During blood feeding, mosquitoes inject saliva containing a mixture of molecules that inactivate or inhibit various components of the hemostatic response to the bite injury as well as the inflammatory reactions produced by the bite, to facilitate the ingestion of blood. However, the molecular functions of the individual saliva components remain largely unknown. Here, we describe anopheline antiplatelet protein (AAPP) isolated from the saliva of Anopheles stephensi, a human malaria vector mosquito. AAPP exhibited a strong and specific inhibitory activity toward collagen-induced platelet aggregation. The inhibitory mechanism involves direct binding of AAPP to collagen, which blocks platelet adhesion to collagen and inhibits the subsequent increase in intracellular Ca2+ concentration ([Ca2+]i). The binding of AAPP to collagen effectively blocked platelet adhesion via glycoprotein VI (GPVI) and integrin α2β1. Cell adhesion assay showed that AAPP inhibited the binding of GPVI to collagen type I and III without direct effect on GPVI. Moreover, intravenously administered recombinant AAPP strongly inhibited collagen-induced platelet aggregation ex vivo in rats. In summary, AAPP is a malaria vector mosquito-derived specific antagonist of receptors that mediate the adhesion of platelets to collagen. Our study may provide important insights for elucidating the effects of mosquito blood feeding against host hemostasis.
Introduction
The saliva of blood-sucking arthropods contains a number of pharmacologically active molecules that counteract the host defenses triggered by blood feeding, such as inhibitors of the clotting cascade, platelet aggregation and vasodilators.1 Mosquitoes search for blood by repeatedly thrusting their mouthparts into the host's deep network of skin vessels. This probing causes vessel laceration by the mouthparts. After laceration of blood vessels, platelets are generally exposed to subendothelial collagen in the damaged vessel walls, and subsequently adhere to the collagen, become activated, release their granular contents and form aggregates, with the result that the injuries are plugged by platelets.2 Thus, platelet activation by collagen is an initial step in normal hemostasis. However, mosquitoes can successfully engorge on their hosts within a half minute because antihemostatic components of their saliva facilitate location of blood vessels.3 Although mosquito saliva is known to show inhibitory activity toward platelet aggregation in response to adenosine diphosphate (ADP), collagen, and thrombin,3 a limited number of saliva components involved in the inhibition of platelet aggregation have been characterized. Specifically, Aedes aegypti apyrase inhibits ADP-induced platelet aggregation by metabolizing ADP released from injured cells,4 while Anopheles albimanus anopheline inhibits thrombin-mediated blood coagulation.5 However, no inhibitors of collagen-induced platelet aggregation have been identified to date
In anopheline mosquitoes, saliva and the salivary glands have been extensively studied due to their direct involvement in the transmission of malaria parasites to human hosts.6,7 Recently, the salivary gland transcriptosome and proteosome (termed the sialome) of anopheline mosquitoes have been reported.8,–10 In a previous study that characterized a salivary gland-specific promoter by using a transgenic Anopheles stephensi,11 we cloned a gene encoding a putative saliva protein that was specifically expressed in the female salivary glands. Sialome searches revealed that the gene product is a homolog of a 30-kDa A aegypti allergen of unknown function12 and well conserved among anopheline mosquitoes as a member of the GE-rich protein family.8,–10,13 In the present paper, we demonstrate that this A stephensi homolog protein specifically inhibits collagen-induced platelet aggregation. Therefore, we have renamed it anopheline antiplatelet protein (AAPP). To the best of our knowledge, this is the first report of a mosquito saliva component that is capable of specifically inhibiting collagen-induced platelet aggregation in vitro and ex vivo.
Methods
Preparation of a salivary gland extract
A stephensi mosquitoes (SDA 500 strain) were maintained at Jichi Medical University as described previously.14 The salivary glands were dissected from unfed female A stephensi mosquitoes, transferred to 1.5-mL conical polypropylene tubes containing cooled PBS and disrupted by sonification on ice. After centrifugation at 15 000g for 5 minutes, the resulting supernatant was assayed for platelet aggregation.
Materials
Fibrillar type I collagen from equine tendon (Horm), for use in platelet aggregation, cell adhesion, and Ca2+ influx assays, was from Nycomed Pharma (Munich, Germany); nonfibrillar type I collagen from rat tail collagen, for use under static conditions, was from BD Biosciences (San Jose, CA); collagen type III, collagen type IV, ADP, bovine serum albumin (BSA), normal goat serum, platelet-activating factor (PAF) and A23187 were from Sigma-Aldrich (St Louis, MO); epinephrine was from Daiichi Pharmaceuticals (Tokyo, Japan); U-46 619 was from Cayman Chemicals (Ann Arbor, MI); convulxin was from Alexis Biochemicals (San Diego, CA). Thrombin receptor-activating peptide (TRAP) was synthesized by Sawadey Technology (Tokyo, Japan). Collagen-related peptide (CRP) was synthesized by Peptide Institute (Osaka, Japan). Antihuman GPVI monoclonal antibody (mAb) OM-2 has previously been described.15 Anti-(human integrin α2-subunit) mAb 6F116 was a generous gift from Dr B. J. Coller (Mount Sinai Hospital, New York, NY).
Recombinant proteins
A cDNA encoding AAPP was cloned from an A stephensi salivary gland cDNA library by immunoscreening with a rabbit anti-A stephensi salivary gland immune serum as described previously.11 The cDNA fragment encoding amino acids 22 to 269 of AAPP, which lacked the signal peptide, was amplified from the aapp cDNA by PCR using the primers pAnSG-F7 (5′-CCATGGCGTCCGACGAGACTACGGATCAAGAA-3′) and pAnSG-R1 (5′-GCGGCCGCCTCTGAATCACGCTTTTCGACGATGC-3′). The PCR product was inserted into the NcoI/NotI sites of pET32-b (+) (Invitrogen, Carlsbad, CA) and expressed as a thioredoxin fusion protein. As a control, recombinant thioredoxin (rTrx) was produced by using pET32-b (+) without any insert. rAAPP and rTrx were produced and solubilized under denaturing conditions using 6 M guanidine-HCl, and then purified by affinity chromatography on a Ni-NTA column (Qiagen, Valencia, CA) followed by dialysis against PBS as described previously.17 The purity of the recombinant proteins was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and the protein yield was estimated by comparison with BSA standards.
