Cell therapy is a novel promising option for treatment of ischemic diseases. Administered endothelial progenitor cells (EPCs) are recruited to ischemic regions and improve neovascularization. However, the number of cells that home to ischemic tissues is restricted. The GTPase Rap1 plays an important role in the regulation of adhesion and chemotaxis. We investigated whether pharmacologic activation of Epac1, a nucleotide exchange protein for Rap1, which is directly activated by cAMP, can improve the adhesive and migratory capacity of distinct progenitor cell populations. Stimulation of Epac by a cAMP-analog increased Rap1 activity and stimulated the adhesion of human EPCs, CD34+ hematopoietic progenitor cells, and mesenchymal stem cells (MSCs). Specifically, short-term stimulation with a specific Epac activator increased the β2-integrin–dependent adhesion of EPCs to endothelial cell monolayers, and of EPC and CD34+ cells to ICAM-1. Furthermore, the Epac activator enhanced the β1-integrin–dependent adhesion of EPCs and MSCs to the matrix protein fibronectin. In addition, Epac1 activation induced the β1- and β2-integrin–dependent migration of EPCs on fibronectin and fibrinogen. Interestingly, activation of Epac rapidly increased lateral mobility of β1- and β2-integrins, thereby inducing integrin polarization, and stimulated β1-integrin affinity, whereas the β2-integrin affinity was not increased. Furthermore, prestimulation of EPCs with the Epac activator increased homing to ischemic muscles and neovascularization-promoting capacity of intravenously injected EPCs in the model of hind limb ischemia. These data demonstrate that activation of Epac1 increases integrin activity and integrin-dependent homing functions of progenitor cells and enhances their in vivo therapeutic potential. These results may provide a platform for the development of novel therapeutic approaches to improve progenitor cell homing.
Introduction
The term vasculogenesis describes the de novo formation of new vessels from angioblasts during embryonic development.1 Vasculogenesis, which can be mediated by circulating bone marrow-derived endothelial progenitor or hematopoietic stem cells, also contributes to postnatal neovascularization of adult ischemic tissues.2,,,–6 Therapeutical administration of endothelial progenitor cells (EPCs) increases neovascularization and improves left ventricular function after myocardial infarction in animal models.7,–9 Moreover, first clinical studies demonstrated a beneficial effect of intracoronary administration of EPCs or bone marrow cells on the left ventricular function in patients after myocardial infarction.10,–12 Progenitor cells are preferentially recruited to sites of ischemia and improve neovascularization by being directly incorporated into vascular structures and differentiating to endothelial cells and/or by eliciting paracrine effects.2,4,6,7,13,14 Both the paracrine effects and the differentiation of progenitor cells to endothelial cells depend on the homing of the progenitor cells to ischemic sites. However, only a small portion of systemically administered EPCs or bone marrow cells are recruited and remaining to ischemic tissues after intracoronary or intravenous infusion.15,–17 This underlines the need for the development of new strategies, to increase homing of systemically administered progenitor cells to ischemic tissues for improvement of progenitor cell-mediated neovascularization in patients with ischemic disorders.
In an in vivo intravital microscopy study, embryonic EPCs arrested within tumor microvessels, extravasated into the interstitium, and incorporated into neovessels, suggesting that adhesion and transendothelial migration are involved in the recruitment of EPCs.18 Recent evidence supports the involvement of integrins for the homing of EPCs to sites of active neovascularization. Specifically, we and other groups found that β2-integrins are mediating the homing of EPC and bone marrow progenitor cells to ischemic tissues.19,20 Moreover, the α4β1-integrin is involved in the homing of bone marrow progenitor cells to sites of active tumor angiogenesis.21 However, the regulation of integrin activity in EPC is unclear. Exchange protein directly activated by cAMP-1 and -2 (Epac-1 and Epac-2) are guanine nucleotide exchange factors for the small GTPases Rap1 and Rap2.22 Rap1 activates integrin activity and regulates integrin- and cadherin-dependent adhesion in many cellular systems.23,24 Epac1 and Epac2 guanine nucleotide exchange factor activity is directly activated by cAMP. Activation of Epac has been shown to increase Rap1 activity and to promote Rap1-dependently the β1-integrin-mediated adhesion.22,25,26 In the last years, significant progress has been achieved in the understanding of the biologic function of Epac by the introduction of a pharmacologic activator, 8-pCPT-2′-O-Me-cAMP, which specifically increases Epac activity without affecting protein kinase A (PKA) activity.22,25,27
Because adhesion and migration are important steps during homing of EPCs and progenitor cells to sites of ischemia, we investigated the effects of Epac activation on integrin-dependent homing functions of progenitor cells in vitro and in vivo. We found that Epac1 is expressed in human EPCs and that its stimulation with a specific pharmacologic activator is capable of increasing integrin activity and integrin-dependent homing functions, such as adhesion and migration. Besides EPC, Epac activation increased adhesion of mesenchymal stem cells (MSCs) and CD34+ hematopoietic progenitor cells. Moreover, short-term preincubation of human EPCs with the pharmacologic Epac activator increased in vivo homing to sites of ischemia and neovascularization-promoting capacity of EPC suggesting that activation of Epac1 is a useful strategy for increasing the therapeutical potential of human EPCs.
Methods
Approval was obtained from the J.W. Goethe University Hospital Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
Materials
Antihuman Epac1 antibody was purchased from Upstate (Schwalbach, Germany). All cAMP-analogs were purchased by Biolog Life Science Institute (Bremen, Germany). 8-pCPT-2′-O-Me-cAMP (8-pCPT-cAMP) is a specific, membrane-permeant Epac activator. 8-Br-2′-O-Me-cAMP activates both PKA and Epac, whereas 6-Bnz-cAMP is a specific activator of PKA. TNFα was purchased from Sigma (Munich, Germany).
