma biomarkers either previously identified in smaller, single-institution studies or hypothesized to be biomarkers for cGVHD. Proteomics performed on a limited sample set of ASCT0031 patients also identified an additional marker, soluble CD13 (sCD13; aminopeptidase N). Evaluations were done to determine the ability of plasma-derived biomarkers to diagnose, classify, and evaluate response to therapy for children with newly diagnosed, extensive cGVHD.
Methods
Patients
Peripheral blood samples were collected and evaluated prospectively for these studies from patients enrolled in the COG trial ASCT0031, a phase 3 randomized, placebo-controlled, double-blind trial evaluating 2 treatment regimens for patients with newly diagnosed extensive cGVHD. Institutional Review Boards at each participating center approved the study, and informed consent was obtained in accordance with the Declaration of Helsinki from parents of patients. Patients with cGVHD received a standard regimen of cyclosporine and alternate-day prednisone with either hydroxychloroquine or placebo. Subjects were between 1 and 29 years old at the time of study entry. Newly diagnosed extensive cGVHD was documented with biopsy confirmation of at least 1 organ system (eg, lip, skin, liver) and either (1) generalized skin involvement; (2) localized skin involvement and/or liver dysfunction, plus at least 1 of the following: liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis; eye involvement (Schirmer test with < 5 mm wetting); involvement of minor salivary glands or oral mucosa on lip biopsy; or involvement of any other target organs; or (3) involvement of at least 2 target organs. Patients with limited cGVHD were excluded from enrollment. Patients enrolled and treated on ASCT0031 formed the experimental group (n = 52). A control group (n = 28) of patients having undergone allo-BMT but who did not have cGVHD were also enrolled in the biology study component of the trial. Healthy volunteer blood donor controls (n = 15) who had not undergone transplantation were evaluated.
A time-matched comparison with the control group was performed for the 52 patients with cGVHD, with days after allo-BMT at the time of cGVHD diagnosis used to divide the experimental group into early onset cGVHD (3-8 months after transplantation) or late onset cGVHD (≥ 9 months after transplantation). This allowed for comparison against blood samples drawn from control participants at 6 months (early controls) and 12 months (late controls) after transplantation, respectively.
Samples evaluated
In experimental patients with cGVHD, peripheral blood was collected at study entry (time of cGVHD diagnosis) and at 2, 6, and 12 months after initiation of study therapy. The sample collected at 2 months was used to correlate with later therapeutic response at 9 months. In control participants, peripheral blood was collected at 6 and 12 months (after transplantation) to address the potential confounding factor of immune reconstitution after BMT. Between 1.0 and 1.5 mL/kg to a maximum of 50 mL peripheral blood was collected in heparinized tubes and shipped at room temperature by overnight courier. The sample was separated into cells and plasma after centrifugation and stored at −80°C until use.
Enzyme-linked immunosorbent assay
Patient plasma samples were examined for sIL-2Rα, MCP-1, IL-6 (BD Biosciences, San Diego, CA), sBAFF, platelet-derived growth factor-AA (PDGF-AA), PDGF-BB (R&D Systems, Minneapolis, MN), TGF-β (Biosource International, Camarillo, CA), and IFN-α (PBL-Biomedical Laboratories, Piscataway, NJ) by sandwich enzyme-linked immunosorbent assay (ELISA). Autoantibody enzyme immunoassay kits for antinuclear antibody (ANA), anti-dsDNA, antimitochondrial, and anticardiolipin antibodies (Bio-Rad Laboratories, Hercules, CA) were performed according to the manufacturer's protocol.
ANA number for each patient specimen was determined using the following formula: OD of test sample / OD of cutoff = ANA no. of test sample.
Proteomics analysis
Plasma samples were depleted of 12 abundant plasma proteins (albumin, IgG, α1-antitrypsin, IgA, IgM, fibrinogen, transferrin, haptaglobin, α1-acid glycoprotein, α2-macroglobin, HDL apoprotein A1, and HDL apoprotein A2) using a 5 mL avian immunoaffinity column (Genway Biotech, San Diego CA).29 Flow-through proteins were collected and precipitated overnight in 10% trichloroacetic acid (EMD Biosciences, Darmstadt, Germany), recovered by centrifugation, washed with cold acetone (4°C), and dissolved in 200 μL of 50% 0.5 M TEAB/50% saturated urea/0.2% SDS overnight at 4°C. Protein quantitation was determined using the bicinchoninic acid assay (BCA; Sigma-Aldrich, St Louis, MO), and the volume required to obtain 100 μg total protein was precipitated in 10 vol high-performance liquid chromatography (HPLC)–grade acetone at −20°C (Sigma-Aldrich, Seelze, Germany) followed by incubation for 16 to 18 hours at −20°C. Protein samples were dissolved in trifluoroacetic acid (TCEP) at a final concentration of 3.3 mM, and cysteine residues were reduced and blocked by the addition of methyl methane to a final concentration of 6.7 mM and incubation for 60 minutes at 60°C. Proteins were digested by the addition of 10 μg per sample of sequencing grade modified trypsin (Promega, Madison, WI) and incubation at 37°C overnight.