Indirect immunofluorescence
The salivary glands were dissected in PBS, fixed in 4% paraformaldehyde for 20 minutes at 4°C and dried on poly-L-lysine–treated slides (MAS Coated Slides; Matsunami, Tokyo, Japan). The glands were then incubated with 10% normal goat serum in PBS for 1 hour at room temperature, followed by incubation with a mouse anti-rAAPP immune serum. After extensive washing, the salivary glands were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Biosource, Camarillo, CA). The salivary glands were mounted onto glass slides, covered with a drop VECTASHIELD (Vector, Laboratories, Burlingame, CA), and examined by fluorescence microscopy.
SDS-PAGE, silver staining, and immunoblotting
Salivary glands were solubilized in Laemmli buffer18 containing 2% 2-mercaptoethanol and boiled for 5 minutes. The proteins were separated by SDS-PAGE using a 10% gel, and then stained with a Silver Stain Kit (GE Healthcare UK, Chalfont St Giles, United Kingdom) or electrophoretically transferred to an Immobilon Transfer Membrane (Millipore, Bedford, MA). For immunoblotting, the membrane was treated with a mouse anti-rAAPP immune serum. A Polypeptide band recognized by the serum was detected with biotinylated antimouse IgG (H + L; Vector Laboratories), followed by color development with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt/nitroblue tetrazolium chloride substrate (Life Technologies, Rockville, MD) as described previously.19
RT-qPCR
Total RNA was isolated from mosquito salivary glands and carcasses using the RNeasy Mini columns (Qiagen). The aapp and ubiquitin mRNAs were quantified by reverse-transcription quantitative polymerase chain reaction (RT-qPCR), with ABI PRISM 7700 Sequence Detection System and the SYBR Green Master Mix Kit (Applied Biosystems, Foster City, CA). The primers used for detection of the aapp and ubiquitin genes were as follows: pAAPP-RT-F1: 5′-CGACCTGGTCGGCAGTATCT-3′, and pAAPP-RT-R1, 5′-CGAGTCGAAGCATTCCTTAATCTT-3′; pAnsUbi-RT-F1, 5′-CGCAAGTGCTACGCTCGTC-3′, and pAnsUbi-RT-R1, 5′-TGGTGTGACCGCACTTCTTC-3′. These primers were designed using Primer Express Software v. 1.5 (Applied Biosystems). The fold induction of the mRNA was determined from the threshold cycle values normalized for the ubiquitin gene expression and then normalized to the value derived from the aapp gene in the salivary glands of male mosquitoes.
Platelet aggregation assay
Platelet aggregation with human platelet-rich plasma (PRP), obtained by centrifuging citrated blood, was investigated as described previously.20 Platelet number was determined by an automatic counter, and PRP were prepared to 3 × 108 cells/mL with platelet-poor plasma (PPP). To measure changes in the light transmission rate, PRP samples (200 μL) were incubated with stirring for 2 minutes at 37°C in the presence or absence of rAAPP, then with 22.2 μL of platelet agonists for 5 minutes at 37°C. The intensity of light transmission over 5 minutes was then measured using an aggregometer (MCM Hematracer 313M, Model:PAM-12C, SSR Engineering, Tokyo, Japan). The baseline was set with PRP and the maximum possible increase in light transmission (platelet aggregation rate: 100%) was set with PPP. The percentage inhibition was calculated based on the maximum aggregation rate of the test samples relative to the appropriate buffer control.
Preparation of human washed platelets
Blood was isolated from an arm vein of healthy human volunteers. The blood was drawn with a 21-G needle into a plastic syringe containing 0.1 vol 3.8% trisodium citrate solution, and centrifuged at 180g for 10 minutes. PRP was removed and mixed with one-fourth volume of ACD buffer (44.8 mM sodium citrate, 20.9 mM citric acid, 74.1 mM glucose, pH 5.0). After the centrifugation, the platelet pellet was resuspended in washing buffer (113 mM NaCl, 4 mM KCl, 24 mM NaH2PO4, 4 mM Na2HPO4, 0.2 mM EGTA, 0.1% glucose pH 6.0), and recentrifuged at 500g for 20 minutes to create a platelet pellet. The final pellet was resuspended in incubation buffer (134 mM NaCl, 12 mM NaHCO3, 0.34 mM NaH2PO4, 2.9 mM KCl, 1 mM CaCl2, 0.8 mM MgCl2, 5 mM Hepes, 5 mM glucose, pH 7.4).
AAPP binding assay
Binding of rAAPP to collagen was measured in 96-well collagen-coated microtiter plates (Nunc, Rochester, NY). After blocking with blocking buffer (PBS containing 5% BSA) for 1 hour at room temperature, various concentrations of rAAPP was added and incubated for 1 hour at room temperature. Binding of rAAPP to collagen was detected using the ExpressDetector Nickel-HRP (KPL, Gaithersburg, MD), which can bind to the His-tag at the C-terminal of rAAPP, according to the manufacturer's protocol. As a control, rTrx was used.
Platelet adhesion assay
Ninety-six–well microtiter plates were coated with 40 μg/mL of collagen for 1 hour at room temperature, followed by blocking with the blocking buffer for 1 hour. Control wells were coated with the blocking buffer only. The wells were rinsed 3 times with PBS. rAAPP was serially diluted, and 50 μL of each dilution was added to the plates and incubated for 30 minutes at room temperature. Next, 50 μL of human washed platelet suspension (6 × 108 cells/mL) was added to each well and incubated for 45 minutes at room temperature. Nonadherent platelets were removed, and the wells were washed 3 times with PBS. The number of adherent platelets was determined using the Dc protein assay kit (Nippon Bio-Rad, Tokyo, Japan).
GPVI-expressing Jurkat cells
The human GPVI cDNA was cloned from a cDNA library of the human placenta (Clontech, Palo Alto, CA) and inserted into a lenti virus vector,21 kindly provided by Dr X. Wu (University of Alabama, Birmingham, AL), and transfected with Jurkat cells. A stable GPVI-expressing Jurkat cell line was established by a method of Kappes et al21 and maintained in RPMI1640 10% FCS medium supplemented with 0.5 μg/mL Puromycin (Sigma-Aldrich). Surface expression of GPVI was confirmed by flow cytometry using antihuman GPVI mAb OM-2. Nontransfected Jurkat cells do not express endogenous GPVI.
Cell adhesion assay
Ninety-six–well microtiter plates were coated with various concentrations of collagen or rAAPP. The wells were blocked with the blocking buffer at room temperature for 1 hour, then rinsed once in PBS. GPVI-expressing Jurkat cells were washed with PBS twice and resuspended in the blocking buffer at 1 × 106 cells/mL. One hundred microliters of cell suspension was added to each well and incubated at room temperature for 1 hour. The wells were washed with PBS 3 times to remove nonadherent cells. Adherent cells were quantified by the BCA protein assay kit (Pierce, Rockford, IL).