Cell culture
Mononuclear cells (MNCs) were isolated by density-gradient centrifugation with Ficoll (Biochrom, Berlin, Germany) from peripheral blood of healthy human volunteers as described previously.28 Immediately after isolation, total MNCs (8 × 106 cells/mL medium; cell density 2.5 × 106 cells/cm2) were plated on culture dishes coated with 10 μg/mL human fibronectin (Sigma) and maintained in endothelial basal medium (Cambrex, Taufkirchen, Germany) supplemented with 1 μg/mL hydrocortisone, 12 μg/mL bovine brain extract, 50 μg/mL gentamycin, 50 ng/mL amphotericin B, 10 ng/mL epidermal growth factor, and 20% fetal calf serum. After 3 days, nonadherent cells were removed and adherent cells were incubated in medium for another 24 hours before initiation of the experiments. EPCs were characterized by dual staining for 1,1′-dioctadecyl–,3,3′,3′–tetramethylindo-carbocyanine-labeled acetyl-low-density lipoprotein (Dil; Cell Systems, Troisdorf, Germany) and lectin and expression of endothelial markers KDR, VE-cadherin, and VWF.15 Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex and cultured in endothelial basal medium supplemented with 1 μg/mL hydrocorti-sone, 12 μg/mL bovine brain extract, 50 μg/mL gentamicin, 50 ng/mL amphotericin-B, 10 ng/mL epidermal growth factor, and 10% fetal calf serum until the third passage. After detachment with trypsin, cells (1.5-2 × 104 cells per well) were seeded and grown in wells of 96-well plates (precoated with gelatin 0.2%, 2 hours, 37°C) for at least 48 hours as described previously. MSCs were isolated by density-gradient centrifugation with Ficoll from bone marrow of healthy human volunteers. Immediately after isolation, cells were maintained in Mesencult medium (StemCell Technologies, St Katharinen, Germany). MSC expressed CD73, CD13 and were negative for the expression of CD45. The CD34+ hematopoietic progenitor cells were isolated from human peripheral blood by immunomagnetic purification.
Cell migration
Transwell membranes (8 μm; Costar, Kaiserslautern, Germany) were coated on both sides with fibronectin (2.5 μg/mL; Roche, Mannheim, Germany) or fibrinogen (2.5 μg/mL; Hemochrom Diagnostica, Essen, Germany) overnight at 4°C. Ex vivo-expanded human EPCs were stained with CellTracker Green 5-chloromethylfluorescein diacetate (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. EPCs were detached by trypsinization, and after neutralization of trypsin, cells were resuspended in serum-free RPMI 1640 (Gibco, Karlsruhe, Germany) containing 0.05% bovine serum albumin (BSA; Sigma). EPC in suspension were stimulated with 8-pCPT-2′-O-Me-cAMP 100 μM for 2 hours, before migration. 8-pCPT-2′-O-Me-cAMP was washed out by centrifugation and resuspension of the cells in serum-free RPMI 1640 containing 0.05% BSA. Then EPCs were incubated in the upper chamber at 37°C in 5% CO2 for 16 to 18 hours. Cells remaining on the upper surface of the transwells membranes were mechanically removed, and cells that had migrated to the lower surface were fixed with 4% formaldehyde and counted in 5 fields using a fluorescence microscope (Axiovert 100; Carl Zeiss, Jena, Germany).
Cell–cell adhesion
Cell–cell adhesion was performed as previously described.19,29 Confluent HUVEC monolayers were used as matrix for the EPC adhesion. Ex vivo-expanded human EPCs were stained with Cell Tracker Green-CMFDA (Molecular Probes) or with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Molecular Probes) and after detachment with trypsin were resuspended in RPMI 1640 with 0.05% albumin. EPCs in suspension were stimulated with the indicated concentrations of 8-pCPT-2′-O-Me-cAMP or phosphate-buffered saline (PBS; control) for 15 minutes at 37°C. Before adhesion, 8-pCPT-2′-O-Me-cAMP was washed out by centrifugation and resuspension of the cells in RPMI 1640 (Gibco) containing 0.05% albumin (Sigma). Then a total of 105 EPC/well (in 100 μL RPMI 1640 with 0.05% albumin) was added to the HUVEC monolayers. In the experiments with the neutralizing integrin antibodies the EPCs were added to the TNFα-stimulated HUVEC monolayers in the presence of blocking monoclonal β2-integrin antibodies (clone TS1/18, 40 μg/mL; Biolegend, San Diego, CA) or murine isotype control antibodies (40 μg/mL; Alexis Biochemicals, Gruenberg, Germany). After incubation for 20 minutes at 37°C, the plates were washed with warm RPMI 1640 to remove nonadherent cells. Adherent EPCs were quantified in triplicates on a fluorescence plate reader (Fluostat; BMG Lab Technologies, Offenburg, Germany). All the antibodies used in the functional experiments contained no sodium azide.
Cell–matrix adhesion
Cell–matrix adhesion was performed as previously described.29,30 Ninety-six–well plates were coated overnight at 4°C with 5 μg/mL soluble recombinant human ICAM-1 (R&D Systems, Wiesbaden, Germany) or 2.5 μg/mL human fibronectin (Roche) in coating buffer (150 mM NaCl, 20 mM Tris HCl, 2 mM MgCl2, pH 9.0) and then blocked for 1 hour at room temperature with 3% (w/v) heat-inactivated (2 hours, 56°C) human serum albumin (Sigma). Ex vivo-expanded human EPCs or MSCs were stained with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (Molecular Probes) or CellTracker Green (Molecular Probes) and after detachment with trypsin were resuspended in RPMI 1640 containing 0.05% human serum albumin. Then cells were stimulated as indicated, with 8-pCPT-2′-O-Me-cAMP for 15 minutes at 37°C and subsequently seeded at 105 cells/well in 100 μL in the coated wells for 15 minutes at 37°C. After washing of nonadhering cells with warm RPMI 1640, adhering cells were quantified in triplicates with a fluorescence plate reader (Fluostat, BMG Lab Technologies or Synergy HT, Biotek, Bad Friedrishall, Germany).