The trypsin-digested samples were dried by speed vacuum and labeled with iTRAQ reagents according to manufacturer's protocol (Applied Biosystems, Foster City, CA). iTRAQ-labeled peptide samples were then pooled and separated by strong ion chromatography on a VISION workstation (Applied Biosystems) equipped with a PolySULFOETHYL A column (100 × 4.6 mm, 5 μm, 300 Å; PolyLC, Columbia, MD). Fractions of 500 μL were collected over an 80-minute gradient divided into 2 linear profiles: (1) 0 to 30 minutes of 5% to 35% buffer B and (2) 30 to 80 minutes of 35% to 100% buffer B. Buffer A: 10 mM monobasic potassium phosphate (Sigma-Aldrich), 25% acetonitrile (EMD Chemicals, Gibbstown, NJ), pH 2.7; buffer B: same as buffer A with the addition of 0.5 M potassium chloride (Sigma-Aldrich). A total of 16 fractions were selected based on λ = 214 nm and subjected to reverse-phase separation. Peptides were first desalted by loading fractions onto a C18 PepMap guard column (300 μm internal diameter [ID] × 5 mm, 5 μm, 100 Å; LC Packings, Amsterdam, the Netherlands) and washing for 15 minutes at 50 μL/minute with mobile phase A (water/acetonitrile/trifluoroacetic acid, 98:2:0.1 [vol/vol]). The trapping column was then switched into the nano flow stream at 200 nL/minute, with peptides being loaded onto a Magic C18 nano LC column (15 cm, 5 μm pore size, 100 Å; Michrom Bioresources, Auburn, CA) for high-resolution chromatography. Peptides were eluted by the following gradient: 0 to 45 minutes with 5% to 15% acetonitrile/water/TFA (98:2:0.1 vol/vol; buffer B); 45 to 100 minutes with 15% to 40% buffer B; and 100 to 105 minutes with 40% to 75% buffer B, and spotted (750 nL per spot) on to matrix-assisted laser desorption ionization (MALDI) ABI 4800 plates (Applied Biosystems) using a Probot microfraction collector (LC Packings). Matrix solution, 3 mg/mL α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich) in 50% acetonitrile and 0.1% TFA, was then added at 0.75 μL per spot. Analysis was done using the 4800 MALDI time-of-flight (TOF)/TOF analyzer (Applied Biosystems) with data collection using 4000 series Explorer V3.5 software (Applied Biosystems). Peak picking and quantitation was completed using GPS Explorer version 3.6 (Applied Biosystems) with an integrated MASCOT server version 2.1.30 Spectra were searched against IPI Human v3.2131 and MSDB build 20063108 sequence databases.32 Protein identification was based on MASCOT's Mowse scoring algorithm33 with a confidence interval of 95% (http://www.matrixscience.com/help/scoring_help.html). iTRAQ reporter ions were measured, a tandem mass spectrometry (MS/MS) level ratio was calculated, and then considered into the overall average ratio of the protein if the peptide spectra meet set criteria such as ion strength and confidence in identification. The mean value for each iTRAQ ratio was determined from all the identified proteins and used as a normalization factor. A change in iTRAQ ratio greater than 20% to 30% was considered significant.
Enzymatic assay of sCD13 (aminopeptidase N)
Plasma samples were tested for sCD13 (aminopeptidase N) activity. Each 5 μL of sample (1:30) was mixed with 5 μL of 24 mM L-leucine-p-nitroaniline (Sigma-Aldrich, Oakville, ON) in a 96-well plate in 140 μL of phosphate buffer solution per well. The release of p-nitroaniline was used to follow aminopeptidase activity in the plasma by measuring the absorbance at 405 nm for 1 hour at 37°C (1 U aminopeptidase N/mL will hydrolyze 1.0 μM L-leucine-p-nitroanilide to L-leucine and p-nitroaniline per minute at pH 7.2 and 37°C).
Evaluation of clinical presentation and therapeutic response
Evaluation of whether biomarkers correlated with either clinical presentation could predict therapeutic response or be used as a surrogate endpoint was performed using data from the clinic study. The physician and data manager at each treating center determined clinical presentation of cGVHD and therapeutic response to treatment. Response to therapy was defined as either a complete response (complete clinical resolution of all cGVHD symptoms, excluding ocular sicca, since cGVHD may irreversibly damage the lacrimal gland) or partial response (complete clinical resolution of cGVHD in at least 1 site, but persistent nonprogressive disease in other sites). Patients were considered unevaluable if they had discontinued the study early due to toxicity, relapse, or infection and were excluded from this analysis. Nonresponders were defined as having only minor responses (clinical improvement with no progression, but no resolution in at least 1 site), mixed responses (clinical improvement in 1 site with progression in other sites), stable disease, or progressive disease. Patients were classified as nonresponders if they had discontinued therapy by 9 months because of progression of disease. Tissue involvement by cGVHD was classified by the individual participating centers and validated by central review. The patient's response status and tissue involvement was confirmed by an expert central review panel that reviewed the data submitted, including digital photographs. The expert panel was blinded to the status of the patient with regards to whether they were enrolled as having cGVHD or non-cGVHD and to whether the patient was as a responder or nonresponder. The expert central review panel made the final determination as to each of these classifications.