GPVI- and α2β1-mediated platelet adhesion assay
Ninety-six–well microtiter plates were coated with insoluble equine tendon fibrillar type I collagen or soluble rat tail type I (nonfibrillar) collagen maintained in acetate buffer, pH 4.5. Various concentrations of rAAPP were added to the 96-well plates immobilized with the fibrillar type I collagen with Tyroad's-Hepes buffer (136.7 mM NaCl, 13.8 mM, NaHCO3, 0.36 mM NaH2PO4·H2O, 2.6 mM KCl, 1.0 mM MgCl2·6H2O, 5.5 mM glucose, 0.25% BSA, pH 7.4) containing 50 μM EDTA. Similarly, various concentrations of rAAPP were added to the 96-well plates immobilized with the nonfibrillar type I collagen with Tyroad-Hepes buffer and incubated for 1 hour. After washing with PBS 3 times, washed platelet suspension (105 cells/μL) were allowed to adhere under static conditions to each well for 1 hour. As positive controls, washed platelet suspension were pre-incubated with antihuman GPVI mAb OM-2 (1 μg/mL) or anti-(human integrin α2-subunit) mAb 6F1 (5 μg/mL). Adherent platelets were quantified fluorimetrically as described by Nakamura et al.22 Results are representative of 3 independent experiments and expressed as the mean of triplicate reading plus or minus SEM for the indicated concentrations.
Measurement of intracellular Ca2+ concentration
Ca2+ concentration ([Ca2+]i) transients were monitored by fura-2 fluorescence. Human PRP was incubated with 5 μM fura-2 AM (Dojindo, Tokyo, Japan) for 60 minutes at 37°C. After 2 washes with the wash buffer, the platelets were resuspended to 3 × 108 cells/mL with the incubation buffer. After a 1-minute incubation with various concentrations of rAAPP, the platelets were stimulated with collagen (0.5 μg/mL) and the fura-2 fluorescence was measured at an excitation wavelength of 340/380 nm and emission wavelength of 500 nm using a Hitachi F-2000 fluorescence spectrophotometer.
Ex vivo platelet aggregation of rats
Platelet aggregation of rats was investigated as described previously.23 Crl:SD rats (Charles River Japan, Tokyo, Japan) were used in this study. An rAAPP solution (0.1, 0.3, or 1.0 mg/kg) or rTrx control solution was administered intravenously into the tail vein. 10 minutes after the administration, 6 mL of blood was sampled from the inferior vena cava of each rat under ether anesthesia using a 21-G needle and a plastic syringe containing 0.1 vol. of 3.18% trisodium citrate solution. Platelets were prepared at 109 cells/mL with autologous PPP. Platelet aggregation was measured 60 minutes after blood collection. All care and handling of the animals was in accordance with the Guidelines for Animal Care and Use prepared by Otsuka Pharmaceutical.
Statistical analyses
Results are presented as the means plus or minus standard error of the mean (SEM). The protein concentrations and inhibitory ratios of platelet aggregation were transformed into logarithms and logits, respectively. IC50 values and 95% confidence limits were calculated by log-logit regression analysis. The SAS system (Release 8.1; SAS Institute Japan, Tokyo, Japan) was employed for these statistical analyses.
Results
AAPP is a predominant protein in the female salivary glands
The complete AAPP cDNA has an ORF encoding 269 amino acid residues. The gene product has characteristic features of acidic secreted proteins (pI = 3.8) containing 10 unique repeats of a 6-amino acid unit (GEEGGA) or related sequences (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). We named this protein anopheline antiplatelet protein (AAPP), according to its biologic function identified in the present study.
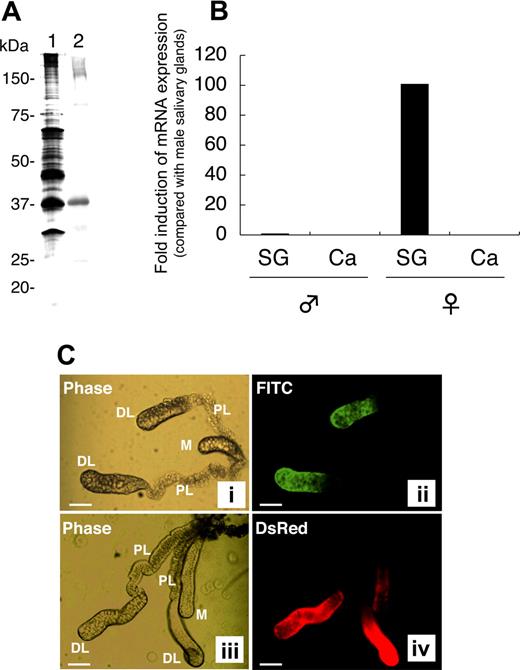
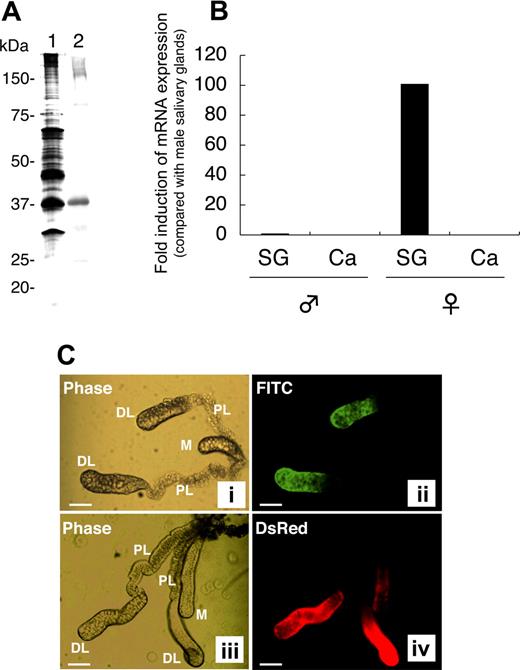
rAAPP was produced as a thioredoxin fusion protein by using an E coli expression system. Anti-rAAPP immune serum specifically reacted with a salivary gland protein with an apparent molecular mass of around 37 kDa (Figure 1A lane 2), which corresponds to one of the most abundant proteins found in the female salivary glands (Figure 1A lane 1). To confirm the tissue- and sex-specificity of AAPP expression, we performed RT-qPCR analysis of the aapp mRNA extracted from the salivary glands and carcasses of male and female adult mosquitoes. Abundant aapp mRNA was detected in the salivary glands of female mosquitoes, compared with the very low expression level (< 1%) in the male salivary glands (Figure 1B). No aapp mRNA was detected in the carcasses of either male or female mosquitoes. These results demonstrate that abundant AAPP specifically expressed in the female salivary gland, suggesting that the protein plays important roles in blood feeding.