Rap1 activity assay
Rap1 activation assays were performed according to the commercial Rap1-activity Assay Kit (Upstate). Briefly, EPCs were detached by trypsinization, and after neutralization of trypsin, cells were resuspended in serum-free RPMI 1640 containing 0.05% BSA (Sigma). EPCs were stimulated with 100μM 8-pCPT-2′-O-Me-cAMP for the indicated times. Then cells were lysed in Rap1 activation lysis buffer. Lysates were clarified by centrifugation, a portion of the cell lysate was reserved for analysis of total Rap1 content, and 500 μl of lysate was incubated with GST-tagged RBD of RalGDS precoupled to glutathione beads (Upstate) to specifically pull down the GTP-bound form of Rap1. Samples were incubated for 45 minutes at 4°C with gentle rotation. Beads were washed 3 times in lysis buffer. Rap1 was detected using Western blotting with antiRap1 antibodies (Upstate).
Detection of integrin activation epitopes
EPCs were harvested with trypsin and after centrifugation were resuspended in RPMI 1640 containing 0.05% heat-inactivated fetal calf serum. For the HUTS21 antibody staining, EPCs were incubated up to 15 minutes at 37°C with phycoerythrin (PE)-conjugated HUTS21 antibody (BD, Heidelberg, Germany) or isotype PE-labeled control antibody (BD, Germany) in the presence of 8-pCPT-2′-O-Me-cAMP 100μM or PBS (control). The β1-integrin subunit expression was evaluated using anti-CD29 FITC-labeled antibody (clone 4B4) (Beckman Coulter, Krefeld, Germany) and isotype FITC-labeled control antibodies (BD). Surface expression was quantified using a FACS CALIBUR (BD; San Diego, CA).
Immunofluorescence staining
After detachment with trypsin, EPCs were resuspended in RPMI 1640. EPCs were stimulated in suspension for 15 minutes at 37°C with 8-pCPT-2′-O-Me-cAMP 100μM or PBS. The reaction was stopped by fixation of the cells in suspension for 15 minutes with 3.3% paraformaldehyde at room temperature. Fixed cells were mounted on poly-L-lysine-coated slides (Sigma) and then blocked with 10% goat serum (DAKO, Hamburg, Germany) containing 2% BSA in PBS for 30 minutes at room temperature. For double staining of CD18 (β2-integrin-subunit) and CD44 or CD29 (β1-integrin-subunit) and CD44, fixed cells were first stained with the TS1/18 antibody (CD18, 20 μg/mL; Biolegend, San Diego, CA) or with the P5D2 antibody (CD29, Chemicon, Schwalbach, Germany) for 1 hour at room temperature followed by Alexa Fluor 546-conjugated goat antimouse antibody (Molecular Probes) (1:400 dilution) for 45 minutes at room temperature. Then, CD44 was detected with a FITC-labeled antihuman-CD44 antibody (1:25 dilution, 1 hour at room temperature, BD). Unbound antibodies were removed by 3 washing steps with PBS. Stained cells were viewed by confocal microscopy (LSM510, Zeiss, Jena, Germany).
Model of hind limb ischemia
The animal experiments were approved from the Regional Board of Land Hessen (Darmstadt, Germany). The proximal femoral artery including the superficial and the deep branch as well as the distal saphenous artery were ligated in 6-week old female nude mice; 48 hours after induction of limb ischemia, the mice were killed. Human EPCs were pretreated, where indicated, with 8-pCPT-2′-O-Me-cAMP 100 μM for 15 minutes at 37°C or PBS, and then washed, to remove 8-pCPT-2′-O-Me-cAMP 100 μM; 2 × 106 CM-Dil-labeled human EPCs were injected in the tail vein of each mouse. After 1 day, the mice were killed and the ischemic muscles were harvested. The number of EPC per high power field was determined using 10-μm cryosections. Nuclei were stained with Topro III (Molecular Probes). Injected human EPCs were identified by the CM-Dil labeling. A total of 10 high-power fields/per mouse was evaluated with confocal microscopy (Zeiss LSM 510) for the presence of EPCs. For the neovascularization experiments 24 hours after induction of hind limb ischemia in nude mice, human EPCs were stimulated with PBS or 8-pCPT-2′-O-Me-cAMP 100μM for 15 minutes at 37°C. After washing of 8-pCPT-2′-O-Me-cAMP, EPCs (nonstimulated or prestimulated with 8-pCPT-2′-O-Me-cAMP) or PBS (no cells) were intravenously injected in the tail vein. After 15 days, mice were killed and the ischemic muscles were harvested. Immunofluorescence staining of cryosections was performed by an antimouse-Laminin antibody from rabbit (Abcam, Cambridge, United Kingdom) followed by an Alexa Fluor 488-conjugated goat antirabbit secondary antibody (Molecular Probes) and a PE-conjugated anti-PECAM-1 antibody (BD). The number of capillaries in relation to muscles was determined using 10-μm cryosections. A total of 10 high-power fields/per mouse was evaluated with confocal microscopy by 2 persons blinded to the treatment of the mice (Zeiss LSM 510).
Statistical analysis
Continuous variables are expressed as mean plus or minus SEM. Comparisons between groups were analyzed by t test (2-sided) or ANOVA (post hoc test: LSD) for experiments with more than 2 subgroups (SPSS software; SPSS, Chicago, IL). P values less than .05 were considered as statistically significant.