Statistical analysis
All analyses included the 2 therapeutic arms (cyclosporin A [CsA], prednisone ± hydroxychloroquine) of the clinical trial for patients with cGVHD as a single group. A biomarker was defined as biologically important if it met 2 criteria: (1) 100% higher or 50% lower compared with control (plus or minus 5%); and (2) a statistically significant P value (P ≤ .05). Sensitivity was calculated as the true positives / (the true positives + false negatives), and specificity was calculated as true negatives / (true negatives + false positives). True positives were those that had an elevated biomarker and evidence of clinical cGVHD, false negatives as those who had no biomarker elevation with clinical cGVHD, and false positives as having biomarker elevation with no clinical cGVHD. Descriptive statistics were generated on all data using Prism version 4 for PC (GraphPad Software, San Diego, CA). Significance of observed changes was determined using Student t test, Fisher exact, chi-square, and t test as deemed appropriate by the statistician (M.K.). The alpha (P) value was set at .05, making all P values less than .05 statistically significant. Receiver operating characteristic (ROC) curves were generated to provide evidence of a marker's discriminating ability for diagnosis and response to treatment, and for estimation of the best level to be used as a cutoff.
Results
Patient characteristics
Patients with and without cGVHD were compared for differences in age, sex, donor source, donor type, and presence of acute GVHD (Table 1). No significant differences were detected, except patients with cGVHD were more likely to have had an unrelated donor transplantation compared with patients without cGVHD (29 [57%] of 51 vs 8 [29%] of 28; P = .02).
Evaluation of soluble biomarkers in cGVHD
Evaluations were performed in a time-dependent manner to match the onset of cGVHD with equivalent time points after transplantation in control participants. This approach was used previously for our analysis of other biomarkers.13 To further evaluate the necessity of this approach in these analyses, an evaluation on the control group was performed on 4 biomarkers that appeared to be elevated in the following analyses. The 6-month and 12-month control values were compared with each other for sBAFF, sIL-2Rα, sCD13, and anti-dsDNA. There was no significant difference between the 6- and 12-month values of sCD13 (P = .67) and sIL-2Rα (P = .33) and anti-dsDNA (P = .35) in non-cGVHD control participants, but there was significant difference for sBAFF (P = .02) between the 2 time points in the control group. Thus, all analyses were performed matching patients with an early onset of cGVHD (3-8 months) with 6-month control participants and late onset cGVHD (≥ 9 months) with 12-month controls.
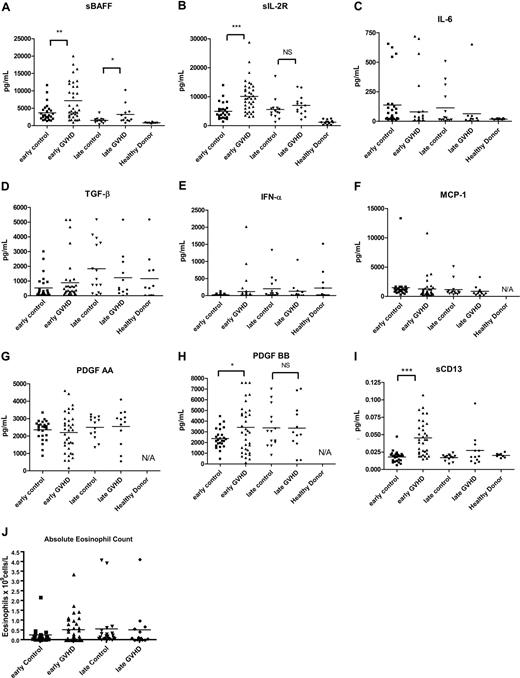
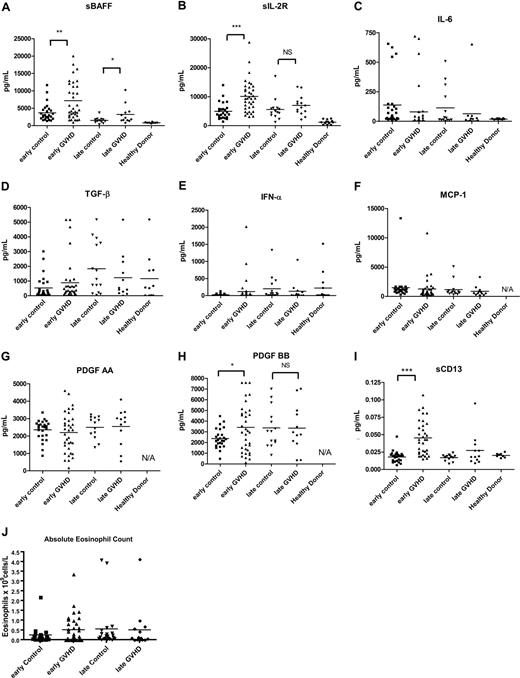
We evaluated a number of known or proposed soluble inflammatory markers in each sample, including sBAFF,20 sIL-2Rα,23-25 IL-6,16,17 TGF-β,18 IFN-α,19 MCP-1,14,15 PDGF-AA, and PDGF-BB.34,35 We found increased levels of sBAFF in patients with cGVHD with both early (P = .003) and late (P = .04) onset cGVHD compared with early and late control participants, respectively (Figure 1A). sIL-2Rα was elevated in early onset cGVHD (P < .001) but not late onset cGVHD (Figure 1B). PDGF-BB was elevated in early onset cGVHD (P = 0.03; Figure 1H); however, the difference did not reach our criteria for 100% higher (± 5%) to make PDGF-BB an important biomarker for early cGVHD. Absolute eosinophil count as a frequently measured indicator was included (Figure 1J). Although the absolute eosinophil count in the early cGVHD group (0.51 ± 0.45 × 109 cells/L) was almost 100% higher then early control participants (0.26 ± 0.45 × 109 cells/L; P = 0.77), the difference was not significant. PDGF-AA, TGF-α, IFN-γ, IL-6, and MCP-1 levels were not statistically different at either early or late cGVHD time points (Figure 1).
