Hematopoiesis initiates within the yolk sac of mammalian embryos in overlapping primitive and definitive waves, each containing erythroid and megakaryocyte progenitors. c-myb–null mouse fetuses lack definitive erythrocytes but contain primitive erythroblasts and hepatic megakaryocytes. However, it is unclear if c-myb–null embryos harbor definitive erythroid or any megakaryocyte progenitors. We determined that c-myb was not expressed in primitive erythroid precursors and that c-myb–null embryos had normal primitive erythroid and megakaryocyte progenitor numbers and kinetics between embryonic day (E) 7.0 and E9.0. While primitive hematopoiesis is c-myb–independent, no definitive erythroid potential was detected in c-myb–null embryos, confirming that definitive erythropoiesis, beginning at E8.25 in the yolk sac, is completely c-myb–dependent. In contrast, reduced numbers of megakaryocyte progenitors with restricted proliferative capacity persist in E10.5 yolk sac and E11.5 liver. Despite this impaired megakaryocyte potential, c-myb–null fetuses had normal platelet numbers at E12.5 but became thrombocytopenic by E15.5, suggesting that c-myb is required for sustained thrombopoiesis.
Introduction
Hematopoietic potential first emerges in mammals within the yolk sac in 2 overlapping waves distinguished by distinct primitive and definitive erythroid lineages. Primitive erythroid progenitors (EryP-CFC) transiently appear in the murine yolk sac between embryonic days 7.25 and 9.0 (E7.25-9.0) and subsequently produce the embryo's first red cells, while definitive erythroid progenitors (BFU-E) arise and expand in the yolk sac between E8.25 and E10.0 and then transition to the liver.1,–3 We recently determined that these primitive and definitive erythroid waves are each hierarchically associated with megakaryocyte potential.4 The appearance of distinct, primitive, and definitive megakaryocyte-erythroid progenitors (MEP) raises the possibility that embryonic megakaryopoiesis may be differentially regulated by the transcriptional programs that regulate primitive and definitive hematopoiesis.
The transcription factor c-myb differentially regulates primitive and definitive erythropoiesis. c-myb–null mouse embryos contain primitive erythroblasts but entirely lack definitive erythrocytes and die of anemia by E15.5, when primitive erythroid cells can no longer sustain the rapidly growing fetus.5 The in vitro culture of c-myb–null embryonic stem (ES) cells recapitulates several hematopoietic defects evident in vivo, including the absence of BFU-E.6 Surprisingly, a small number of CFU-E were found in c-myb–null ES cell cultures and in yolk sacs of E8.5 c-myb–null embryos.6,7 It remains unclear if these “CFU-E” represent residual definitive erythroid potential or decreased primitive erythroid potential.
In contrast to the complete absence of definitive erythropoiesis, megakaryocyte cells are found, albeit in reduced numbers, in the livers of c-myb–null fetuses.5,7 Their origin is unclear because no megakaryocyte progenitors were found in c-myb–null fetuses.7 In the adult, several c-myb hypomorphic mutants display both thrombocytosis and anemia, suggesting that c-myb may regulate the lineage decision between erythropoiesis and megakaryopoiesis.8,–10 In order to shed further light on the role of c-myb in the regulation of primitive and definitive hematopoiesis, we have investigated the emergence of erythroid and megakaryocyte lineages in c-myb–null mouse embryos.
Methods
Embryonic tissues were isolated from timed pregnancies as previously described4,11 and genotyped with c-myb primers.5 In situ hybridization was performed as described12 with phosphorus-33 [33P]-labeled antisense transcripts (bp 819-3353 of c-myb, accession no. M12848)13 and viewed with an Optiphot microscope (10× objective, numeric aperture 0.5; Nikon, Melville, NY). Hematopoietic progenitor assays, with immunohistochemical identification of megakaryocyte and erythroid cells were performed as previously described,4 viewed with an Eclipse TE2000-S microscope (Nikon, 10×, 20×, and 40× objectives with numeric apertures 0.30, 0.40, and 0.60, respectively), and photographed with SPOT RT-slider (Diagnostic Instruments, Sterling Heights, MI).