Results
Activation of Epac stimulates adhesion of EPC, MSC, and CD34+ hematopoietic progenitor cells
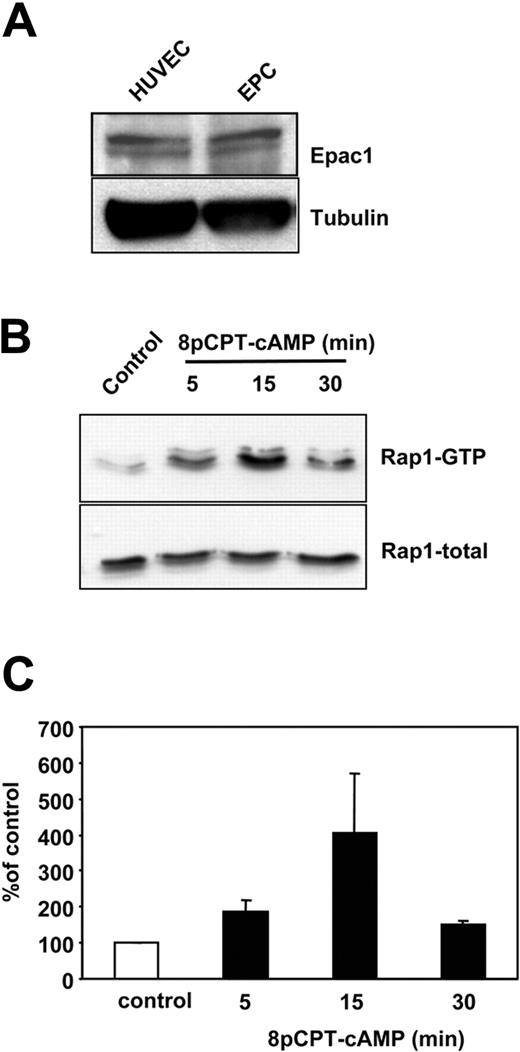
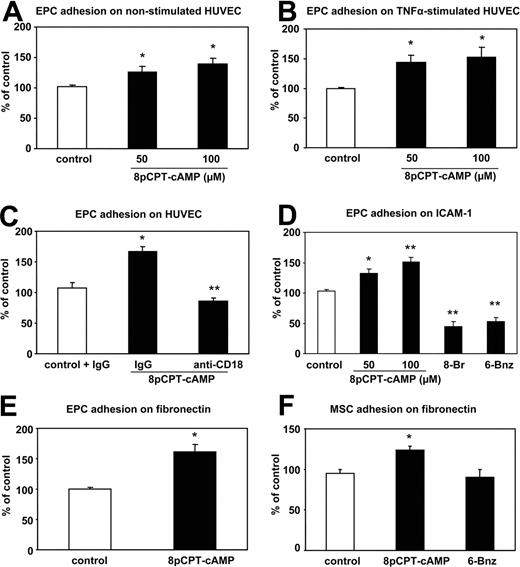
To assess the role of Epac1 on the integrin-dependent homing function of progenitor cells, we used 3 different progenitor cell populations: human ex vivo–expanded EPCs, human CD34+ hematopoietic progenitor cells, and human MSCs. The endothelial phenotype of the ex vivo–cultivated EPCs was confirmed by immunostaining, fluorescence-activated cell sorting analysis and functional response to shear stress as previously described.7,31,32 As assessed by Western blot, EPC express Epac1 (Figure 1A). Because Epac1 is an established guanine nucleotide exchange factor for the small GTPase Rap1, we next investigated whether stimulation of Epac 1 can increase Rap1 activity in progenitor cells. Indeed, stimulation of EPC with a specific Epac activator, 8-pCPT-2′-O-Me-cAMP, time-dependently increased the active GTP-bound form of Rap1 with a maximum after 15 minutes of stimulation (Figure 1B,C). These data demonstrate that human progenitor cells express functional Epac1 and that 8-pCPT-2′-O-Me-cAMP is able to activate Rap1 in progenitor cells. Next, we assessed the biologic effects of Epac1 activation on human EPCs, MSCs, and CD34+ hematopoietic progenitor cells. We first explored whether activation of Epac1 may affect vasculogenesis-related functions of progenitor cells. While stimulation with 8-pCPT-2′-O-Me-cAMP did not affect proliferation, apoptosis, and endothelial differentiation of EPCs (data not shown), preincubation of EPCs with 8-pCPT-2′-O-Me-cAMP dose-dependently increased the adhesion of EPCs to mature endothelial cell monolayers (with or without prestimulation with TNFα; Figure 2A,B). Because β2-integrins mediate EPC adhesion to HUVECs,19 we studied the effect of a neutralizing integrin antibody on the 8-pCPT-2′-O-Me-cAMP-induced EPC adhesion to TNFα-stimulated HUVECs. The inhibitory β2-integrin antibody abolished the pro-adhesive effect of 8-pCPT-2′-O-Me-cAMP, suggesting that Epac-induced adhesion of EPC to HUVEC is mediated by the β2-integrins (Figure 2C). To further decipher the 8-pCPT-2′-O-Me-cAMP-induced stimulation of progenitor cell–endothelial cell interactions, we investigated the effect of Epac activation on EPC and CD34+ hematopoietic progenitor cell adhesion to immobilized recombinant human ICAM-1, which functions as the major endothelial-cell ligand for β2-integrins.33 Short-term stimulation of progenitor cells with 8-pCPT-2′-O-Me-cAMP dose-dependently and significantly increased the adhesion of EPC (Figure 2D) and the adhesion of CD34+ hematopoietic progenitor cells to ICAM-1 (180.2% ± 28.7% vs control). We then investigated whether activation of Epac is capable of increasing the β1-integrin-dependent progenitor cell adhesion to fibronectin.33 Remarkably, short-term stimulation of EPC with 8-pCPT-2′-O-Me-cAMP significantly increased the adhesion of human EPC (Figure 2E) and of human MSC to fibronectin (Figure 2F). Interestingly, 6-Bnz-cAMP, another cAMP analog, which specifically activates PKA,34 or 8-Br-cAMP, which activates both, PKA and Epac, led to a significant inhibition of EPC adhesion to ICAM-1, whereas 6-Bnz-cAMP had no effect on the MSC adhesion to fibronectin, suggesting that Epac- but not PKA activation is able to activate integrin-dependent adhesion (Figure 2D,F). Taken together, these data demonstrate that specific activation of Epac can increase both β1- and β2-integrin-dependent adhesion of distinct progenitor/stem cell populations to matrix proteins.