Soluble biomarkers in cGVHD. Soluble factors were tested for (A) sBAFF, (B) sIL-2Rα, (C) IL-6, (D) TGF-β, (E) IFN-α, (F) MCP-1, (G) PDGF-AA, and (H) PDGF-BB by ELISA. Patients with early onset (3-8 months) cGVHD (▴) were compared with 6-month controls (■), patients with late onset (≥ 9 months) cGVHD (♦) were compared with 12-month controls (▾). (I) sCD13 activities were analyzed by the protocol described under “Methods.” (J) Absolute eosinophil counts were from center-derived complete peripheral blood counts. Bars indicate mean values for each group. ***P < .001; **P < .01; *P < .05. NS indicates not significant.
Soluble biomarkers in cGVHD. Soluble factors were tested for (A) sBAFF, (B) sIL-2Rα, (C) IL-6, (D) TGF-β, (E) IFN-α, (F) MCP-1, (G) PDGF-AA, and (H) PDGF-BB by ELISA. Patients with early onset (3-8 months) cGVHD (▴) were compared with 6-month controls (■), patients with late onset (≥ 9 months) cGVHD (♦) were compared with 12-month controls (▾). (I) sCD13 activities were analyzed by the protocol described under “Methods.” (J) Absolute eosinophil counts were from center-derived complete peripheral blood counts. Bars indicate mean values for each group. ***P < .001; **P < .01; *P < .05. NS indicates not significant.
Proteomic analysis of plasma for additional markers
To expand possible factors that might correlate with cGVHD, we tested 8 cGVHD (4 early and 4 late onset) samples and 8 control samples (4 6-month samples and 4 12-month samples) by proteomic analysis and found sCD13 (aminopeptidase N) was more than 1.5 times higher than control samples in 3 of 8 cGVHD samples. To validate the proteomic analysis, an enzyme activity assay for sCD13 was performed on the 52 cGVHD and 28 control samples. The analyses revealed higher sCD13 activity in early (P < .001) but not late (P = .12) onset cGVHD as compared with control (Figure 1I).
Evaluation of autoimmune antibodies in cGVHD
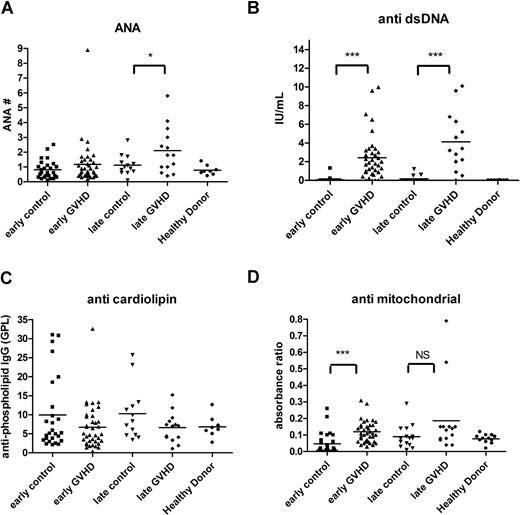
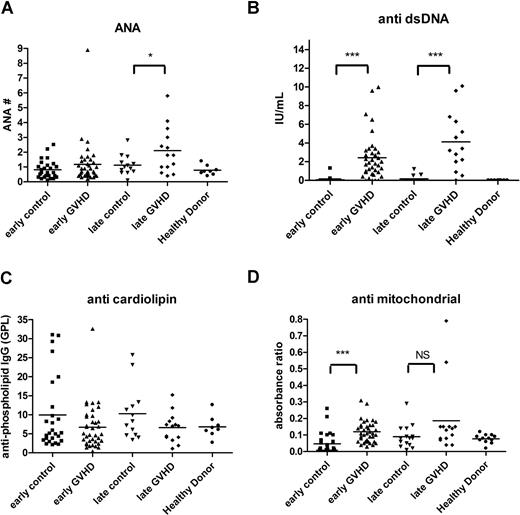
Analyses were then performed for autoantibodies previously shown to be associated with cGVHD, including ANA,36-38 anti-dsDNA antibody,39 antimitochondrial antibody,38 and anticardiolipin antibody.38 ANA positivity was similar between control patients without cGVHD and patients with cGVHD (P = .26). ANA levels, however, were significantly higher in patients with late cGVHD compared with late control patients (P = .05; Figure 2A), and barely met our criteria as a biomarker at being 95% higher than the mean of the control value. Anti-dsDNA levels were significantly higher for both early (P < .001) and late (P < .001) cGVHD compared with non-cGVHD time-matched control participants (Figure 2B). No differences in anticardiolipin antibody levels could be detected (Figure 2C). Antimitochondrial antibody (Figure 2D) was elevated in patients with early onset cGVHD (P < .001), but was not greater than 95% of the control.
Autoantibodies in cGVHD. Autoimmune autoantibodies such as ANA (A), anti-dsDNA antibody (B), anticardiolipin antibody (C), and antimitochondrial antibody (D) were measured by ELISA, and patients with early onset (3-8 months) cGVHD (▴) were compared with 6-month controls (■); patients with late onset (≥ 9 months) cGVHD (♦) were compared with 12-month controls (▾). Bars indicate mean values for each group. ***P < .001. NS indicates not significant.