Female salivary gland–specific expression of AAPP. (A) Western blotting for AAPP in the salivary glands of female mosquitoes. A homogenate of 2 pairs of female salivary glands was subjected to 10% SDS-PAGE. Lane 1, silver staining; lane 2, Western blotting using a mouse anti-rAAPP immune serum. (B) RT-qPCR of aapp mRNA. Total RNA was isolated from salivary glands (SG) and carcasses (Ca) of male and female mosquitoes. The expression of aapp mRNA was examined for its tissue- and sex-specificity using RT-qPCR. The fold induction was determined from threshold cycle values normalized for ubiquitin mRNA expression and then normalized to the values of each gene from the male salivary gland mRNA. (C) Immunostaining of salivary glands with the anti-rAAPP immune serum. The paired salivary glands of mosquitoes are present in the thorax flanking the esophagus. Each gland consists of 3 lobes: a distal lateral lobe (DL), proximal lateral lobe (PL), and medial lobe (M). (i) phase-contrast image of the salivary glands of a wild-type mosquito; (ii) immunostaining of the salivary glands of a wild-type mosquito with the anti-rAAPP immune serum; (iii) phase-contrast image of the salivary glands of a DsRed transgenic mosquito; and (iv) DsRed expression in the salivary glands of a DsRed transgenic mosquito. Scale bars: 100 μm.
Female salivary gland–specific expression of AAPP. (A) Western blotting for AAPP in the salivary glands of female mosquitoes. A homogenate of 2 pairs of female salivary glands was subjected to 10% SDS-PAGE. Lane 1, silver staining; lane 2, Western blotting using a mouse anti-rAAPP immune serum. (B) RT-qPCR of aapp mRNA. Total RNA was isolated from salivary glands (SG) and carcasses (Ca) of male and female mosquitoes. The expression of aapp mRNA was examined for its tissue- and sex-specificity using RT-qPCR. The fold induction was determined from threshold cycle values normalized for ubiquitin mRNA expression and then normalized to the values of each gene from the male salivary gland mRNA. (C) Immunostaining of salivary glands with the anti-rAAPP immune serum. The paired salivary glands of mosquitoes are present in the thorax flanking the esophagus. Each gland consists of 3 lobes: a distal lateral lobe (DL), proximal lateral lobe (PL), and medial lobe (M). (i) phase-contrast image of the salivary glands of a wild-type mosquito; (ii) immunostaining of the salivary glands of a wild-type mosquito with the anti-rAAPP immune serum; (iii) phase-contrast image of the salivary glands of a DsRed transgenic mosquito; and (iv) DsRed expression in the salivary glands of a DsRed transgenic mosquito. Scale bars: 100 μm.
To further analyze the location of AAPP, the salivary glands were immunostained with an anti-rAAPP immune serum. AAPP was specifically detected in the distal lateral lobes of the salivary glands but not in the medial lobes or proximal lateral lobes (Figure 1C panel 2). Trace amounts of AAPP were rarely detected in the medial lobes (data not shown). These AAPP expression sites are consistent with our previous study showing that the aapp promoter can strongly drive the expression of Discosoma sp red fluorescent protein (DsRed) at the same regions of the salivary glands in a DsRed transgenic mosquito (Figure 1C panel 4).11
AAPP inhibits platelet aggregation via a collagen activation pathway
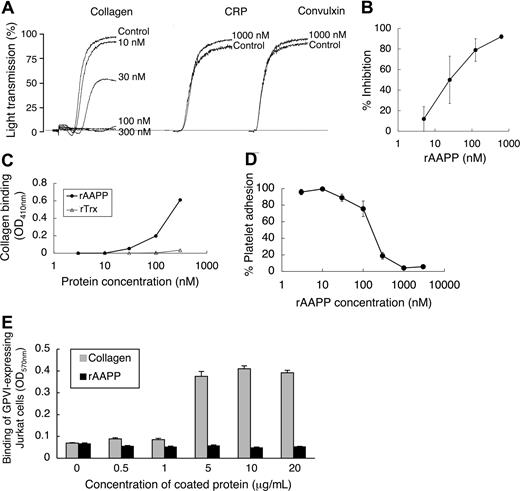
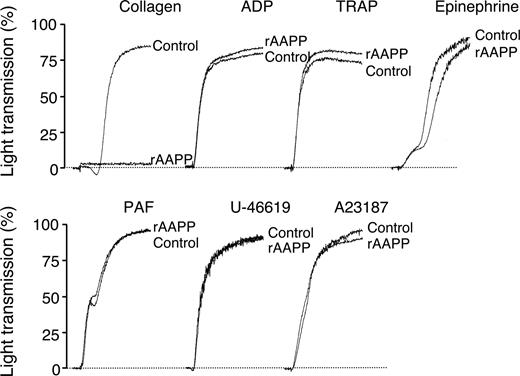
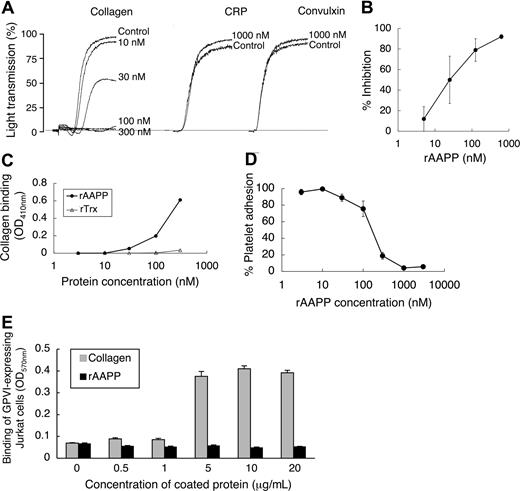
To investigate the biologic functions of AAPP, we focused on its effect on platelet aggregation using various platelet aggregation agonists. AAPP inhibited collagen-induced human platelet aggregation (Figure 2), but had no effects on ADP-, TRAP-, epinephrine-, PAF-, U-46 619- or A23187-induced platelet aggregation. The IC50 of rAAPP for collagen-induced platelet aggregation was approximately 25 nM (Figure 3A,B). Collagen induces platelet aggregation by interacting with the immunoglobulin-like receptor GPVI24,25 on the surface of platelets. GPVI plays the central role in platelet-collagen interactions by activating different adhesive receptors.26 2 specific agonists were used to examine the interaction between rAAPP and GPVI: the synthetic, triple-helical, collagen-related peptide (CRP) based on a repeated GPO (glycine-proline-hydroxyproline) motif,27 and convulxin, a multimeric C-type lectin-like toxin from the venom of a rattlesnake28,29 These 2 molecules activate platelets selectively via GPVI. Figure 3A shows that rAAPP had no effect on CRP- or convulxin-induced platelet aggregation at concentrations of up to 1000 nM.