Expression of Epac1 in EPCs. (A) Expression of Epac1 in HUVECs and EPCs as assessed by Western blot. (B) Human EPCs were stimulated for 5, 15, and 30 minutes in suspension with 8-pCPT-2′-O-Me-cAMP 100 μM. The level of GTP-bound (active) Rap1 was assessed. A representative blot from 3 independent experiments is shown. (C) Densitometric analysis of Rap1 activation in EPC from 3 independent experiments. Error bars represent SEM.
Expression of Epac1 in EPCs. (A) Expression of Epac1 in HUVECs and EPCs as assessed by Western blot. (B) Human EPCs were stimulated for 5, 15, and 30 minutes in suspension with 8-pCPT-2′-O-Me-cAMP 100 μM. The level of GTP-bound (active) Rap1 was assessed. A representative blot from 3 independent experiments is shown. (C) Densitometric analysis of Rap1 activation in EPC from 3 independent experiments. Error bars represent SEM.
Effect of 8-pCPT-2′-O-Me-cAMP on the adhesion of EPCs on HUVEC or matrix proteins. (A,B) EPCs were preincubated in suspension for 15 minutes with the indicated concentrations of 8-pCPT-cAMP or PBS (control). After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were added to the HUVEC monolayers. (A) Nonstimulated HUVECs (n = 9). (B) TNFα stimulated HUVECs (n = 6, *P < .05 vs control). (C) EPCs were preincubated in suspension with 8-pCPT-2′-O-Me-cAMP (100 μM) for 15 minutes. After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were added to TNFα-stimulated HUVECs in the presence of blocking monoclonal β2-integrin antibodies or murine isotype control antibodies and incubated for 20 minutes at 37°C (n = 3, *P < .05 vs control + IgG; **P< .01 vs 8-pCPT-cAMP + IgG). (D) EPCs were stimulated with the indicated 8-pCPT-2′-O-Me-cAMP concentrations for 15 minutes before adhesion or with 8-Br-cAMP and 6-Bnz-cAMP. Adhesion of EPC to ICAM-1-coated plates was detected after 15 minutes (n = 9, *P < .05 vs control; **P< .01 vs contol). (E) EPCs were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) for 15 minutes before adhesion. Adhesion of EPC to fibronectin-P-coated plates was detected after 15 minutes (n = 7, *P < .05 vs control). (F) MSCs were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or 6-Bnz-cAMP (100 μM) for 15 minutes before adhesion. Adhesion of EPC to fibronectin-coated plates was detected after 15 minutes (n = 5, *P < .05 vs control). Error bars represent SEM.
Effect of 8-pCPT-2′-O-Me-cAMP on the adhesion of EPCs on HUVEC or matrix proteins. (A,B) EPCs were preincubated in suspension for 15 minutes with the indicated concentrations of 8-pCPT-cAMP or PBS (control). After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were added to the HUVEC monolayers. (A) Nonstimulated HUVECs (n = 9). (B) TNFα stimulated HUVECs (n = 6, *P < .05 vs control). (C) EPCs were preincubated in suspension with 8-pCPT-2′-O-Me-cAMP (100 μM) for 15 minutes. After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were added to TNFα-stimulated HUVECs in the presence of blocking monoclonal β2-integrin antibodies or murine isotype control antibodies and incubated for 20 minutes at 37°C (n = 3, *P < .05 vs control + IgG; **P< .01 vs 8-pCPT-cAMP + IgG). (D) EPCs were stimulated with the indicated 8-pCPT-2′-O-Me-cAMP concentrations for 15 minutes before adhesion or with 8-Br-cAMP and 6-Bnz-cAMP. Adhesion of EPC to ICAM-1-coated plates was detected after 15 minutes (n = 9, *P < .05 vs control; **P< .01 vs contol). (E) EPCs were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) for 15 minutes before adhesion. Adhesion of EPC to fibronectin-P-coated plates was detected after 15 minutes (n = 7, *P < .05 vs control). (F) MSCs were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or 6-Bnz-cAMP (100 μM) for 15 minutes before adhesion. Adhesion of EPC to fibronectin-coated plates was detected after 15 minutes (n = 5, *P < .05 vs control). Error bars represent SEM.
Stimulation of Epac increases EPC migration
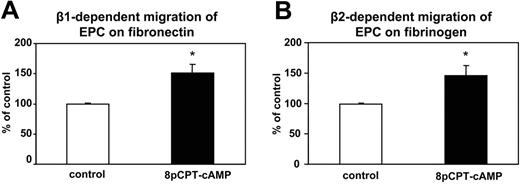
Besides adhesion, progenitor cell migration is another essential step during homing to ischemic tissues.35 We assessed the effect of Epac1 activation on the integrin-dependent matrix migration of EPC on matrix proteins. Preincubation of EPCs with 8-pCPT-2′-O-Me-cAMP increased the β1-integrin–dependent migration on fibronectin and the β2-integrin–dependent adhesion to fibrinogen (Figure 3A,B). These data suggest that activation of Epac can increase the integrin-dependent migratory capacity of human EPC on matrix proteins.
Effect of 8-pCPT-2′-O-Me-cAMP on the migration of EPC on matrix proteins. EPC migration assays on fibronectin (n = 19) (A) or fibrinogen (n = 11) (B) were performed. EPCs were stimulated in suspension with addition of PBS (control) or with 8-pCPT-2′-O-Me-cAMP 100μM. After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were allowed to migrate. Data are presented as mean migrated cells (% of control) plus or minus SEM. * indicates Pless than .05 vs control.
Effect of 8-pCPT-2′-O-Me-cAMP on the migration of EPC on matrix proteins. EPC migration assays on fibronectin (n = 19) (A) or fibrinogen (n = 11) (B) were performed. EPCs were stimulated in suspension with addition of PBS (control) or with 8-pCPT-2′-O-Me-cAMP 100μM. After washing out 8-pCPT-2′-O-Me-cAMP, EPCs were allowed to migrate. Data are presented as mean migrated cells (% of control) plus or minus SEM. * indicates Pless than .05 vs control.