Ter119+ and Ter119− cells were isolated from E9.0 yolk sac (16-20 somite pairs [sp]) and E15.5 liver using IMag Beads (BD Biosciences, San Jose, CA). RNA and cDNA were prepared with RNeasy (Qiagen, Valencia, CA) and SuperScript III First-Strand (Invitrogen, Carlsbad, CA), respectively, and quantitative polymerase chain reaction (qPCR) was performed as previously described.11
Results and discussion
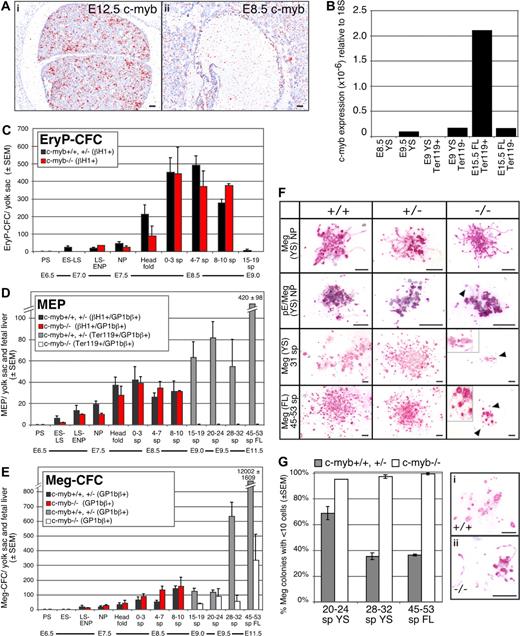
To investigate the role of c-myb in the emergence of the hematopoietic system, we first examined its expression in sites of hematopoietic differentiation. c-myb is strongly expressed in tissues that support definitive hematopoiesis14 such as the fetal liver (Figure 1Ai). However, c-myb transcripts did not accumulate above background in E8.5 yolk sac blood islands (Figure 1Aii). We next isolated Ter119+ and Ter119− cell populations from E15.5 liver and E9.0 yolk sac. c-myb transcripts were highly enriched in purified fetal liver-derived definitive erythroid precursors (Figure 1B). In contrast, no c-myb transcripts were detected in purified E9.5 yolk sac–derived primitive erythroid precursors or in whole E8.5 yolk sacs (Figure 1B).
Emergence of the erythroid and megakaryocyte lineages in wildtype and c-myb–null mouse fetuses. (A) Expression of c-myb in E12.5 (i) and E8.5 (ii) mouse embryos analyzed by in situ hybridization. c-myb transcripts accumulated in E12.5 fetal liver but were not detected above background in E8.5 yolk sac blood islands. Size bars indicate 50 μm. (B) Quantitation by qPCR of c-myb transcripts in sites of hematopoiesis and in purified erythroid cell populations: (1) E8.5 yolk sac (YS), (2) E9.5 yolk sac, (3) Ter119-positive cells from E9.0 yolk sac (16-20 sp), (4) Ter119− cells from E9.0 yolk sac (16-20 sp), (5) Ter119+ cells from E15.5 fetal liver (FL), and (6) Ter119− cells from E15.5 fetal liver. (C-E) Early ontogeny of primitive erythroid progenitors (EryP-CFC), bipotential megakaryocyte/erythroid progenitors (MEP), and megakaryocyte progenitors (Meg-CFC) between E6.5 and E11.5 of gestation in c-myb–null (−/−) and combined wildtype (+/+) and c-myb-heterozygous (+/−) mouse embryos. Mean (± SEM) of each progenitor type is graphed. Wildtype and c-myb-heterozygous embryos revealed similar progenitor numbers and temporal kinetics. EryP-CFC-derived colonies contain βH1-globin+ primitive erythroid cells, MEP-derived colonies contain GP1Bβ+megakaryocytes and either primitive erythroid (βH1-globin+ cells through 19 sp) or definitive erythroid (Ter119+ cells > 19 sp), and Meg-CFC-derived colonies contain GP1Bβ+ megakaryocytes. Embryonic stages and (number of embryos examined at each stage): PS, primitive-streak (2); ES-LS, early primitive streak to late primitive streak (9); NP, neural plate (21); head fold, (16) and number of somite pairs; 0 to 3 sp (16); 4 to 7 sp (21); 8 to 10 sp (5); 15 to 19 sp (12); 20 to 24 sp (18); 28 to 32 sp, (15) 45 to 59 sp. (9). (F) Morphology of Meg-CFC-derived colonies (Meg) and primitive MEP-derived colonies (pE/Meg) from wildtype (+/+), c-myb-heterozygous (+/−), and c-myb–null (−/−) embryos. Megakaryocytes (red/pink stain) are labeled with rat anti–mouse GP1bβ (Emfret Analytics, Würzburg, Germany), and primitive erythroid cells (blue/purple stain) are labeled with rabbit anti–mouse βH1-globin16 as described in Tober et al.4 All cultures were grown for 10 days. Arrows and higher magnification insets highlight proplatelet formation. Size bars indicate 50 μm. (G) Mean percentage (± SEM) of Meg-CFC-derived colonies (composed of GP1bβ-positive cells) consisting of fewer than 10 megakaryocytes in combined wild-type (+/+) and c-myb–heterozygous (+/−;  ) and c-myb–null (−/−, ▭) mouse embryos. Representative small colonies from wild-type (i) and c-myb–null (ii) E10.5 cultures are shown. Proplatelet formation and platelet-sized fragments are evident. Size bars indicate 50 μm. Images were processed with lens defect correction and brightness/contrast optimization in Photoshop (Adobe, San Jose, CA) with Fovea Pro plug-in (Reindeer Graphics, Ashville, NC).