AAPP specifically inhibits collagen-induced platelet aggregation. PRP were incubated with 1000 nM rAAPP for 2 minutes followed by the addition of various platelet aggregation agonists, collagen (1.5 μg/mL), ADP (8 μM), TRAP (30 μM), epinephrine (0.5 μg/mL), PAF (0.125 μg/mL), U46619 (1 μM), and A23187 (20 μM). Tracings are representative results of a typical experiment.
AAPP specifically inhibits collagen-induced platelet aggregation. PRP were incubated with 1000 nM rAAPP for 2 minutes followed by the addition of various platelet aggregation agonists, collagen (1.5 μg/mL), ADP (8 μM), TRAP (30 μM), epinephrine (0.5 μg/mL), PAF (0.125 μg/mL), U46619 (1 μM), and A23187 (20 μM). Tracings are representative results of a typical experiment.
AAPP specifically inhibits collagen-induced platelet aggregation by binding to collagen. (A) AAPP does not inhibit GPVI agonist-mediated platelet aggregation. PRP was incubated with the indicated concentrations of AAPP for 2 minutes followed by the addition of collagen (2 μg/mL). In the presence of 1000 nM rAAPP, PRPs were stimulated by CRP (0.5 μg/mL) or convulxin (10 ng/mL). As a control, the indicated concentrations of rAAPP were incubated in the absence of CRP or convulxin. Tracings are representative results of a typical experiment. (B) Dose-dependent inhibition of collagen-induced platelet aggregation by AAPP. PRPs were incubated with various amounts of rAAPP for 2 minutes followed by the addition of collagen. Results are means plus or minus SEM from 3 independent experiments. (C) Binding of AAPP to immobilized collagen. Various concentrations of rAAPP or rTrx were incubated in 96-well collagen-coated plates for 1 hour. After washing, bound proteins were detected using the Nickel-HRP. (D) Effect of AAPP on platelet adhesion to collagen. Washed platelet suspension and the indicated amounts of rAAPP were added to 96-well collagen-coated plates and incubated for 1 hour. After washing, adherent platelets were detected by the Dc protein assay kit. Results are means plus or minus SEM from 3 independent experiments. (E) GPVI binds to collagen but not AAPP. The indicated amounts of collagen or rAAPP were coated in 96-well plates. GPVI-expressing Jurkat cells were added to each well and incubated for 1 hour. After washing, adhesion cells were measured by the BCA protein assay. Results are representative of 3 independent experiments and expressed as the mean of tripli-cate reading plus or minus SEM for the indicated concentrations.
AAPP specifically inhibits collagen-induced platelet aggregation by binding to collagen. (A) AAPP does not inhibit GPVI agonist-mediated platelet aggregation. PRP was incubated with the indicated concentrations of AAPP for 2 minutes followed by the addition of collagen (2 μg/mL). In the presence of 1000 nM rAAPP, PRPs were stimulated by CRP (0.5 μg/mL) or convulxin (10 ng/mL). As a control, the indicated concentrations of rAAPP were incubated in the absence of CRP or convulxin. Tracings are representative results of a typical experiment. (B) Dose-dependent inhibition of collagen-induced platelet aggregation by AAPP. PRPs were incubated with various amounts of rAAPP for 2 minutes followed by the addition of collagen. Results are means plus or minus SEM from 3 independent experiments. (C) Binding of AAPP to immobilized collagen. Various concentrations of rAAPP or rTrx were incubated in 96-well collagen-coated plates for 1 hour. After washing, bound proteins were detected using the Nickel-HRP. (D) Effect of AAPP on platelet adhesion to collagen. Washed platelet suspension and the indicated amounts of rAAPP were added to 96-well collagen-coated plates and incubated for 1 hour. After washing, adherent platelets were detected by the Dc protein assay kit. Results are means plus or minus SEM from 3 independent experiments. (E) GPVI binds to collagen but not AAPP. The indicated amounts of collagen or rAAPP were coated in 96-well plates. GPVI-expressing Jurkat cells were added to each well and incubated for 1 hour. After washing, adhesion cells were measured by the BCA protein assay. Results are representative of 3 independent experiments and expressed as the mean of tripli-cate reading plus or minus SEM for the indicated concentrations.
Direct binding of AAPP to collagen interferes with adhesion of platelets to collagen
When rAAPP was incubated with immobilized collagen in a 96-well plate, rAAPP effectively bound to collagen in a dose-dependent manner (Figure 3C). Moreover, the rAAPP binding strongly interfered with adhesion of platelets to the immobilized collagen in a dose-dependent manner (Figure 3D). The IC50 of the platelet adhesion blocking was 250 nM, and 1000 nM rAAPP completely prevented detectable platelet adhesion to collagen. Next, we examined the possible involvement of AAPP in interactions with GPVI. Figure 3E shows that GPVI-expressing Jurkat cells bound to collagen type I–coated plate, but not to AAPP-coated plate, indicating that AAPP dose not involved in direct interaction with GPVI. This result is consistent with the data that rAAPP had no effect on 2 GPVI agonists (Figure 3A).
AAPP acts as an antagonist of receptors that mediate adhesion of platelets to collagen
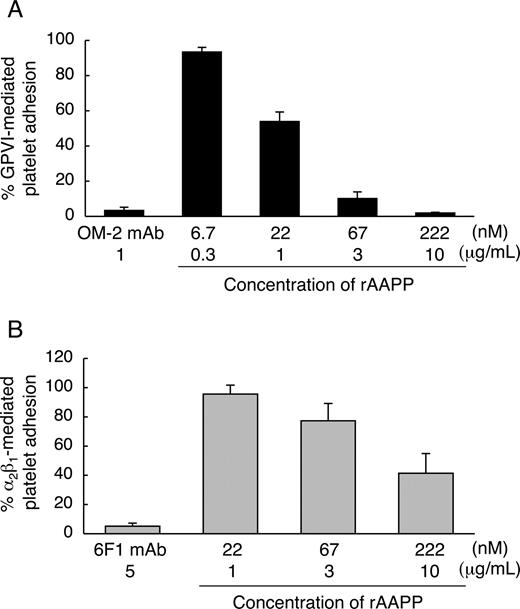
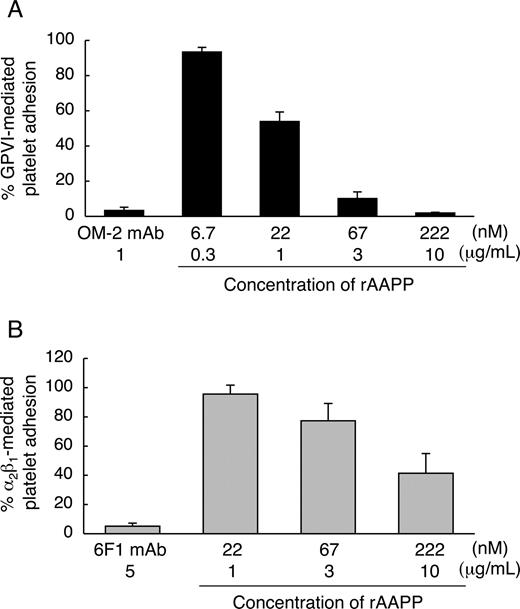
We found that the AAPP-mediated platelet aggregation inhibition is due to interfere with collagen-platelet interaction by direct binding of AAPP to collagen. Collagen induces platelet aggregation by interacting with GPVI24,25 and integrin α2β130,31 on the surface of platelets. GPVI- and integrin α2β1-mediated platelet adhesion are well characterized. Integrin α2β1 is known to mediate platelet adhesion to collagen in a Mg2+-dependent manner.32 Human platelets adhere to soluble monomeric collagen in a Mg2+-dependent manner, while they adhere to insoluble polymeric collagen in a Mg2+-independent manner.33 The studies with anti-integrin α2β1 and anti-GPVI antibodies indicate that integrin α2β1 mediates the platelet adhesion to soluble monomeric collagen in a Mg2+-dependent manner,22,34,35 and that GPVI mediates the platelet adhesion to insoluble fibrillar collagen in a Mg2+-independent manner.22 Therefore, to address whether AAPP interferes with platelet adhesion by selectively binding to collagen receptors, we examined the effects of AAPP on platelet adhesion to soluble collagen in the presence of Mg2+ (integrin α2β1-mediation) and to insoluble collagen in the absence of Mg2+ (GPVI-mediation). Various concentrations of rAAPP were incubated with insoluble fibrillar type I collagen immobilized in a 96-well plate before adding platelet suspension under static conditions in the absence of Mg2+. Figure 4A shows that GPVI-mediated platelet adhesion was abolished by increasing amounts of rAAPP. The IC50 was approximately 22 nM, and complete inhibition was reached with 222 nM (10 μg/mL). As a positive control, a mouse mAb specific for human GPVI, OM-2 (1 μg/mL), strongly inhibited the platelet adhesion (> 95%). Next, we examined the ability of rAAPP on the binding of integrin α2β1 under static conditions. In the presence of Mg2+, integrin α2β1-mediated platelet adhesion to nonfibrillar type I collagen was reduced by increasing amounts of rAAPP (IC50≈222 nM) (Figure 4B). As a positive control, a mouse mAb specific for human integrin α2-subunit, 6F1 (5 μg/mL), strongly inhibited the platelet adhesion (> 95%). Thus, AAPP effectively inhibited both GPVI- and integrin α2β1-mediated platelet adhesion to collagen.
AAPP inhibits both GPVI- and integrin α2β1-mediated platelet adhesion to collagen. The indicated concentrations of rAAPP were incubated in 96-well plates coated with fibrillar type I collagen in the absence of Mg2+ (A) or with nonfibrillar type I collagen in the presence of Mg2+ (B) for 1 hour. After washing, washed platelet suspensions were allowed to adhere under static conditions to each well for 1 hour. After washing, adhesion platelets were quantified fluorometrically. As positive controls, washed platelet suspension were preincubated with antihuman GPVI mAb, OM-2 (1 μg/mL) (A) or anti-(human integrin α2-subunit) mAb, 6F1 (5 μg/mL) (B). Results are representative of 3 independent experiments and expressed as the mean of triplicate reading plus or minus SEM for the indicated concentrations.
AAPP inhibits both GPVI- and integrin α2β1-mediated platelet adhesion to collagen. The indicated concentrations of rAAPP were incubated in 96-well plates coated with fibrillar type I collagen in the absence of Mg2+ (A) or with nonfibrillar type I collagen in the presence of Mg2+ (B) for 1 hour. After washing, washed platelet suspensions were allowed to adhere under static conditions to each well for 1 hour. After washing, adhesion platelets were quantified fluorometrically. As positive controls, washed platelet suspension were preincubated with antihuman GPVI mAb, OM-2 (1 μg/mL) (A) or anti-(human integrin α2-subunit) mAb, 6F1 (5 μg/mL) (B). Results are representative of 3 independent experiments and expressed as the mean of triplicate reading plus or minus SEM for the indicated concentrations.
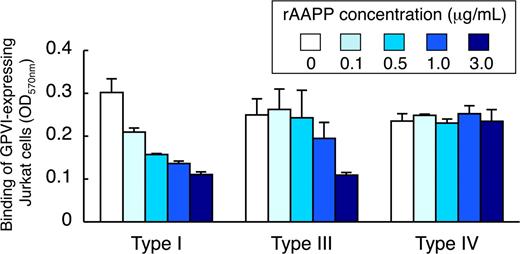
AAPP inhibits GPVI-mediated platelet adhesion to collagen type I, III, but not type IV
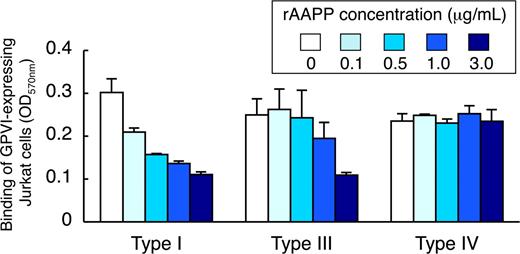
Because different types of collagen are among the most thrombogenic component of the vessel wall responsible for the initiation of platelet adhesion, we examined the ability of rAAPP to bind to these collagens. Various concentrations of rAAPP were incubated either with collagen type I, III or IV immobilized in a 96-well plate. The binding of rAAPP to each collagen was evaluated by studying the inhibition of GPVI-expressing Jurkat cells to binding to each collagen. Figure 5 shows that rAAPP inhibited the binding of GPVI-expressing Jurkat cells to collagen types I and III in a dose-dependent manner, but not to type IV, indicating that AAPP competes with GPVI for the binding to collagen types I and III, but not to type IV.
AAPP inhibits GPVI-mediated platelet adhesion to collagen types I, III, but not type IV. Different types of collagen (types I, III, and IV) were coated in 96-well plates. The indicated concentrations of rAAPP were added to each well and incubated for 1 hour. After washing, GPVI-expressing Jurkat cells were allowed to adhere to each well for 1 hour. After washing, adhesion cells were quantified fluorometrically. The top box indicates the concentration of rAAPP (μg/mL). Results are representative of 3 independent experiments and expressed as the mean of triplicate reading plus or minus SEM for the indicated concentrations.
AAPP inhibits GPVI-mediated platelet adhesion to collagen types I, III, but not type IV. Different types of collagen (types I, III, and IV) were coated in 96-well plates. The indicated concentrations of rAAPP were added to each well and incubated for 1 hour. After washing, GPVI-expressing Jurkat cells were allowed to adhere to each well for 1 hour. After washing, adhesion cells were quantified fluorometrically. The top box indicates the concentration of rAAPP (μg/mL). Results are representative of 3 independent experiments and expressed as the mean of triplicate reading plus or minus SEM for the indicated concentrations.
AAPP inhibits the collagen-induced increase in [Ca2+]i
Because an increase in [Ca2+]i is considered to play a pivotal role in platelet aggregation, we investigated the possible involvement of AAPP in the regulation of [Ca2+]i. Addition of collagen to a human platelet suspension in the presence of 1 mM CaCl2 resulted in an increase in [Ca2+]i (Figure 6 control). To determine the effects of AAPP on the collagen-induced increase in [Ca2+]i, platelets were incubated with various concentrations of rAAPP for 1 minute before their activation with collagen. Even at a low concentration (30 nM), rAAPP drastically decreased the collagen-induced change in [Ca2+]i, and 300 nM rAAPP completely abolished the change (Figure 6).
Effect of AAPP on [Ca2+]i in the early phase of collagen stimulation. Fura-2 AM-loaded platelets were incubated with 1 mM CaCl2 for 5 minutes. Collagen (0.5 μg/mL) was added after a 60-second incubation with rAAPP (30, 100 or 300 nM), and the fura-2 fluorescence was determined. Tracings are representative results of a typical experiment.
Effect of AAPP on [Ca2+]i in the early phase of collagen stimulation. Fura-2 AM-loaded platelets were incubated with 1 mM CaCl2 for 5 minutes. Collagen (0.5 μg/mL) was added after a 60-second incubation with rAAPP (30, 100 or 300 nM), and the fura-2 fluorescence was determined. Tracings are representative results of a typical experiment.
Intravenous administration of AAPP inhibits collagen-induced platelet aggregation ex vivo in rats
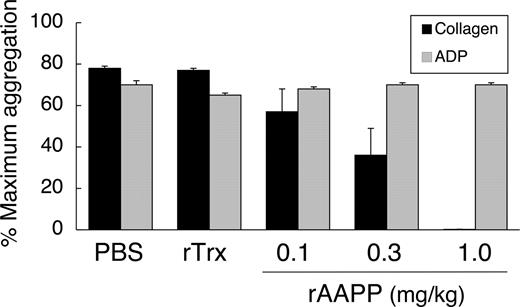
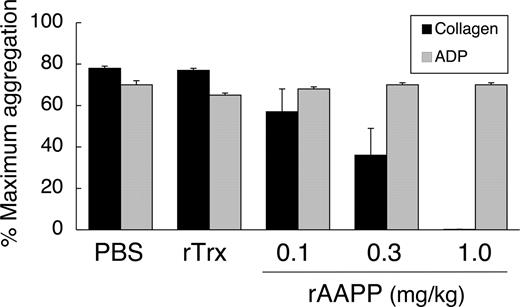
rAAPP also exhibited identical inhibition of collagen-induced aggregation of rat platelets in vitro (data not shown). The in vivo effects of rAAPP were tested by giving SD rats a bolus intravenous injection of rAAPP (0.1–1.0 mg/kg) and performing ex vivo aggregation studies on citrated PRP. The ED50 for the aggregation inhibition was 0.3 mg/kg, and rAAPP at 1.0 mg/kg prevented all detectable collagen-induced platelet aggregation (Figure 7). ADP-induced aggregation remained unaltered, even as concentrations as high as 1.0 mg/kg. There was no significant change in platelet aggregation after rTrx injection, which was used as a negative control.
Inhibition of ex vivo collagen-induced aggregation of rat platelets after injection of AAPP. SD rats were administered rAAPP (0.1, 0.3, or 1.0 mg/kg) intravenously, and blood was drawn 10 minutes after the administration. Treatment with rAAPP results in inhibition of platelet aggregation in response to collagen, compared with treatment with PBS and rTrx, but has no effect on the ADP response. Results are means plus or minus SEM for 3 animals in each group.
Inhibition of ex vivo collagen-induced aggregation of rat platelets after injection of AAPP. SD rats were administered rAAPP (0.1, 0.3, or 1.0 mg/kg) intravenously, and blood was drawn 10 minutes after the administration. Treatment with rAAPP results in inhibition of platelet aggregation in response to collagen, compared with treatment with PBS and rTrx, but has no effect on the ADP response. Results are means plus or minus SEM for 3 animals in each group.
Discussion
In the present study, we have identified and characterized an abundant saliva protein, AAPP, from the A stephensi mosquito. AAPP has characteristic features of acidic secreted proteins with a glycine and glutamic acid-rich region, and is specifically expressed in the distal lateral lobes of the adult female salivary glands. This expression pattern is consistent with the finding in our previous study on transgenic mosquitoes that the strong aapp promoter specifically drives foreign gene expression in the distal lateral lobes.11 We have demonstrated that AAPP is unique in that it specifically blocks platelet adhesion to collagen and subsequent platelet aggregation.
Regarding the mechanism by which AAPP specifically inhibits collagen-induced platelet aggregation, AAPP directly binds to collagen I and III, and interferes with the interaction between collagen and its receptor GPVI on platelets, thereby inhibiting an increase in [Ca2+]i, which is an important second messenger in the platelet activation cascade. The current view of collagen-induced platelet activation is that collagen initially interacts with GPVI and integrin α2β1, and platelet activation signals are then conveyed by GPVI (reviewed in Nieswandt and Watson36 ). However, we excluded the possibility of direct interactions between AAPP and these molecules by the following pieces of evidence: (1) the 2 GPVI receptor agonists, CRP and convulxin did not compete with AAPP for platelet aggregation; (2) GPVI-expressing Jurkat cells did not bind to AAPP; and (3) the sequence motifs of the specific α2β1 ligand, GFOGER-GPP,37 within the collagen triple-helical region and the RGD fibrinogen receptor antagonist were not contained in the AAPP sequence. Our recent investigations have shown that, although the GE-rich region located in the N-terminal half of AAPP shows 65% similarity with the triple-helix region of the α-1 chain of human collagen IV (Figure S1B), this region is involved in the binding neither to GPVI (Figure 5E) nor collagen (S.Y. and T.S., unpublished data, July 2005). Further studies are currently in progress to identify AAPP receptor on collagen by using truncated rAAPPs.