Activation of Epac increases integrin activity in EPCs
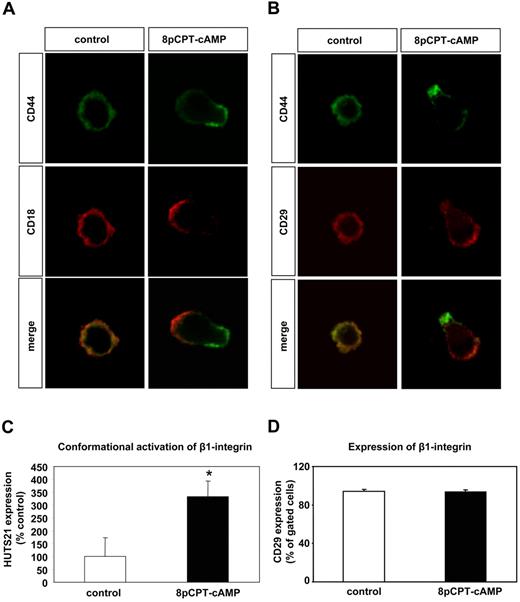
Because short-term stimulation of progenitor cells with 8-pCPT-2′-O-Me-cAMP rapidly increases adhesion and migration of progenitor cells, we hypothesized that stimulation of Epac1 may affect integrin activity in progenitor cells. Integrin activity is regulated by affinity and valency changes.36,37 First, we investigated whether 8-pCPT-2′-O-Me-cAMP can affect the distribution of integrins (in the absence of integrin ligands) on the surface of human EPCs, thereby affecting integrin valency/avidity. EPCs were incubated in suspension for 15 minutes in the presence or absence of 8-pCPT-2′-O-Me-cAMP, then immediately fixed and seeded on poly-L-lysine-coated glass slides. Immunofluorescence staining was performed for integrins and CD44. CD44 is an adhesion molecule, which localizes at the rear of lymphocytes on stimulation with chemokines.38 In the absence of 8-pCPT-2′-O-Me-cAMP, the β2-integrin and CD44 were homogeneously distributed on the EPC surface. Strikingly, activation of Epac in EPCs resulted in polarization of the β2-integrin-subunit (CD18) and of CD44 (Figure 4A). In addition, 8-pCPT-2′-O-Me-cAMP induced the polarization of the β1-integrin-subunit (CD29; Figure 4B). These data demonstrate that activation of Epac induces the lateral motility of the β1- and β2-integrins on the EPC surface, thereby increasing integrin valency.
Effect of 8-pCPT-cAMP on the lateral motility of integrins and CD44 on the cell surface of EPC. (A,B) EPCs in suspension (in the absence of integrin ligands) were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) for 15 minutes. Then EPCs were fixed in suspension and subsequently were mounted on poly-L-lysine-coated slides. Immunofluorescence was performed for the β2-integrin-subunit CD18 (red fluorescence) (A) or the β1-integrin-subunit CD29 (red fluorescence) (B) and CD44 (green fluorescence), and the stained cells were viewed by confocal microscopy. Micrographs were acquired with an LSM 510 confocal microscope (Zeiss, Jena, Germany) fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM 5 image acquisition software (Zeiss). (C) For detection of an activation-dependent epitope on β1-integrins, EPCs were incubated for 15 minutes at 37°C with PE-conjugated HUTS21 antibody or isotype PE-labeled control antibody in the presence of 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) (n = 8, *P < .05 vs control). (D) For detection of the β1-integrin subunits, EPCs were incubated for 15 minutes at room temperature with FITC-conjugated CD29 antibody or an isotype control FITC-labeled antibody in the presence of 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) (n = 3). Error bars represent SEM.
Effect of 8-pCPT-cAMP on the lateral motility of integrins and CD44 on the cell surface of EPC. (A,B) EPCs in suspension (in the absence of integrin ligands) were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) for 15 minutes. Then EPCs were fixed in suspension and subsequently were mounted on poly-L-lysine-coated slides. Immunofluorescence was performed for the β2-integrin-subunit CD18 (red fluorescence) (A) or the β1-integrin-subunit CD29 (red fluorescence) (B) and CD44 (green fluorescence), and the stained cells were viewed by confocal microscopy. Micrographs were acquired with an LSM 510 confocal microscope (Zeiss, Jena, Germany) fitted with a Plan-Neofluar 40×/1.3 NA oil objective and LSM 5 image acquisition software (Zeiss). (C) For detection of an activation-dependent epitope on β1-integrins, EPCs were incubated for 15 minutes at 37°C with PE-conjugated HUTS21 antibody or isotype PE-labeled control antibody in the presence of 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) (n = 8, *P < .05 vs control). (D) For detection of the β1-integrin subunits, EPCs were incubated for 15 minutes at room temperature with FITC-conjugated CD29 antibody or an isotype control FITC-labeled antibody in the presence of 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS (control) (n = 3). Error bars represent SEM.
Then, we investigated the effect of Epac activation on integrin affinity by using specific antibodies, which recognize activation-dependent epitopes on integrins. While the stimulation of EPC with 8-pCPT-2′-O-Me-cAMP had no effect on the total protein expression of the β1-integrin (CD29; Figure 4D), it significantly enhanced the expression of the activation-dependent epitope HUTS21 on the β1-integrin (Figure 4C). However, 8-pCPT-2′-O-Me-cAMP did not increase the expression of the activation-dependent epitopes mAb24 and CBRM1/5 on the β2-integrins (data not shown). Taken together, these data demonstrate that activation of Epac can increase the activity of both β1- and β2-integrins. However, although Epac activation can stimulate the lateral motility/valency of both β1- and β2-integrins, it can only increase the affinity of β1-integrins in progenitor cells.