) and c-myb–null (−/−, ▭) mouse embryos. Representative small colonies from wild-type (i) and c-myb–null (ii) E10.5 cultures are shown. Proplatelet formation and platelet-sized fragments are evident. Size bars indicate 50 μm. Images were processed with lens defect correction and brightness/contrast optimization in Photoshop (Adobe, San Jose, CA) with Fovea Pro plug-in (Reindeer Graphics, Ashville, NC).
Emergence of the erythroid and megakaryocyte lineages in wildtype and c-myb–null mouse fetuses. (A) Expression of c-myb in E12.5 (i) and E8.5 (ii) mouse embryos analyzed by in situ hybridization. c-myb transcripts accumulated in E12.5 fetal liver but were not detected above background in E8.5 yolk sac blood islands. Size bars indicate 50 μm. (B) Quantitation by qPCR of c-myb transcripts in sites of hematopoiesis and in purified erythroid cell populations: (1) E8.5 yolk sac (YS), (2) E9.5 yolk sac, (3) Ter119-positive cells from E9.0 yolk sac (16-20 sp), (4) Ter119− cells from E9.0 yolk sac (16-20 sp), (5) Ter119+ cells from E15.5 fetal liver (FL), and (6) Ter119− cells from E15.5 fetal liver. (C-E) Early ontogeny of primitive erythroid progenitors (EryP-CFC), bipotential megakaryocyte/erythroid progenitors (MEP), and megakaryocyte progenitors (Meg-CFC) between E6.5 and E11.5 of gestation in c-myb–null (−/−) and combined wildtype (+/+) and c-myb-heterozygous (+/−) mouse embryos. Mean (± SEM) of each progenitor type is graphed. Wildtype and c-myb-heterozygous embryos revealed similar progenitor numbers and temporal kinetics. EryP-CFC-derived colonies contain βH1-globin+ primitive erythroid cells, MEP-derived colonies contain GP1Bβ+megakaryocytes and either primitive erythroid (βH1-globin+ cells through 19 sp) or definitive erythroid (Ter119+ cells > 19 sp), and Meg-CFC-derived colonies contain GP1Bβ+ megakaryocytes. Embryonic stages and (number of embryos examined at each stage): PS, primitive-streak (2); ES-LS, early primitive streak to late primitive streak (9); NP, neural plate (21); head fold, (16) and number of somite pairs; 0 to 3 sp (16); 4 to 7 sp (21); 8 to 10 sp (5); 15 to 19 sp (12); 20 to 24 sp (18); 28 to 32 sp, (15) 45 to 59 sp. (9). (F) Morphology of Meg-CFC-derived colonies (Meg) and primitive MEP-derived colonies (pE/Meg) from wildtype (+/+), c-myb-heterozygous (+/−), and c-myb–null (−/−) embryos. Megakaryocytes (red/pink stain) are labeled with rat anti–mouse GP1bβ (Emfret Analytics, Würzburg, Germany), and primitive erythroid cells (blue/purple stain) are labeled with rabbit anti–mouse βH1-globin16 as described in Tober et al.4 All cultures were grown for 10 days. Arrows and higher magnification insets highlight proplatelet formation. Size bars indicate 50 μm. (G) Mean percentage (± SEM) of Meg-CFC-derived colonies (composed of GP1bβ-positive cells) consisting of fewer than 10 megakaryocytes in combined wild-type (+/+) and c-myb–heterozygous (+/−;  ) and c-myb–null (−/−, ▭) mouse embryos. Representative small colonies from wild-type (i) and c-myb–null (ii) E10.5 cultures are shown. Proplatelet formation and platelet-sized fragments are evident. Size bars indicate 50 μm. Images were processed with lens defect correction and brightness/contrast optimization in Photoshop (Adobe, San Jose, CA) with Fovea Pro plug-in (Reindeer Graphics, Ashville, NC).
) and c-myb–null (−/−, ▭) mouse embryos. Representative small colonies from wild-type (i) and c-myb–null (ii) E10.5 cultures are shown. Proplatelet formation and platelet-sized fragments are evident. Size bars indicate 50 μm. Images were processed with lens defect correction and brightness/contrast optimization in Photoshop (Adobe, San Jose, CA) with Fovea Pro plug-in (Reindeer Graphics, Ashville, NC).
These results suggested that c-myb may not regulate the emergence of the primitive erythroid and primitive megakaryocyte lineages. To test this hypothesis, we assayed the temporal appearance of erythroid and megakaryocyte progenitors in staged myb-null, myb-heterozygote, and wild-type murine embryos. As shown in Figure 1C-E, colonies derived from EryP-CFC (βH1-globin+), Meg-CFC (GP1Bβ+) and bipotential primitive MEPs (GP1Bβ/βH1-globin+) were first detected at primitive streak stages (E7-7.5) and persisted until approximately 15 sp (E9.0). All 3 primitive hematopoietic progenitors revealed similar numbers and temporal kinetics in c-myb–null fetuses compared with c-myb–heterozygotes and wild-type embryos (Figure 1C-E). The morphology of the colonies was also similar for each of the genotypes, with the megakaryocyte component consisting of small numbers of cells capable of extensive proplatelet formation (Figure 1F ▶). These results support the concept that primitive hematopoiesis is at least bilineage in nature and indicate that the primitive erythroid and primitive megakaryocyte lineages in the mammalian embryo are not regulated by c-myb.
No primitive erythroid (βH1-globin+) colonies were detected after 15 to 19 sp,2,4 therefore we used Ter119 positivity to detect definitive erythroid cells in colonies later in development. No definitive erythroid progenitors were detected in c-myb–null embryos at all time points examined using either collagen- or methylcellulose-based assays2 (data not shown), nor were colonies detected after 15 to 19 sp that contained both erythroid and megakaryocyte cells in c-myb–null conceptuses (Figure 1D). These results indicate that definitive erythroid potential, which normally emerges in the yolk sac beginning at E8.25, is completely c-myb–dependent.
In contrast to the definitive erythroid lineage, megakaryocyte progenitors were detected in c-myb–null embryos beyond 15 to 19 sp (Figure 1E □). However, their numbers in the yolk sac at E10.5 and the fetal liver at E11.5 were significantly lower than normal (Figure 1E ▩), indicating that the absence of c-myb did not induce definitive-MEP to preferentially adopt a megakaryocyte fate. Meg-CFC from c-myb–null tissues produced small colonies, typically containing fewer than 6 megakaryocytes (Figure 1F, bottom 2 rows) and almost all contained fewer than 10 megakaryocytes (Figure 1G). In contrast, a majority of Meg-CFC from wildtype and c-myb-heterozygous embryos at E10.5 to E11.5 generated large colonies containing more than 10 megakaryocytes (Figure 1F bottom 2 rows and Figure 1G). Despite diminished colony size, most megakaryocytes within the c-myb–null colonies showed advanced stages of cell maturation with proplatelet formation and platelet-sized fragments (Figure 1Gi,ii). These findings indicate that c-myb is not required for terminal maturation of these embryonic megakaryocytes.
It is currently unclear if the Meg-CFC present at E10.5 to E11.5 in the c-myb–null yolk sac and fetal liver represent a persistence of primitive megakaryopoiesis or a restricted form of definitive megakaryopoiesis. The small colony size, comparable with that of primitive-Meg-CFC, supports the former possibility and suggests that c-myb may be absolutely necessary, not only for definitive erythropoiesis, but also for definitive megakaryopoiesis emerging from the yolk sac. However, there are similar numbers of Meg-CFC in c-myb–null fetuses as definitive-MEP in wildtype and c-myb heterozygotes (Figure 2A). This finding, and the prolonged temporal persistence of Meg-CFC in the yolk sac and liver of c-myb–null fetuses, supports the alternative concept that a restricted form of definitive megakaryopoiesis emerges in c-myb–null fetuses and that c-myb is necessary for the extensive proliferative capacity of definitive megakaryocyte progenitors. In this scenario, hematopoietic progenitors that would normally have become MEP are unable to take on an erythroid fate but retain the capability of becoming Meg-CFC with extremely restricted proliferative capacity.