Autoantibodies in cGVHD. Autoimmune autoantibodies such as ANA (A), anti-dsDNA antibody (B), anticardiolipin antibody (C), and antimitochondrial antibody (D) were measured by ELISA, and patients with early onset (3-8 months) cGVHD (▴) were compared with 6-month controls (■); patients with late onset (≥ 9 months) cGVHD (♦) were compared with 12-month controls (▾). Bars indicate mean values for each group. ***P < .001. NS indicates not significant.
Evaluation of the effect of acute GVHD and steroid treatment on biomarker elevation at diagnosis
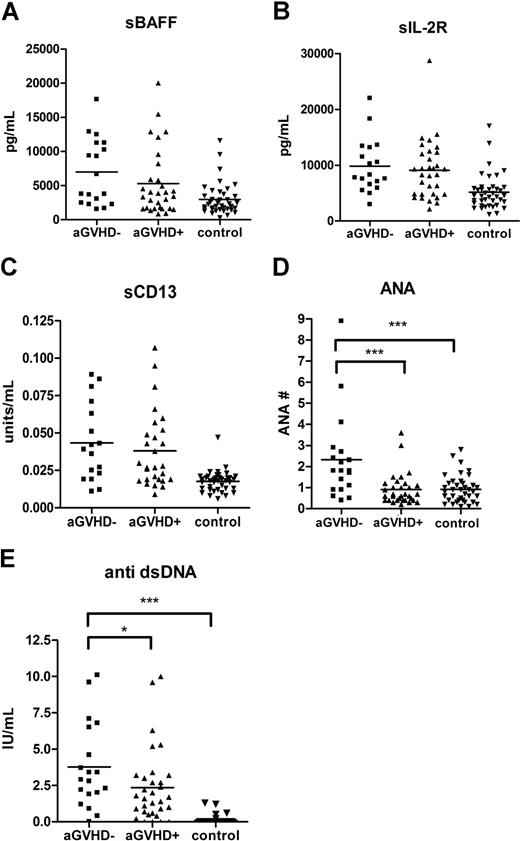
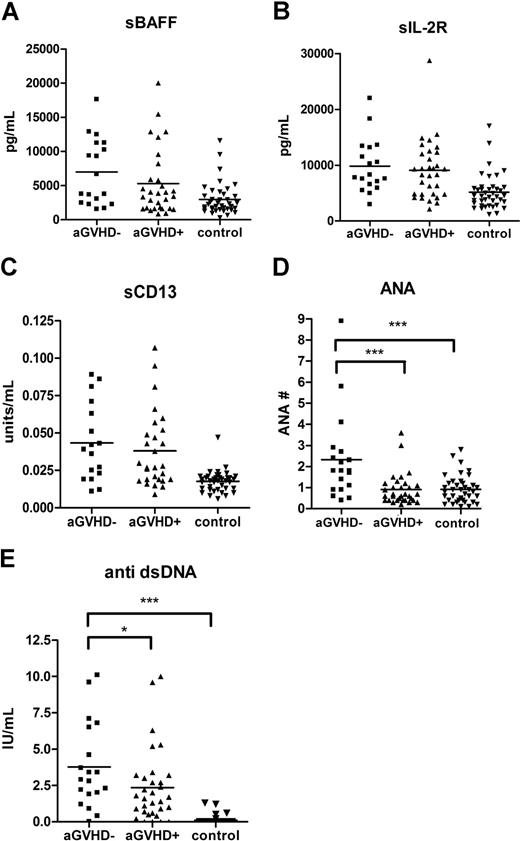
Acute GVHD is a known risk factor for the development of cGVHD.37 To exclude the possibility that the biomarkers reflected residual changes due to previous acute GVHD, an analysis was performed evaluating whether prior history of acute GVHD had an effect on the identified cGVHD biomarkers. Patients with de novo cGVHD (no previous acute GVHD) had higher levels of both ANA (P < .001) and anti-dsDNA (P = .04) compared with patients with a prior history of acute GVHD (Figure 3). There were no differences in levels of sBAFF, sIL-2Rα, or sCD13 between patients with and without a history of acute GVHD.
The levels of autoantibodies were lower in patients with acute GVHD history. A total of 5 markers (sIL-2Rα, sBAFF, sCD13, anti-dsDNA antibody, and ANA) in patients with de novo cGVHD (■) were compared with patients with a previous history of acute GVHD (▴) and non-GVHD control (▾).
The levels of autoantibodies were lower in patients with acute GVHD history. A total of 5 markers (sIL-2Rα, sBAFF, sCD13, anti-dsDNA antibody, and ANA) in patients with de novo cGVHD (■) were compared with patients with a previous history of acute GVHD (▴) and non-GVHD control (▾).
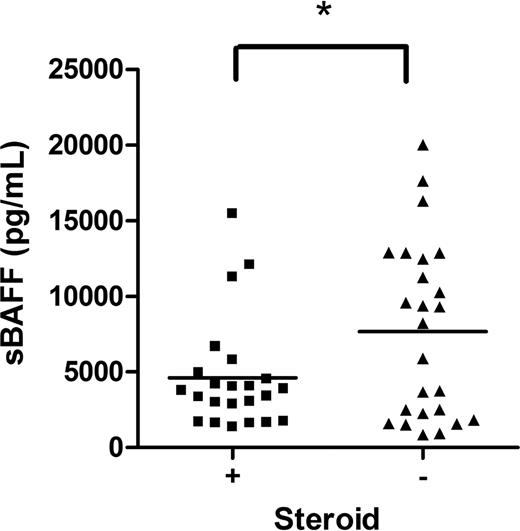
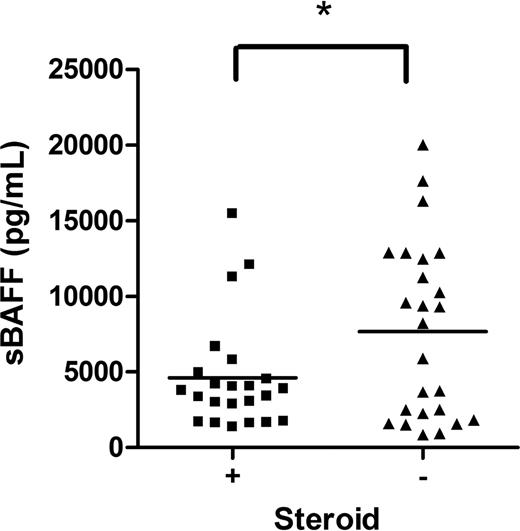
Inclusion criteria for study enrollment allowed the use of prednisone at a dose of less than 1 mg/kg per day (or the equivalent of another steroid) for up to 1 week for symptom management during the consent and evaluation process. Since many of the patients were on steroids upon study entry, we evaluated the effect of steroids on the values of each biomarker. Subjects on corticosteroids at the onset of cGVHD had decreased levels of sBAFF (Figure 4; P < .05), but no differences in levels of sIL-2Rα, anti-dsDNA, or sCD13 compared with patients not on corticosteroids (data not shown).
The effect of steroid therapy on sBAFF. sBAFF levels at the onset were compared between those on steroids at diagnosis (■) and on no steroids (▴).
The effect of steroid therapy on sBAFF. sBAFF levels at the onset were compared between those on steroids at diagnosis (■) and on no steroids (▴).
Correlation of biomarkers with cGVHD organ involvement
It was possible that biomarkers that did not correlate with the onset of cGVHD overall did correlate with specific organ manifestations of cGVHD. All 13 biomarkers were evaluated for their association with specific organ involvement in cGVHD (Table 2). Levels of sBAFF and sCD13 were significantly higher in patients with hepatic cGVHD (P = .004 and P < .001, respectively). Anti-dsDNA levels were higher in patients with joint (P < .001), sclerodermatous (P < .01), and ocular (P < .01) cGVHD. Elevated sBAFF was significantly associated with lichenoid skin rash and joint cGVHD (P < .05), elevated IL-6, MCP-1 with joint cGVHD (P < .05), and elevated anticardiolipin antibody with ocular involvement (P < .05). None of the markers evaluated were associated (P ≤ .05) with gastrointestinal (oral, esophageal, or other parts of the gastrointestinal [GI] tract), pulmonary, or musculoskeletal cGVHD (data not shown).
Diagnostic sensitivity and specificity of biomarkers in cGVHD
We evaluated each of the previously identified markers for early cGVHD by receiver operating characteristic (ROC) analysis to identify a cut-off value and sensitivity that corresponded to a greater than 90% specificity (Table 3). Analysis of the area under the curve (AUC) for the early cGVHD group showed that all were close to or greater than 0.70 for anti-dsDNA (0.66), IL2Rα (0.83), sCD13 (0.77), and sBAFF (0.70). We found that the sensitivity was relatively low, ranging between 42% and 53%. We estimated a conservative cut-off value for each biomarker with what should be considered to be positive for anti-dsDNA (2.0 IU/mL), IL2Rα (10 ng/mL), sCD13 (0.43 U/mL), and sBAFF (7.5 ng/mL) at 90% specificity.
We analyzed the 4 biomarkers in combination to see whether sensitivity can be increased (Table 4). We found that overall sensitivity was relatively high at 84% (38 of 45 patients) if 1 or more of the 4 markers were positive in patients with cGVHD and 56% if a criterion of 2 or more of the 4 markers was used. Specificity was high (100%) with 26 of 26 patients at 6 months and 14 of 14 true negatives at 12 months for cGVHD when 1 or fewer biomarkers were positive. The positive predictive value of patients with 2 or more of 4 biomarkers was 100%.
Ability of biomarkers to act as prognostic markers or surrogate endpoints for therapeutic response
Biomarkers may be useful as a prognostic indicator and allow for assignment to more or less intensive therapeutic approaches. A biomarker would ideally be prognostic and be able to identify patients who would either require less/more intensive therapy or, as is likely for cGVHD, likely to respond to a specific/selective immune modulatory treatment. All of the biomarkers, including the 4 biomarkers identified as having diagnostic capability (sBAFF, sIL2-Rα, sCD13, and anti-dsDNA), were evaluated for their ability at the time of diagnosis to predict response to cGVHD therapy at a time point earlier than evaluation of clinical response (Table 5). Of the 52 patients, only 40 were evaluable as responders (complete or partial response) or nonresponders (mixed response or no response). The other 12 patients were not evaluable due to withdrawal from treatment or relapse of malignancy. Only IL-2Rα had a significant difference between responders (7612 pg/mL) versus nonresponders (11 010 pg/mL; P = .03). Although significant, the difference was less than 50% of the responder group. Since the removal of the nonevaluable patients from the analysis may have affected the analysis, we compared the biomarker levels in patients evaluable for response versus those who were not. Although there was no significant difference for sCD13, anti-dsDNA, and sIL2Rα, there was a significant difference in the sBAFF concentrations between the evaluable patients (7144 ± 5288 pg/mL) compared with the nonevaluable patients (2566 ± 2551 pg/mL; P < .001).
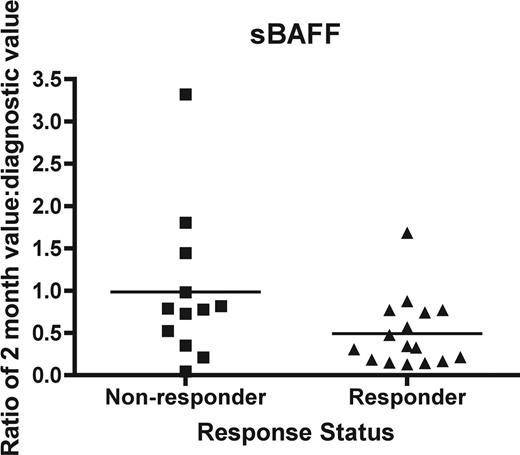
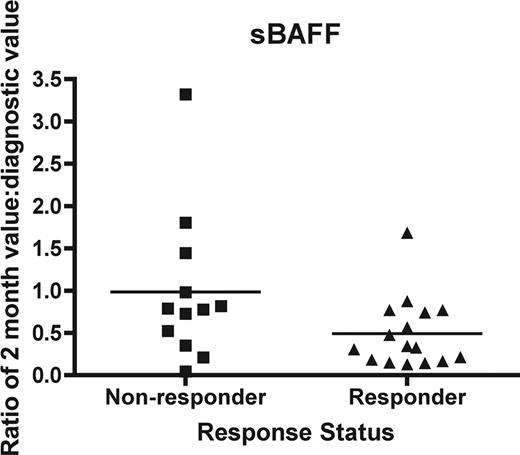
Since clinical response of cGVHD often cannot be assessed until 3 months or more after initiation of therapy, a biomarker may be useful as a surrogate endpoint of therapeutic response at an early time point 1 and 2 months after start of therapy. We evaluated the change in the 2-month concentration as a ratio compared with that at diagnosis for IL2Rα, sBAFF, sCD13, and anti-dsDNA in both responders and nonresponders. Clinical response was evaluated at 9 months of therapy. We found that there was no significant difference between responders and nonresponders for sCD13 (P = .21), IL-2Rα (P = .95), or anti-dsDNA (P = .85). However, there was a significantly lower ratio of sBAFF, 0.49 ± 0.10 (mean ± SEM) versus 0.98 ± 0.26 for nonresponders (P = .05; Mann-Whitney; Figure 5).
The ability of biomarker changes at 2 months to act as a surrogate endpoint. Soluble BAFF was measured as a ratio of the 2-month value compared with the initial value for each patient. Nonresponders (■) were compared with responders (▴) as determined by a clinical response at 9 months. The difference is significant (P = .05).
The ability of biomarker changes at 2 months to act as a surrogate endpoint. Soluble BAFF was measured as a ratio of the 2-month value compared with the initial value for each patient. Nonresponders (■) were compared with responders (▴) as determined by a clinical response at 9 months. The difference is significant (P = .05).
Discussion
We have evaluated a number of biomarkers previously shown or hypothesized to be associated with cGVHD from patients enrolled in a large multicenter clinical trial. This study has numerous strengths. First, stringent enrollment criteria and frequent monitoring for response with confirmation from an expert review panel ensured accuracy of clinical status. Secondly, only newly diagnosed extensive cGVHD was evaluated. Previous studies examining correlative biology have tended to group patients with limited and extensive cGVHD, as well as evolving, newly diagnosed, and established disease together. Finally, we used time-matched controls (early cGVHD matched with 6-month, and late cGVHD matched with 12-month after allo-BMT controls, respectively) to allow correction for immune reconstitution of each biomarker, an issue frequently not addressed in previous such studies.
We show that early onset cGVHD is characterized by elevated sIL-2Rα, sBAFF, sCD13, and anti-dsDNA levels compared with control patients. Elevation of sIL-2Rα in patients with early onset cGVHD is consistent with previous findings26,27 and suggests high levels of T-cell activation.23-28 Elevated sBAFF in early onset cGVHD also suggests contribution from B cells,20 since sBAFF is associated with T helper 1 (Th1) responses and B-cell activation and expansion.31,40 By comparison, in late onset cGVHD the biomarkers sBAFF, anti-dsDNA, and ANA are higher, suggesting that in late onset cGVHD, B-cell activation is more predominant. The fact that ANA and anti-dsDNA appear to be markers for de novo cGVHD suggests that early and late onset cGVHD may be mediated by different patterns of T- and B-cell activation.
The role of B cells in cGVHD pathogenesis has been investigated in mice10,11 and humans.12 B cells from patients with cGVHD have both a higher percentage of CD86 expression following CpG stimulation (positive CpG response) and increased TLR9 high-expressing B cells in their peripheral blood.13 TLR7/9 signaling is required for autoantibody production,41,42 consistent with our observation of elevated ANA and anti-dsDNA antibodies in cGVHD.
CD13 (aminopeptidase N) is a type II integral membrane protein with both receptor function and enzyme activity. CD13 is expressed on myeloid cells, activated T cells, and B cells,43 plays an important role in the early interaction between human cytomegalovirus (CMV) and target cells,44 and alters T-cell function through degradation of peptides bound to major histocompatibility complex class II molecules.45 Although CD13 is a membrane-bound protein, human plasma contains significant amounts of an active soluble form (sCD13), suggesting activated cells may release this protein.46 The presence of autoantibodies against CD13 following CMV infection has been shown to correlate with the development of cGVHD.47 If CD13 can be confirmed to have a functional role in cGVHD, a specific inhibitor, actinonin, may be useful as a therapeutic agent.
Elevated sIL-2Rα, sBAFF, sCD13, and anti-dsDNA levels were, by our defining criteria, very specific. However, at the levels determined to be sufficiently specific, sensitivity was relatively low at 42% to 56%. This is likely due to the heterogeneity of cGVHD. We did evaluate whether we could increase our sensitivity if 2 or more of the 4 biomarkers were positive (as defined by the ROC analysis; Table 3), and found we could increase sensitivity to 84%. The addition of the recently identified CpG B-cell response, identified from this same patient population,13 was performed in a subpopulation (due to sample limitations), and we found the addition of the CpG response as a fifth factor increased the sensitivity, with 18 (95%) of 19 patients with cGVHD having 2 or more biomarker positivity. Thus, introduction of additional markers may increase the sensitivity of biomarkers, but needs to be validated in larger numbers.
Since the plasma was collected from peripheral blood samples that had been shipped at room temperature for 24 hours, it is possible that the results may be affected by release of cytokines and cellular proteins from lymphocytes, neutrophils, or platelets. Transport of samples will occur with multicenter trials and central laboratories. However, this pragmatic approach is similar to what occurs in clinical practice and focused on the identification of robust biomarkers. We reasoned that any marker that met our criteria of being either 100% higher or 50% lower than the control and significantly different in a relatively small sample size would have the highest probability of diagnostic and prognostic ability in the real world situation of the clinic.
Using biomarkers to predict response to therapy for cGVHD is attractive clinically and raises the possibility that therapy could be changed according to biomarker levels. We saw that a greater than 50% drop of sBAFF values at 2 months after initiation of therapy may predict response. Previously, we have seen that drops in TLR9 high-expressing, CpG-responsive B cells after 2 months of therapy may predict response.13 Since we used a relatively late time point of 9 months after initiation of treatment for determination of therapeutic response, many patients were considered unevaluable due to removal from protocol for toxicity, infection, or relapse of malignancy. This did not affect results for sCD13, anti-dsDNA, and IL-2Rα. There were significantly lower concentrations of sBAFF at diagnosis in nonevaluable patients, raising the possibility that low sBAFF concentrations may correlate with infection, relapse, or toxicity, the reasons that the patients were unevaluable. Larger validation studies are required before these markers could be used for decision-making or risk assignment.
We demonstrated that certain biomarkers are predictive for specific clinical manifestations of cGVHD. As in other autoimmune disorders, anti-dsDNA is elevated in ocular (sicca), sclerodermatous, and joint and anticardiolipin antibodies in ocular cGVHD. This suggests that B cells may have a more dominant role in these manifestations. Elevated sBAFF is correlated with hepatic cGVHD48 and has a biologic effect that augments B-cell numbers and function to pathologic levels.49 The fact that the markers we evaluated correlated with some cGVHD manifestations but not others suggests that other yet to be determined biomarkers may correlate with other pathologies. PDGF-BB, in collaboration with TGF-β, is reported to be associated with scleroderma and lung fibrosis,35 likely by regulation of collagenase gene expression,34 but we did not correlate it with lung cGVHD. Since we collected clinical data at the onset of cGVHD, our measurement of lung involvement, which many times develops as a later manifestation of cGVHD, may be less than the actual incidence. More recent data show that autoantibodies against PDGF receptor play a role in sclerodermatous cGVHD.50
One application of biomarkers is to identify potential targets for cGVHD therapy. Monoclonal antibodies against the B-cell antigen CD20 (eg, rituximab) can deplete autoreactive B cells in autoimmune disorders; however, marginal zone and B1 B cells are resistant to therapy.51 Therapies blockading sBAFF, which affect the entire B-cell population, may be better and are being evaluated in autoimmune disease clinical trials.52 Similarly, targeting CD13+ cell populations or function may represent an alternative approach.
In conclusion, we have identified biomarkers that can be used to aid in the diagnosis, evaluation, and response to therapy in patients with cGVHD and may identify particular forms of cGVHD. We found evidence that early and late onset cGVHD may be mediated by different patterns of biomarkers indicative of different B- and T-cell activation patterns. Once validated in larger studies, these biomarkers may allow for improved classification, early response evaluation, and ultimately to direct future cGVHD treatment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant R01-CA84137 from the National Cancer Institute of the National Institutes of Health and by the Canadian Institutes of Health Research/Wyeth Clinical Research Chair in Transplantation.
National Institutes of Health
Authorship
Contribution: H.F. was the primary author, designed and performed research, and wrote the paper; G.C. and K.S. designed and performed research and wrote the paper; S.A. and H.S. performed research; A.K. performed research and wrote the paper; M.K. and Z.C. acted as COG statistician and analyzed data; R.M. designed and performed research and wrote the paper; A.B. performed research and assisted in analysis; F.G., S.A.G., and D.A.W. designed and performed research and wrote the paper; A.L.G. was the primary investigator of clinical study, designed and performed research, and wrote the paper; and K.R.S., as senior author, designed and performed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirk R. Schultz, Department of Pediatrics, Division of Hematology/Oncology/Bone Marrow Transplantation, University of British Columbia, BC Children's Hospital, 4480 Oak Street, Vancouver, BC V6H 3V4, Canada; e-mail: kschultz@interchange.ubc.ca.