Stimulation of Epac increases in vivo homing and neovascularization-promoting capacity of human EPCs
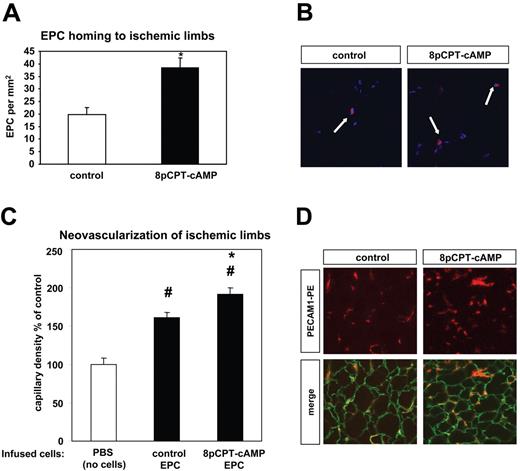
Stimulation of Epac by 8-pCPT-2′-O-Me-cAMP activates β1- and β2-integrins in EPC and increases integrin-dependent functions such as adhesion and migration in vitro. Therefore, we questioned whether activation of Epac by 8-pCPT-2′-O-Me-cAMP may affect EPC homing at sites of ischemia in vivo. Indeed, prestimulation of EPC with 8-pCPT-2′-O-Me-cAMP significantly enhanced the homing of intravenously injected EPC to ischemic limbs in the murine model of hind limb ischemia (Figure 5A,B). Prestimulation of EPC with 8-pCPT-2′-O-Me-cAMP before infusion significantly increased the neovascularization of ischemic muscles compared with nonstimulated EPC in the model of hind limb ischemia (Figure 5C,D).
Effect of 8-pCPT-cAMP on in vivo homing and neovascularization capacity of EPC. (A) CM-Dil-labeled EPCs in suspension were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS for 15 minutes. After a washing step, EPCs were resuspended in PBS and injected in the tail vein in nude mice 2 days after the induction of hind limb ischemia. The number of EPCs was assessed in the ischemic muscles by microscopy (* P < .05 vs control, control, n = 4; 8-pCPT-cAMP, n = 5; data are mean ± SEM). (B) Representative images of the homing experiment demonstrated in panel A. Infused EPCs were identified as CM-Dil-labeled cells (red fluorescence); the blue fluorescence indicates nuclear staining (Topro III). (C) EPCs in suspension were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS for 15 minutes at 37°C. After a washing step, 8-pCPT-2′-O-Me-cAMP-stimulated-EPCs, nonstimulated EPCs (control EPCs), or PBS (control, no cells) were injected in the tail vein in nude mice one day after the induction of hind limb ischemia. After 15 days, the ischemic muscles were harvested and the capillary density was assessed by microscopy as described in “Model of hind limb ischemia” (#P < .05 vs PBS, no cells; *P < .05 vs control EPCa; PBS, no cells: n = 7, control EPCs: n = 13, 8-pCPT-cAMP-stimulated EPCs: n = 12; data are mean ± SEM). (D) Representative images from ischemic muscles from nude mice intravenously injected with 8-pCPT-cAMP-stimulated EPCs or control (nonstimulated) EPCs (experiment in panel C). The laminin staining (green fluorescence) indicates the myocytes; the PECAM-1 staining (red fluorescence) indicates the capillaries. Images were acquired as in Figure 4.
Effect of 8-pCPT-cAMP on in vivo homing and neovascularization capacity of EPC. (A) CM-Dil-labeled EPCs in suspension were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS for 15 minutes. After a washing step, EPCs were resuspended in PBS and injected in the tail vein in nude mice 2 days after the induction of hind limb ischemia. The number of EPCs was assessed in the ischemic muscles by microscopy (* P < .05 vs control, control, n = 4; 8-pCPT-cAMP, n = 5; data are mean ± SEM). (B) Representative images of the homing experiment demonstrated in panel A. Infused EPCs were identified as CM-Dil-labeled cells (red fluorescence); the blue fluorescence indicates nuclear staining (Topro III). (C) EPCs in suspension were stimulated with 8-pCPT-2′-O-Me-cAMP (100 μM) or PBS for 15 minutes at 37°C. After a washing step, 8-pCPT-2′-O-Me-cAMP-stimulated-EPCs, nonstimulated EPCs (control EPCs), or PBS (control, no cells) were injected in the tail vein in nude mice one day after the induction of hind limb ischemia. After 15 days, the ischemic muscles were harvested and the capillary density was assessed by microscopy as described in “Model of hind limb ischemia” (#P < .05 vs PBS, no cells; *P < .05 vs control EPCa; PBS, no cells: n = 7, control EPCs: n = 13, 8-pCPT-cAMP-stimulated EPCs: n = 12; data are mean ± SEM). (D) Representative images from ischemic muscles from nude mice intravenously injected with 8-pCPT-cAMP-stimulated EPCs or control (nonstimulated) EPCs (experiment in panel C). The laminin staining (green fluorescence) indicates the myocytes; the PECAM-1 staining (red fluorescence) indicates the capillaries. Images were acquired as in Figure 4.
Discussion
The present study provides novel evidence for the regulation of integrin-dependent homing functions of progenitor cells by Epac1. Specifically, our investigations revealed that: (1) EPC express Epac1; (2) activation of Epac1 rapidly increases the β1- and β2-integrin–dependent adhesion of EPCs, CD34+ hematopoietic progenitor cells, and MSC; (3) pharmacologic activation of Epac1 increases EPC migration; and (4) pharmacologic stimulation of Epac1 induces a differential mode of integrin activation by increasing the lateral motility of β1- and β2-integrins, on the cell surface but enhancing selectively the affinity of β1-integrins. Finally, our data demonstrated that prestimulation of EPC with the Epac activator is able to enhance in vivo homing of EPC to sites of ischemia and their neovascularization-promoting capacity. Therefore, the present study provides insights into the regulation of integrin activity in progenitor cells and unravels a new possibility to activate integrin-dependent homing in progenitor cells by stimulation of Epac.