Comparison of megakaryocyte colony numbers and thrombopoiesis in c-myb–null and combined wildtype and c-myb-heterozygous embryos. (A) The number of MEP (± SEM) in staged wildtype and c-myb-heterozygous mouse embryos ( ) compared with the number of Meg-CFC (± SEM) in similarly staged c-myb–null embryos (▭). Data derived from Figures 1C and D. YS = yolk sac, FL = fetal liver. (B) Total platelet numbers (± SEM) in c-myb–null (−/−, dashed line) and combined wild-type (+/+) and c-myb-heterozygous (+/−) (solid line) mouse embryos at E12.5 and E15.5 of mouse gestation. Platelets and blood cells, obtained from 3 to 5 μL of whole blood diluted in PBS/0.1M EDTA/0.1 mg/mL heparin (Sigma-Aldrich, St Louis, MO), were counted on a hemacytometer at 40× magnification using phase optics. Total platelet counts were based on platelet concentration × total blood cell measurements.16
) compared with the number of Meg-CFC (± SEM) in similarly staged c-myb–null embryos (▭). Data derived from Figures 1C and D. YS = yolk sac, FL = fetal liver. (B) Total platelet numbers (± SEM) in c-myb–null (−/−, dashed line) and combined wild-type (+/+) and c-myb-heterozygous (+/−) (solid line) mouse embryos at E12.5 and E15.5 of mouse gestation. Platelets and blood cells, obtained from 3 to 5 μL of whole blood diluted in PBS/0.1M EDTA/0.1 mg/mL heparin (Sigma-Aldrich, St Louis, MO), were counted on a hemacytometer at 40× magnification using phase optics. Total platelet counts were based on platelet concentration × total blood cell measurements.16
Comparison of megakaryocyte colony numbers and thrombopoiesis in c-myb–null and combined wildtype and c-myb-heterozygous embryos. (A) The number of MEP (± SEM) in staged wildtype and c-myb-heterozygous mouse embryos ( ) compared with the number of Meg-CFC (± SEM) in similarly staged c-myb–null embryos (▭). Data derived from Figures 1C and D. YS = yolk sac, FL = fetal liver. (B) Total platelet numbers (± SEM) in c-myb–null (−/−, dashed line) and combined wild-type (+/+) and c-myb-heterozygous (+/−) (solid line) mouse embryos at E12.5 and E15.5 of mouse gestation. Platelets and blood cells, obtained from 3 to 5 μL of whole blood diluted in PBS/0.1M EDTA/0.1 mg/mL heparin (Sigma-Aldrich, St Louis, MO), were counted on a hemacytometer at 40× magnification using phase optics. Total platelet counts were based on platelet concentration × total blood cell measurements.16
) compared with the number of Meg-CFC (± SEM) in similarly staged c-myb–null embryos (▭). Data derived from Figures 1C and D. YS = yolk sac, FL = fetal liver. (B) Total platelet numbers (± SEM) in c-myb–null (−/−, dashed line) and combined wild-type (+/+) and c-myb-heterozygous (+/−) (solid line) mouse embryos at E12.5 and E15.5 of mouse gestation. Platelets and blood cells, obtained from 3 to 5 μL of whole blood diluted in PBS/0.1M EDTA/0.1 mg/mL heparin (Sigma-Aldrich, St Louis, MO), were counted on a hemacytometer at 40× magnification using phase optics. Total platelet counts were based on platelet concentration × total blood cell measurements.16
Hypomorphic mutations of c-myb result in increased megakaryocytes in the fetus and thrombocytosis in the adult,8,–10,15 however, it is not known if absent or reduced levels of c-myb cause thrombocytosis in the embryo. We recently determined that platelets begin to circulate in mouse embryos between E10.5 to E11.5.4 Examination of c-myb–null fetuses revealed normal numbers of circulating platelets at E12.5 (Figure 2B), supporting the concept that the first fetal platelets are ultimately derived from primitive-Meg-CFC. Platelet numbers in wild-type and c-myb–heterozygous fetuses increase 2.6-fold between E12.5 and E15.5. In contrast, platelet numbers increased only 1.7-fold in c-myb–null fetuses (Figure 2B), suggesting that c-myb is required for sustained fetal thrombopoiesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Brindle for providing c-myb–heterozygous mice5 and Paul Kingsley for helpful assistance.
This work was supported by funding from the National Institutes of Health (HL59484 and DK09361).
National Institutes of Health
Authorship
Contributions: J.T. performed research, analyzed data, and wrote the paper; K.E.M. performed in situ hybridization studies; J.P. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Palis, MD, Dept. of Pediatrics, University of Rochester Medical Center, Box 703, 601 Elmwood Avenue, Rochester, NY 14642; e-mail: James_Palis@urmc.rochester.edu.