To develop new therapeutic agents for the treatment of cardiovascular and ischemic disorders, a number of new exogenous factors have recently been identified from animal sources, and the search is still on for novel factors that interfere with platelet aggregation and blood coagulation (reviewed in Kini38 ). Although some factors effectively affect platelet function and aggregation in vitro, most of them fail to retain their biochemical and pharmacologic characteristics in vivo. To date, limited numbers of drugs have been developed or are in the process of being developed from animal sources, for example, desmoteplase (a plasminogen activator) from vampire bat saliva,39,–41 calin (a plasminogen activator) from Hirudo medicinalis leech saliva42,43 ; and hirudin (an anticoagulant) from H medicinalis leech saliva.44,45 Our finding that intravenously administered rAAPP strongly inhibited collagen-mediated platelet aggregation ex vivo in rats may provide a potential use of AAPP for developing new drugs that maintain the flow of blood.
Malaria transmitted by anopheline mosquitoes is the worst health problem in the world, and kills 1 to 2 million people every year. Our recent studies on malaria survey have shown that approximately 90% of people living in the Solomon Islands, where malaria is hyperendemic, have high levels of antibodies against AAPP and the antibody levels are positively correlated with the antibody levels against malaria antigens (data not shown). From the aspect of malaria epidemiology, this information may provide important insights regarding bite exposure or monitoring the inroads of anopheline mosquitoes, thereby leading to successful prediction of the emergence of malaria. In addition, it is also of great interest to address whether high levels of anti-AAPP antibodies affect mosquito biting behavior and/or malaria infection. These studies on mosquito-malaria biology are now in progress in our laboratory.
In conclusion, we have demonstrated for the first time that AAPP is a malaria vector mosquito–derived specific antagonist of receptors that mediate the adhesion of platelets to collagen. AAPP shows no similarity to any other protein in the GenBank database, indicating that it is a novel antiplatelet aggregation protein that acts via an alternative pathway. The female salivary gland–specific AAPP expression pattern and its high levels of expression suggest that AAPP may play a critical role in facilitating location of blood vessels and preventing host hemostasis during feeding. In fact, more than 50% of antiplatelet aggregation activity in the salivary glands is brought about by AAPP (S.Y. and T.S., unpublished data, June 2005). Importantly, AAPP is an attractive molecule for developing a novel antithrombosis drug. Further studies are currently underway to understand the molecular details of the AAPP-collagen interaction and evaluate the pharmacologic effectiveness in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank C. Seki, Y. Shimada, and K. Araki for excellent assistance with the ELISAs and handling of the mosquitoes and mice. We also thank Otsuka Pharmaceutical researchers (H. Hayashi and Y. Nagamura) for help with the rat platelet aggregation study.
This work was supported by grants from the Ministry of Education, Culture, Sports and Science of Japan (16590345 and 18390130) and Otsuka Pharmaceutical.
Authorship
Contribution: S.Y., T.S., M.N., L.T., B.S., J.K., H.W., E.L., and H.M. designed and performed the research; S.Y. and T.S. interpreted and analyzed the data; and S.Y. and T.S. wrote the paper.
Conflict-of-interest disclosure: S.Y. is a grant receiver from Otsuka Pharmaceutical, and T.S. and M.N. are employees of Otsuka Pharmaceutical. All other authors declare no competing financial interests.
Correspondence: Shigeto Yoshida, Division of Medical Zoology, Department of Infection and Immunity, Jichi Medical University, 3311-1 Yakushiji, Shimotsuke, Tochigi 329-0498, Japan; e-mail: shigeto@jichi.ac.jp.
References
Author notes
S.Y. and T.S. contributed equally to this study.


![Figure 6. Effect of AAPP on [Ca2+]i in the early phase of collagen stimulation. Fura-2 AM-loaded platelets were incubated with 1 mM CaCl2 for 5 minutes. Collagen (0.5 μg/mL) was added after a 60-second incubation with rAAPP (30, 100 or 300 nM), and the fura-2 fluorescence was determined. Tracings are representative results of a typical experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-06-097824/3/m_zh80040815130006.jpeg?Expires=1769171781&Signature=peB8bwhcuicN9gaXc6rp1bQUoawzmfa8hUu~d9itGnG~HRFY~HmKquDa6v1JXFfk3vuoG3PkZ4lLm90LS4PUi2IUHk5u6TjRjMRP0GiO-ZETNOVf3BFXPtabtUudBGYt65vtNzllmzcwQBiuojGm6o5M4W6Hth5lc9WJb4SWaQxzLB7yfvtt5cv~UL0~2QkRNd6Ijg4NpuK9qtYxuXoxBUcOIjyeXUqpXkA2rCGb9UFoFC7npLWX9h5XLwP9SnQLcQxZGpNkxzBxzylV5Q2I6aadl0ya6gqKGmkv4JbjdTJjnkPFKxO4NIe2r6dkRNSIZ-Szsxc7WsbsvMSH4flw6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Effect of AAPP on [Ca2+]i in the early phase of collagen stimulation. Fura-2 AM-loaded platelets were incubated with 1 mM CaCl2 for 5 minutes. Collagen (0.5 μg/mL) was added after a 60-second incubation with rAAPP (30, 100 or 300 nM), and the fura-2 fluorescence was determined. Tracings are representative results of a typical experiment.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/4/10.1182_blood-2007-06-097824/3/m_zh80040815130006.jpeg?Expires=1769250091&Signature=tbOSITopPQTBC2CyFhhvVixD8smIjZSLB6s~5w9db7z75jY94HwN7yEkSA6pZhXMAIeRwG5-H0tQNZu4Z-0Ye4230ySJKAqPaOwZPjCDsqstTw0tEu-QTO4RZeSkjQQ1h1uxd7fAQJAVxF9l9rOsoH0htJgHK2RkO228CDrNpy6Vq5N5xUm7KJ0HJqg-lS8R18vX7RzUGBUgcqbmL6P3MLTestJeIpPOv4onqodWL1FSfxw2b~sWNp~A1gsuvva-ngrOZ1x4n1P15G4~RdbBNPNCiZ7~9~Q1a6fUtyUFGMYHUu4as6HMr44GWmVK~jS0MSBTT6cwG8v76SUdrBFPLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)