The present work demonstrates that activation of Epac rapidly enhances adhesion of progenitor cells via stimulating integrin activity. Prestimulation with 8-pCPT-2′-O-Me-cAMP increased β1-integrin–dependent adhesion of EPC and MSC to the β1-integrin-ligand fibronectin. Previous studies demonstrated that activation of Epac1 increased β1-integrin–dependent adhesion of ovarial carcinoma cells and monocytes.22,25,39 Remarkably, in the present work, we demonstrated for the first time that, besides β1-integrin–dependent adhesion, activation of Epac1 also stimulates β2-integrin–dependent adhesion of human EPCs to HUVEC monolayers and to ICAM-1, the major β2-integrin–ligand. In addition, pharmacologic activation of Epac1 increased the β2-integrin–dependent adhesion of CD34+ hematopoietic progenitor cells to ICAM-1. These effects seem to be specifically mediated by Epac because activators of PKA reduced EPC adhesion to ICAM-1. This observation is consistent with a reported inhibitory role of PKA on the β2-integrin–dependent adhesion of leukocytes.40
Besides adhesion, prestimulation with 8-pCPT-2′-O-Me-cAMP increased the migration of adult human peripheral blood-derived EPC on fibronectin and fibrinogen. In line with these results, a previous study demonstrated that activation of Epac1 enhances chemokine-induced migration of monocytes on fibronectin.39
To understand the stimulating effect of Epac activation on integrin activity, we separately studied the effects of 8-pCPT-2′-O-Me-cAMP on the integrin distribution on the cell surface (motility/valency) and on the integrin affinity of β1- and β2-integrins in EPC. Strikingly, 8-pCPT-2′-O-Me-cAMP affected the distribution of β1- and β2-integrins on the surface of EPC (integrin valency) in the absence of integrin ligands. Specifically, stimulation of EPC in suspension with 8-pCPT-2′-O-Me-cAMP led to a polarized distribution of β1- and β2-integrins and CD44 on the EPC surface with β1-integrins and β2-integrins segregating to the pole opposite to the CD44-localization. This distribution pattern is reminiscent of the polarization of leukocytes,38,41 in which CD44 is localized at the uropod (rear of migrating cells).38,41 These data indicate for the first time that activation of Epac induces the lateral mobility of integrins on the cell surface in the absence of ligands, thereby increasing integrin valency. Because cell polarization is essential for cell migration,42 it is conceivable that the 8-pCPT-2′-O-Me-cAMP–induced polarization of integrins may also be involved in the promigratory effect of 8-pCPT-2′-O-Me-cAMP on progenitor cells. In line with these results, β2-integrins are localized at the leading front of migrating human EPC on stimulation with a gradient of chemokines (G.C. and E.C., unpublished data, August 2007). Interestingly, while 8-pCPT-2′-O-Me-cAMP affected the distribution of both β1- and β2-integrins on the surface of EPCs in suspension, it also stimulated the expression of an activation-dependent epitope on β1-integrins, indicating an increase in integrin affinity. In contrast, the expression of 2 distinct activation-dependent epitopes on β2-integrins was not affected (data not shown). Taken together, we provide evidence that activation of Epac increases the lateral motility and the affinity of β1-integrins but enhances only the lateral motility of β2-integrins. In line with these data, activation of Epac1 was shown to increase the expression of an activation epitope of β1-, but not of β2-integrins in monocytes, although the effect of Epac activation on the integrin distribution on the cell surface was not investigated in this study.39 These differential responses suggest that Epac activation increases β1- and β2-integrin–dependent adhesion by distinct pathways. A possible molecular explanation could be that activation of the Epac pathway may affect β1- and β2-integrins via distinct effector proteins. In this regard, it is still an open question how 8-pCPT-2′-O-Me-cAMP regulates integrin affinity and avidity changes in progenitor cells. 8-pCPT-2′-O-Me-cAMP time-dependently increased the activity of the small GTPase Rap1 in EPC, which is involved in the stimulation of integrin affinity and avidity.23,43 In addition, the Epac1-induced adhesion in ovarial carcinoma cells was shown to be mediated by Rap1.25 Established Rap1 effector proteins, which could mediate Rap1-induced adhesion, are RAPL and RIAM.41,44 In addition, activation of Epac1 was shown to increase R-Ras activity.45 Further studies are required to clarify the mechanism of 8-pCPT-2′-O-Me-cAMP–induced integrin-activation in progenitor cells.
In accordance with our in vitro data regarding the proadhesive effect of Epac1 activation on 3 different progenitor cell populations (EPCs, MSCs, and CD34+ hematopoietic progenitor cells), short-term prestimulation of EPCs with 8-pCPT-2′-O-Me-cAMP enhanced the in vivo homing of EPCs to ischemic muscles in the murine model of hind limb ischemia. This observation indicates that activation of Epac1 could be a novel strategy to increase integrin-dependent homing functions of progenitor cells and their recruitment to ischemic tissues. In addition, short-term prestimulation of EPC with 8-pCPT-2′-O-Me-cAMP significantly increased the neovascularization-promoting capacity of infused EPCs, suggesting that activation of Epac may be a useful strategy to enhance the therapeutical potential of progenitor cells. In line with these results, integrin activation by activating β2-integrin-antibodies or by HMGB1 is sufficient to increase homing and neovascularization capacity of EPCs.19,35 Taken together, increased integrin-dependent homing functions of progenitor/stem cells by pharmacologic activation of Epac may be of therapeutic relevance for the treatment of patients with ischemic disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peggy Müller for excellent technical assistance, Ariane Fischer for the animal experiments, and Dr N. Hogg for providing us the antibody mAb24.
This work was supported by Deutsche Forshungsgemeinschaft (DFG; Transregional Collaborative Research Center SFB/TR23, Project A2; E.C., S.D.).
Authorship
Contribution: G.C. performed and designed experiments, and corrected the manuscript; E.C. designed and performed experiments, and wrote the manuscript; U.K. performed cell isolation procedures; A.M.Z. corrected the manuscript; and S.D. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanouil Chavakis, Molecular Cardiology, Department of Internal Medicine III, J. W. Goethe University of Frankfurt, Theodor Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: Chavakis@em.uni-frankfurt.de.
References
Author notes
G.C. and E.C. contributed equally to the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal