Abstract
Answers to questions about frequency and repertoire of immune cells, relative contributions made by different types of immune cells toward the total Epstein-Barr virus (EBV)–directed response and the variation of such responses in healthy persons have been elusive because of disparities in assays, antigen presenting cells, and antigenic sources used in previous experiments. In this study, we addressed these questions using an assay that allowed direct comparison of responses generated by different types of cells of the immune system. This short-term (20-hour) ex vivo assay measured interferon-γ production by blood cells in response to autologous EBV-transformed lymphoblastoid cell lines (LCLs). Our experiments defined the variation in responses among persons and clearly distinguished 10 healthy EBV-immune from 10 healthy EBV-naive persons. In EBV-immune persons, 33% of responding cells were CD4+, 43.3% were CD8+, and 12.9% were γ-δ T cells. LCL-reactive CD8+ T cells were only 1.7-fold more frequent than similarly reactive CD4+T cells. Responses by γ-δ T cells were 6-fold higher in seropositive than in seronegative persons. Our findings emphasize the importance of CD4+ and γ-δ T-cell responses and have implications for immunotherapy and for identifying defects in T-cell populations that might predispose to development of EBV-associated lymphomas.
Introduction
The importance of T cells in the prevention of Epstein-Barr virus (EBV)–lymphoproliferative diseases (LPD) or lymphomas is highlighted by the development of such tumors only in patients with deficient cell-mediated immunity. The essential role of T cells is evident from successful prophylaxis and therapy of EBV-LPD with adoptive transfer of T cells expanded after exposure to lymphoblastoid cell lines (LCLs) immortalized by EBV.1,2 In healthy EBV-seropositive patients, EBV-specific CD8+ T-cell responses have been mapped to epitopes derived from latent antigens, including EB nuclear antigen (EBNA) 3A, EBNA3B, and EBNA3C. Subdominant CD8+ T-cell responses to latent membrane protein (LMP) 2 and EBNA1 have also been described.3-6 More recently, CD8+ T-cell reactivities directed against lytic antigens such as Bam Z Epstein-Barr replication activator (ZEBRA), Bam R transactivator (RTA), and Bam M transactivator (MTA) have also been identified.7,8
Far less is known about CD4+ T-cell responses to EBV. Studies on EBV-specific CD4+ T-cell responses have focused on T-cell lines and clones generated from peripheral blood mononuclear cells (PBMCs) using EBV-LCL, and dendritic cells (DC) loaded with EBV peptides or recombinant proteins.9-16 The targets of such CD4+ T cells are EBNA1, EBNA3A, EBNA3B, and EBNA3C. Very little is known about CD4+ T-cell responses to EBV lytic antigens. Studies using MHC class I tetramers (for CD8+ T-cell responses) and EBV antigens (for CD4+ T-cell responses) have demonstrated that EBV-specific CD8+ memory T cells are approximately 10-fold more abundant than EBV-specific CD4+ memory T cells in PBMCs.12,17-23 However, these studies were performed using different assays, different antigen presenting cells, and different sources of antigens, making comparisons between responses by different cell types specious. Because of the lack of a uniform assay, there is little consensus regarding the relative contribution by each cell type toward responses directed against EBV.
Although cell lines derived from reactivation of PBMCs with autologous EBV-LCL are routinely used for adoptive immunotherapy to treat EBV-lymphomas,1,2 the frequency, repertoire, and variation in the peripheral blood of such reactivities have not been systematically and simultaneously examined among EBV-immune and EBV-naive persons. To fill this void, we used a short-term uniform assay that allowed direct comparisons of responses directed against EBV, by different types of immune cells. Using an experimental system that examines T-cell and natural killer (NK)– cell responses in PBMCs reactivated by autologous EBV-LCL among healthy EBV-seropositive and EBV-seronegative patients, we asked the following 4 questions: (1) What is the frequency of cells responding to EBV in the peripheral blood of healthy EBV-seropositive and -seronegative patients? (2) What is the repertoire of cells responding to EBV in the peripheral blood of healthy EBV-seropositive and -seronegative patients? (3) How much variation is there in the magnitude of responses by immune cells among healthy patients and between healthy EBV-seropositive and -seronegative patients? (4) What is the relative contribution made by each type of immune cell toward the total EBV-directed response in healthy EBV-seropositive patients? An additional objective of this study was to develop an assay that could detect functional CD4+ and CD8+ T-cell responses to EBV latent and lytic antigens among patients with diverse HLA class I and class II backgrounds and thus be of clinical utility.
Methods
Study subjects
Ten healthy EBV-seropositive volunteers between the ages of 19 and 36 years and 10 healthy EBV-seronegative volunteers between the ages of 14 and 55 years were identified by Western blot analyses for antibodies to EBNA1 and small viral capsid antigen using the B95-8 cell line.24 Results were confirmed by a fluorescence-activated cell sorting (FACS)–based assay using HH514-16 cells treated with sodium butyrate.25 The use of human subjects in this study was approved by the Human Investigation Committee at Yale University. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Isolation and stimulation of mononuclear cells
PBMCs were isolated from venous blood as described.26 Cells either were used fresh or were frozen in liquid nitrogen in 90% fetal bovine serum and 10% dimethyl sulfoxide. CD4+ and CD8+ T cells were enriched by negative selection using an EasySep Human CD4+ T cell or Human CD8+ T-cell enrichment kit, respectively (StemCell Technologies, Vancouver, BC) using the manufacturer's instructions. Mononuclear cells were incubated at 37°°C in the presence of 5% CO2 at 2 × 106/mL in RPMI 1640 containing 10% fetal bovine serum either alone or in the presence of 2 × 105 autologous LCLs (PBMC/LCL = 10:1; in experiments shown in the figures) or 4 × 105 autologous LCL (PBMC/LCL = 5:1; in experiment shown in Figure 1). Brefeldin A was added for the last 4 hours of incubation at a final concentration of 5 μg/mL. Cells were harvested, counted, and subjected to staining with appropriate antibodies.
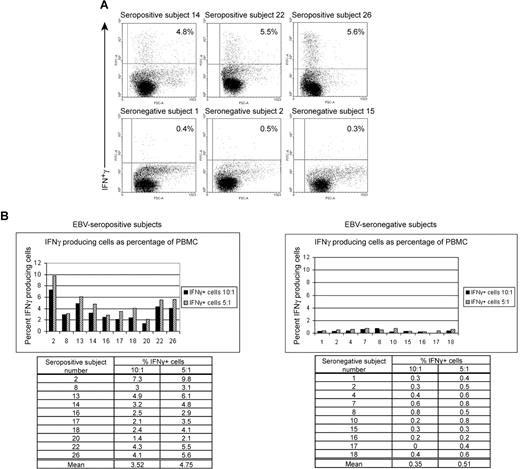
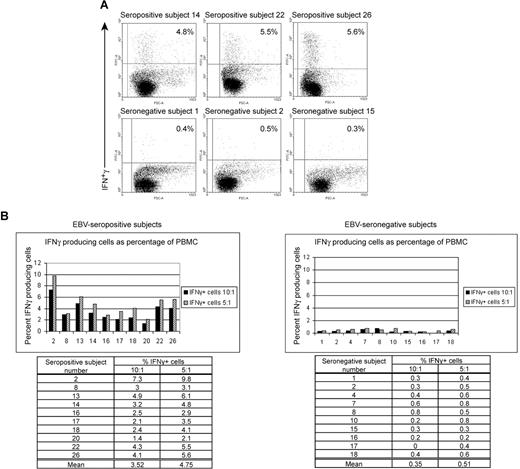
Healthy EBV-seropositive patients can be distinguished from healthy EBV-seronegative patients based on production of IFNγ by PBMC in response to autologous LCL. (A) FACS dotplots of PBMC from healthy EBV-seropositive subjects 14, 22, and 26 or healthy EBV-seronegative subjects 1, 2, and 15 producing IFNγ by intracellular staining after 20 hours of culture with autologous LCL (PBMC/LCL = 5:1). (B) Comparison of percentage of PBMCs producing IFNγ between 10 healthy EBV-seropositive and 10 healthy EBV-seronegative patients after culture with autologous LCL at ratios of 10:1 or 5:1 (PBMC/LCL).
Healthy EBV-seropositive patients can be distinguished from healthy EBV-seronegative patients based on production of IFNγ by PBMC in response to autologous LCL. (A) FACS dotplots of PBMC from healthy EBV-seropositive subjects 14, 22, and 26 or healthy EBV-seronegative subjects 1, 2, and 15 producing IFNγ by intracellular staining after 20 hours of culture with autologous LCL (PBMC/LCL = 5:1). (B) Comparison of percentage of PBMCs producing IFNγ between 10 healthy EBV-seropositive and 10 healthy EBV-seronegative patients after culture with autologous LCL at ratios of 10:1 or 5:1 (PBMC/LCL).
Generation and culture of LCLs
LCLs were generated from PBMCs using standard protocols.27 Briefly, PBMCs were resuspended at 5 × 106/mL in the presence of 20 nM FK506. Supernatant from the marmoset B-cell line B95-8 was used as the source of EBV.26 Cells were fed on day 12 of culture followed by weekly feedings thereafter. Cell lines were obtained by 3 to 4 weeks.
Seven-color staining and analysis and LCLs
After 20 hours of culture, PBMCs or PBMCs + LCLs were harvested and resuspended at 107 cells/mL. A mixture of saturating concentrations of different fluorochrome–conjugated monoclonal antibodies against human cell surface molecules was added to cells. Antibodies included anti-CD3-Alexa Fluor 680, anti-CD4-Peridinin chlorphyll protein, anti-CD8-Allophycocyanin (APC)-Cy7, anti-CD45RA-APC, anti-CD62L-PE, anti-CD23-PE, anti-CD56-PE, anti-γ-δ TCR-PE, and anti-CCR7-PE-Cy7. Murine Ig at 1 mg/mL was included in the mixture to inhibit nonspecific binding. Cells were incubated with antibodies for 40 minutes on ice. The reactivity of antibodies to human lymphoid cell surface molecules was compared with the reactivity of isotype control antibodies consisting of monoclonal murine IgG1-Alexa Fluor 680, IgG1-Peridinin chlorphyll protein, IgG1-APC-Cy7, IgG2a-APC, IgG1-PE, and rat IgG2a-PE-Cy7. Cells were washed twice and fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences PharMingen, San Diego, CA). Cells were then incubated with 4 μg of anti-interferon gamma (IFN)γ-biotin diluted in 100 μL Cytofix/Cytoperm buffer for 30 minutes on ice. Cells were washed twice in Cytofix/Cytoperm buffer and incubated with 0.6 μg of avidin-fluorescein isothiocyanate (FITC) diluted in 100 μL Cytofix/Cytoperm buffer for 30 minutes on ice. Murine monoclonal IgG1-biotin followed by avidin-FITC was used as isotype–matched negative control for staining with anti-IFNγ antibody. Antibodies were purchased from BD Biosciences PharMingen, Sigma-Aldrich (St Louis, MO), or Dako (Ft Collins, CO). Data from 100 000 cells stained with each antibody mixture was acquired on a LSR II (BD Biosciences, San Jose, CA) flow cytometer. Gates were set on live lymphocytes based on their forward- and side-scatter profiles. Percent IFNγ-producing PBMCs when incubated with LCLs was determined by subtracting the percentage IFNγ-producing cells by PBMCs incubated alone. The same principle was used when determining percent IFNγ-producing T cells or NK cells.
Detection of lytically infected LCL
Lytically infected LCLs were detected using a method that we have described.25 Briefly, B95-8–derived- and endogenous virus–derived LCLs were incubated with a reference EBV-seropositive serum or a reference EBV-seronegative serum as negative control25 followed by incubation with goat antihuman IgG-FITC (Sigma-Aldrich). Percent lytically infected cells in each type of LCLs were determined by subtracting percent lytically infected cells detected by the negative control serum when added to the corresponding type of LCLs.
Statistical analysis
Unpaired t test was used to compare the means of 2 groups of interest.
Results
Healthy EBV-seropositive subjects can be distinguished from healthy EBV-seronegative subjects based on IFNγ response directed against autologous LCL
We examined cell-mediated immune responses directed against EBV using a system that closely resembled interactions between cellular components of the immune system and EBV-infected cells in vivo. In healthy EBV-seropositive patients, B cells are latently infected with EBV and express 6 nuclear antigens (EBNA1, 2, 3A, 3B, 3C, and 4), 2 membrane proteins (LMP1 and LMP2), and 2 types of noncoding RNA molecules (EBERs, BART).28,29 B cells exhibiting this pattern of viral gene expression are commonly referred to as latency type III cells and are regularly present in the secondary lymphoid organs30 but not in peripheral blood of healthy EBV-seropositive patients.28,31 Thus, cell-mediated immunity to EBV is expected to be directed against such latency type III cells and function to prevent their outgrowth. Therefore, we established a 20-hour assay to assess cell-mediated immune responses in PBMCs directed against autologous LCL generated in vitro. LCLs are B-cell lines that are latently infected with EBV, have a type III viral latency gene expression pattern,32 and allow simultaneous examination and comparison of responses by several cell types of the immune system to EBV in a single assay.
Ten healthy EBV-seropositive patients and 10 healthy EBV-seronegative patients were identified. LCLs were established from each patient. Figure 1A,B shows the percentages of PBMCs expressing IFNγ in response to autologous LCLs when mixed at ratios of 10:1 and 5:1 (PBMC/LCL) in a 20-hour period of culture. Dotplot analyses of IFNγ secretion by PBMC from 3 representative EBV-seropositive patients and 3 representative EBV-seronegative patients are shown in Figure 1A. At a ratio of 5:1, an average of 4.7% (range, 2.1%-9.8%) of PBMCs in healthy patients persistently infected with EBV responded to autologous LCL. In contrast, an average of 0.5% (range, 0.3%-0.8) of PBMCs in healthy EBV-seronegative patients produced IFNγ (Figure 1B). Similar responses were observed at a ratio of 10:1 (Figure 1B). For both ratios, the difference in means was statistically significant with a P value of .001. Similar fractions of PBMC from healthy EBV-seronegative and -seropositive patients produced IFNγ in response to stimulation by phytohemagglutinin for 20 hours (data not shown). Thus, healthy EBV-seropositive patients could be distinguished from healthy EBV-seronegative patients based on a 10-fold higher frequency of PBMCs responding to autologous LCL by producing IFNγ. There was no overlap in the frequency of cells responding to autologous LCL between the 2 groups.
CD4+ and CD8+ T cells in healthy EBV-seropositive subjects make nearly equal contributions to the pool of IFNγ-secreting cells in response to autologous LCL
After culture with LCLs, cells were subjected to flow cytometric examination by surface staining for their phenotypic identity and intracellular IFNγ production. Figure 2A shows that, in healthy EBV-seropositive patients, an average of 0.9% (range, 0.5%-1.7%) of PBMCs were CD8+ T cells, which produced IFNγ in response to autologous LCL. An average of 0.84% (range, 0.3%-2.2%) of PBMCs produced IFNγ and were identified as CD4+ T cells. An average each of 0.26% of PBMC responded to autologous LCL and were identified as γ-δ T cells and NK (CD56+, CD3−) cells, respectively (range, 0.09%-0.6% for γ-δ T cells and 0%-0.8% for NK cells; Figure 2A). In healthy EBV-seronegative patients, averages of 0.07%, 0.17%, 0.06%, and 0.06% of PBMCs that produced IFNγ could be costained for markers for CD8+, CD4+, γ-δ T cells, and NK cells, respectively (Figure 2B). However, the numbers of IFNγ-producing cells in healthy EBV-seronegative patients were too few to draw any conclusions.
CD4+ T cells, CD8+ T cells, γ-δ T cells, and NK cells in healthy EBV-seropositive patients respond to autologous LCL after culture for 20 hours. Percentage of PBMCs producing IFNγ and stained for CD4, CD8, γ-δ TCR, and CD56 (NK) markers were measured in 10 healthy EBV-seropositive (A) and healthy EBV-seronegative patients. (B) PBMCs were incubated with autologous LCL at a ratio of 10:1 (PBMC/LCL) followed by cell surface marker staining and intracellular staining for IFNγ. CD4+ T cells and CD8+ T cells compose a major fraction of IFNγ-producing cells (C) or of percent IFNγ-producing cells (D) in response to autologous LCL in healthy EBV-seropositive patients.
CD4+ T cells, CD8+ T cells, γ-δ T cells, and NK cells in healthy EBV-seropositive patients respond to autologous LCL after culture for 20 hours. Percentage of PBMCs producing IFNγ and stained for CD4, CD8, γ-δ TCR, and CD56 (NK) markers were measured in 10 healthy EBV-seropositive (A) and healthy EBV-seronegative patients. (B) PBMCs were incubated with autologous LCL at a ratio of 10:1 (PBMC/LCL) followed by cell surface marker staining and intracellular staining for IFNγ. CD4+ T cells and CD8+ T cells compose a major fraction of IFNγ-producing cells (C) or of percent IFNγ-producing cells (D) in response to autologous LCL in healthy EBV-seropositive patients.
Figure 2C shows that, in healthy persistently infected patients, we were able to account for 22.5% to 78.3% of all IFNγ-producing cells when cells were costained for T-cell and NK-cell markers. All the IFNγ-producing cells could not be identified presumably because of down-regulation of cell surface markers. When the sum of identifiable responding T (CD8+, CD4+, γ-δ+) cells and NK cells was used as the denominator, we found that an average of 43.3% (range, 14.4%-61%) of IFNγ-secreting cells were CD8+ T cells and an average of 33% (range, 14%-68.8%) of IFNγ-secreting cells were CD4+ T cells (Figure 2D). The proportion of responding cells that were CD8+ or CD4+ T cells differed among patients. For example, in healthy EBV-seropositive subjects 14 and 20, up to two-thirds of the IFNγ-secreting cells were represented by CD4+ and CD8+ T cells, respectively (Figure 2D). γ-δ T cells and NK cells contributed an average of 12.9% (range, 4.5%-24.7%) and 10.8% (range, 0%-26.1%) of IFNγ-producing cells, respectively (Figure 2D). Thus, both adaptive and innate arms of the immune system contributed to the IFNγ response directed against autologous LCLs. CD4+ and CD8+ T cells contributed to the bulk of the response, with nearly equal contributions each from CD4+ and CD8+ T cells. Less than 1% of LCL (CD23+) produced IFNγ when incubated with autologous PBMCs (data not shown).
EBV-specific CD4+ T cells are maintained at almost equal frequencies as EBV-specific CD8+ T cells
MHC class I tetramer-based and EBV antigen-based studies have shown that EBV-specific CD8+ T-cell responses are maintained at 10-fold higher frequencies than EBV-specific CD4+ T-cell responses.12,17-23 We examined this issue by enumerating the proportion of each cell type (CD8+ and CD4+ T cells) in peripheral blood that produced IFNγ in response to autologous LCLs. Figure 3A shows dotplot analyses of the fractions of CD8+ T cells and CD4+ T cells that produced IFNγ in 3 representative healthy EBV-seropositive patients. Figure 3B demonstrates that an average of 4.5% (range, 2.1%-7.4%) of CD8+ T cells and an average of 2.6% (range, 0.9%-3.9%) of CD4+ T cells produced IFNγ. Therefore, EBV-specific CD4+ T-cell responses were detected at nearly equal frequencies as EBV-specific CD8+ T-cell responses.
Comparison of fractions of T cells and NK (CD56+) cells from healthy EBV-seropositive patients who respond to autologous LCL. (A) Dotplot depiction of IFNγ-producing cells after application of gates on CD8+, CD4+, and γ-δ TCR+ cells. The percentages in the right upper quadrants indicate the fraction of the cell type that produced IFNγ. (B) Larger fractions of circulating γ-δ T cells, and NK cells than those of circulating CD8+ and CD4+ T cells respond to autologous LCL in healthy EBV-seropositive patients.
Comparison of fractions of T cells and NK (CD56+) cells from healthy EBV-seropositive patients who respond to autologous LCL. (A) Dotplot depiction of IFNγ-producing cells after application of gates on CD8+, CD4+, and γ-δ TCR+ cells. The percentages in the right upper quadrants indicate the fraction of the cell type that produced IFNγ. (B) Larger fractions of circulating γ-δ T cells, and NK cells than those of circulating CD8+ and CD4+ T cells respond to autologous LCL in healthy EBV-seropositive patients.
A larger proportion of γ-δ T cells and NK cells respond to autologous LCL than CD4+ and CD8+ T cells
The comparison of IFNγ responses contributed by each cell type was extended to γ-δ T cells and NK cells. An average of 21.7% (range, 8.1%-36.4%) of γ-δ T cells and an average of 38.9% (range, 0%-61.5%) of NK cells produced INFγ in response to autologous LCL (Figure 3B). Thus in some patients, up to one-third of γ-δ T cells were triggered to produce IFNγ in response to autologous LCLs and contributed up to 12.9% of IFNγ-producing cells in PBMCs (Figure 2D). In contrast, although a lower proportion of CD8+ T cells (mean, 4.5%) and CD4+ T cells (mean, 2.6%) were stimulated to produce IFNγ (Figure 3B), the total number of responding CD8+ and CD4+ T cells were greater than γ-δ T cells and NK cells.
The frequency of CD4+ and CD8+ T-cell response directed against autologous LCLs was confirmed using mononuclear cell fractions enriched for CD4+ and CD8+ T cells from healthy EBV-seropositive subjects
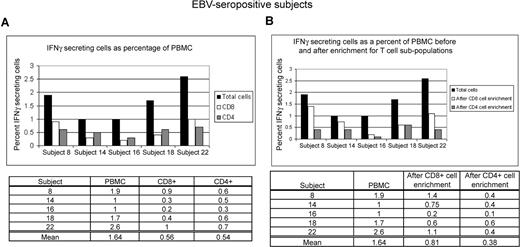
We asked whether the measurement of frequency of responding cells was influenced by the presence of accessory cell types within the PBMC population. Parallel experiments, using unfractionated PBMCs incubated with autologous LCL followed by staining for CD4 and CD8 cell-surface markers, were performed alongside experiments in which mononuclear cell fractions enriched for CD4+ and CD8+ T cells were incubated with autologous LCLs. Figure 4 shows that responses made by CD8+ T cells represented an average of 0.6% of total PBMCs without enrichment (Figure 4A) and 0.8% of total PBMCs after enrichment (Figure 4B) of CD8+ T cells before incubation with autologous LCLs. In comparison, CD4+ T-cell responses represented an average of 0.5% of total PBMCs without enrichment (Figure 4A) and 0.4% of total PBMCs after enrichment (Figure 4B) of CD4+ T cells before incubation with autologous LCLs. These experiments provided additional evidence about the identity and frequencies of T-cell subpopulations within PBMC responsive to LCL.
Peripheral mononuclear cells enriched in CD8+ T cells or CD4+ T cells from healthy EBV-seropositive patients produce responses that are comparable with those produced by CD8+ T cells and CD4+ T cells in total PBMCs. (A) Total PBMCs from 5 healthy EBV-seropositive patients were incubated with autologous LCL (PBMC/LCL = 10:1) followed by staining for CD4 and CD8 markers and intracellular IFNγ. (B) Cells enriched in CD8+ or CD4+ T cells were incubated with autologous LCL (T cells/LCL = 10:1) in parallel with incubation of total PBMCs with autologous LCL (as in panel A) followed by intracellular staining for IFNγ. Data presented show percent of total PBMC producing IFNγ after correction for fraction of CD8+ and CD4+ T cells present in total PBMCs before enrichment.
Peripheral mononuclear cells enriched in CD8+ T cells or CD4+ T cells from healthy EBV-seropositive patients produce responses that are comparable with those produced by CD8+ T cells and CD4+ T cells in total PBMCs. (A) Total PBMCs from 5 healthy EBV-seropositive patients were incubated with autologous LCL (PBMC/LCL = 10:1) followed by staining for CD4 and CD8 markers and intracellular IFNγ. (B) Cells enriched in CD8+ or CD4+ T cells were incubated with autologous LCL (T cells/LCL = 10:1) in parallel with incubation of total PBMCs with autologous LCL (as in panel A) followed by intracellular staining for IFNγ. Data presented show percent of total PBMC producing IFNγ after correction for fraction of CD8+ and CD4+ T cells present in total PBMCs before enrichment.
In healthy EBV-seropositive subjects, CD4+ and CD8+ effector memory T cells make nearly equal contributions to the pool of IFNγ-producing cells in response to autologous LCL
We determined the memory phenotype of CD4+ and CD8+ T cells that produced IFNγ in response to culture with autologous LCL. Cells were stained for CD4 and CD8 molecules and surface memory markers as described by Sallusto et al.33 Responding cells were divided into the following categories with respect to memory phenotype: naive (CD45RA+, CD62L+, CCR7+), central memory (CM; CD45RA−, CD62L+, CCR7+), effector memory (EM; CD45RA−, CD62L−, CCR7−), and CD8+EMRA+ (CD45RA+, CD62L−, CCR7−). Figure 5A shows that, in healthy EBV-seropositive patients, an average of 15.9% (range, 5%-43.3%) of all IFNγ-producing cells were CD8+ T cells with an EM phenotype. An average of 3.6% (range, 0.5%-10.1%) of all IFNγ-producing cells were CD8+EMRA+. Naive and CM CD8+ T cells made negligible contributions to the pool of IFNγ-producing cells (Figure 5A). With respect to CD4+ T cells, an average of 11.5% (range, 2.8%-39.6%) of IFNγ-producing cells had EM phenotype (Figure 5B). Smaller contributions were made by naive and CM CD4+ T cells to the pool of IFNγ-producing cells (Figure 5B). In some patients, up to 40% of the IFNγ-producing cells were either CD4+ or CD8+ T cells with EM phenotype (Figure 5A,B). Thus, CD4+ and CD8+ EM cells made nearly equal contributions to the pool of IFNγ-secreting cells in response to autologous LCLs.
Responses directed against autologous LCL are primarily made up memory T cells. PBMCs from 10 healthy EBV-seropositive patients were incubated with autologous LCL (PBMC/LCL = 10:1) for 20 hours followed by cell surface staining for CD8+ T cells (A) or CD4+ T cells (B) and phenotypic markers of memory, and intracellular IFNγ. Memory phenotypes were defined as following: naive: CD45RA+ CD62L+ CCR7+; central memory: CD45RA− CD62L+ CCR7+; effector memory: CD45RA− CD62L− CCR7−; CD8+ EMRA+: CD45RA+ CD62L− CCR7−. (C) Responding CD8+ and CD4+ T cells are mostly memory cells. (D,E) Larger fractions of effector memory and EMRA+ CD8+ T cells (D) respond to autologous LCL, whereas effector memory and naive CD4+ T cells (E) respond to autologous LCL.
Responses directed against autologous LCL are primarily made up memory T cells. PBMCs from 10 healthy EBV-seropositive patients were incubated with autologous LCL (PBMC/LCL = 10:1) for 20 hours followed by cell surface staining for CD8+ T cells (A) or CD4+ T cells (B) and phenotypic markers of memory, and intracellular IFNγ. Memory phenotypes were defined as following: naive: CD45RA+ CD62L+ CCR7+; central memory: CD45RA− CD62L+ CCR7+; effector memory: CD45RA− CD62L− CCR7−; CD8+ EMRA+: CD45RA+ CD62L− CCR7−. (C) Responding CD8+ and CD4+ T cells are mostly memory cells. (D,E) Larger fractions of effector memory and EMRA+ CD8+ T cells (D) respond to autologous LCL, whereas effector memory and naive CD4+ T cells (E) respond to autologous LCL.
After accounting for EM and CM cells (and EMRA+ for CD8+ T cells), we found that an average of 92.5% (range, 86%-100%) of identifiable responding CD8+ T cells and an average of 76.6% (range, 46%-100%) of identifiable responding CD4+ T cells had memory phenotype (Figure 5C). Therefore, both EBV-specific CD8+ and CD4+ T-cell responses were markedly enriched in the memory pool. Figure 5D shows that an average of 8.1% (range, 3.7%-14.9%) of the pool of CD8+ EM T cells and an average of 5.4% (range, 2.5%-11.6%) of CD8+EMRA+ cells in blood responded to autologous LCLs. In comparison, an average of 3.4% (range, 2.4%-4.7%) of CD4+ EM T cells responded to autologous LCL (Figure 5E). Thus, significant fractions of CD8+ and CD4+ T-cell effector memory pools were directed toward EBV-infected cells.
Distribution of IFNγ-producing cells directed against B95-8 virus–derived LCL are similar to those directed against endogenous virus–derived LCL
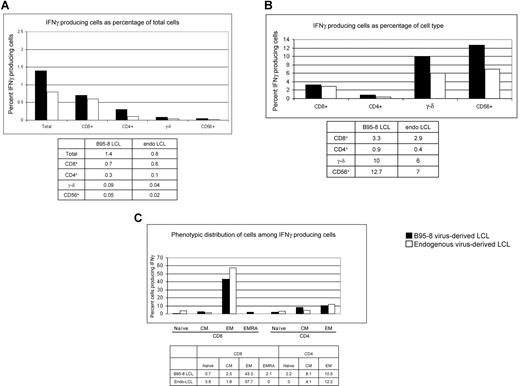
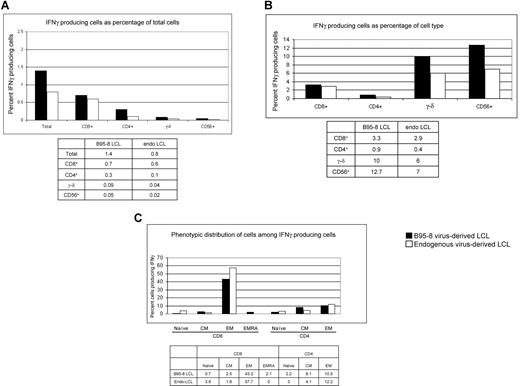
In experiments presented in Figures 1 to 5, we tested IFNγ production in response to autologous LCLs generated after infection with B95-8 virus, a laboratory strain of EBV. In vivo, latently infected B cells are infected with one or more strains of EBV.34-36 To determine whether responses directed against B95-8 virus–derived LCLs were comparable with those directed against latently infected B cells derived from “endogenous” virus, we generated 2 lymphoblastoid cell lines from EBV-seropositive subject 20. One cell line was derived from endogenous virus, by allowing outgrowth of latently infected B cells obtained during acute infectious mononucleosis. The other cell line was generated at the same time by infecting B cells obtained during acute infectious mononucleosis with B95-8. Responses by autologous PBMCs obtained 2 months after resolution of primary infection were assessed against each LCL. Figure 6A shows that more cells produced IFNγ in response to B95-8–derived LCL compared with endogenous virus-derived LCL (1.4% vs 0.8%, respectively); 3.3% and 2.9% of CD8+ T cells responded to B95-8–derived LCL and endogenous virus-derived LCLs, respectively (Figure 6B); 0.9% and 0.4% of CD4+ T cells responded to B95-8–derived LCL and endogenous virus-derived LCL, respectively (Figure 6B). Responding γ-δ T cells (10% and 6% directed against B95-8–derived LCL and endogenous virus-derived LCL, respectively) and responding NK cells (12.7% and 7% directed against B95-8–derived LCL and endogenous virus-derived LCL, respectively) made up small fractions of PBMC (Figure 6A,B). Although the identity of the responding cells were similar irrespective of the virus strain from which LCLs were derived, the magnitude of responses directed against B95-8–derived LCL was 1.5- to 2-fold higher than those directed against endogenous virus-derived LCLs.
Comparison of the magnitude of responses and types of cells producing IFNγ following culture of PBMC from healthy EBV-seropositive subject 20 with autologous LCL derived from B95-8 virus or endogenous virus (PBMC/LCL = 10:1). Data indicate percent IFNγ-producing cells as percentage of total PBMC (A) or as percentage of CD8+ T cells, CD4+ T cells, γ-δ+ T cells, and CD56+ (NK) cells (B). (C) Distribution of responding cells by memory phenotype.
Comparison of the magnitude of responses and types of cells producing IFNγ following culture of PBMC from healthy EBV-seropositive subject 20 with autologous LCL derived from B95-8 virus or endogenous virus (PBMC/LCL = 10:1). Data indicate percent IFNγ-producing cells as percentage of total PBMC (A) or as percentage of CD8+ T cells, CD4+ T cells, γ-δ+ T cells, and CD56+ (NK) cells (B). (C) Distribution of responding cells by memory phenotype.
We found that 43.3% of IFNγ-producing cells directed against B95-8–derived LCL were CD8+ EM cells and 57.7% of IFNγ-producing cells directed against endogenous virus-derived LCLs were CD8+ EM cells (Figure 6C); 10.5% and 12.2% of IFNγ-producing cells were CD4+ EM cells directed against B95-8–derived LCL and endogenous virus-derived LCL, respectively (Figure 6C). Thus, the bulk of the response was made by T cells with EM phenotype and the magnitude of responses was similar regardless of the virus strain from which LCLs were derived.
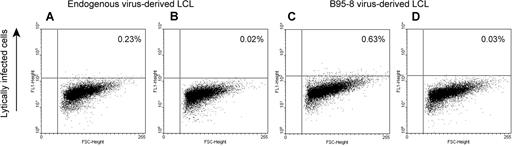
We observed that 2.1% of responses elicited by B95-8–derived LCLs were CD8+ EMRA+ cells, but no CD8+ EMRA+ cells were reactivated by endogenous virus-derived LCL. Hislop et al37 have found that, during persistent infection with EBV, CD8+ T cells directed against EBV lytic cycle antigens but not latent antigens often acquire CD45RA.37 To determine whether B95-8–derived LCLs had higher numbers of lytically infected cells compared with endogenous virus-derived LCLs, we examined both cell lines using a recently described flow cytometry–based assay.25 Figure 7 shows that 0.2% of endogenous virus-derived LCLs were lytically infected, whereas 0.6% of B95-8–derived LCLs were lytically infected. Thus, 3 times as many cells in the B95-8–derived cell line were lytically infected compared with the endogenous virus-derived cell line. This finding is consistent with the possibility that at least some of the CD8+ EMRA+ responses were directed against lytically infected B95-8–derived cells.
LCLs derived from B95-8 virus have a larger fraction of lytically infected cells than LCLs derived from endogenous virus. LCLs derived from endogenous virus (A,B) and LCLs derived from B95-8 virus (C,D) from healthy EBV-seropositive subject 20 were fixed and permeabilized followed by incubation with reference EBV-seropositive serum (A,C) or reference EBV-seronegative serum (B,D) and FITC-conjugated antihuman IgG.
LCLs derived from B95-8 virus have a larger fraction of lytically infected cells than LCLs derived from endogenous virus. LCLs derived from endogenous virus (A,B) and LCLs derived from B95-8 virus (C,D) from healthy EBV-seropositive subject 20 were fixed and permeabilized followed by incubation with reference EBV-seropositive serum (A,C) or reference EBV-seronegative serum (B,D) and FITC-conjugated antihuman IgG.
Discussion
In the present study, using a short-term ex vivo assay that measures cellular immune responses to autologous LCL in a single assay, we have been able to directly compare responses made by different types of immune cells and made several novel observations about EBV-specific cellular immunity in healthy patients persistently infected with EBV. First, CD4+ and CD8+ T cells from peripheral blood contribute nearly equally to this response. Second, whereas reactive T cells with CM and EM phenotype are both detectable in peripheral blood, T cells with EM phenotype make a larger contribution to EBV-specific responses. Third, EBV-specific CD4+ and CD8+ T cells are maintained at nearly equal frequencies in peripheral blood of healthy patients. Fourth, a significantly higher fraction of γ-δ cells in peripheral blood of EBV-seropositive patients respond compared with those in EBV-seronegative patients. Fifth, a similar enhanced response by NK cells is observed in healthy EBV-seropositive patients compared with EBV-seronegative patients. Sixth, healthy EBV-seropositive patients can be distinguished from healthy EBV-seronegative patients by the presence of robust cellular immune responses to EBV-LCL. Examination of T cells in blood that respond to autologous LCL may allow identification of patients at risk for development of EBV-LPD.
An unanswered question has been the relative contribution made by CD8+ and CD4+ T-cell responses to host immune control of EBV-infected cells. Lack of information regarding EBV lytic antigen epitopes presented on HLA class II molecules, differences in EBV antigen sources, and variability of techniques used to determine the reactivity of T-cell subsets against EBV have delayed finding an answer to the question of relative contributions by CD8+ and CD4+ T cells. In this study, using LCLs, an excellent surrogate cell type for in vivo latently infected B cells in secondary lymphoid organs of healthy patients31,38-40 and in peripheral blood of EBV-LPD patients,41-43 we measured CD4+ and CD8+ T-cell reactivities to EBV in parallel. In healthy patients persistently infected with EBV, we found that CD8+ and CD4+ T cells make nearly equal contributions to the adaptive immune response to EBV (Figure 2D). Equivalent contributions made by CD8+ and CD4+ T cells toward immune responses to EBV was again evident in the contributions made by cells of EM phenotype within CD8+ and CD4+ T-cell subsets (Figure 5A,B). One reason that fewer responding cells with CM phenotype were detected may be that 20 hours of exposure to antigen is not sufficient to activate such memory T cells. It is likely that those responding cells with CM phenotype belonged to the pre-Th1 CM subset, as has been demonstrated by Heller et al22 for EBNA1-specific CD4+ CM cells. The presence of T-cell responses with both EM and CM phenotypes in our study (Figure 5B) as well as in experiments by other investigators22,44 may be explained by the persistent nature of infection by EBV.
Use of a single assay for different types of effector cells has allowed us to visit the question of EBV-specific functional CD4+ and CD8+ T-cell frequencies. We found that EBV-specific CD8+ T cells were maintained at only 1.7-fold higher frequency compared with EBV-specific CD4+ T cells (Figure 3A,B). This finding is consistent with that of Nikiforow et al9,26 who demonstrated that, in healthy persistently infected patients, CD4+ T cells controlled the outgrowth of B cells infected with EBV in vitro, emphasizing their role in the control of EBV. However, it contradicts the prevailing dogma that EBV-specific CD8+ T-cell reactivities are maintained at 10-fold higher frequencies than EBV-specific CD4+ T-cell reactivities.12,17-23 We think that a 20-hour exposure of T cells to EBV-LCL is not long enough for proliferation of T cells and amplification of the responses. Therefore, the frequencies that we measured are likely to approximate closely the frequencies in vivo. In addition, the frequencies were very similar in experiments in which mixed effectors and effectors enriched for CD4+ and CD8+ T cells were examined (Figure 4).
The disparity between our findings and those made by others may be explained by differences in experimental approaches. Earlier studies of CD4+ T-cell frequencies to EBV lytic cycle antigens have been limited to a few exogenously provided lytic cycle antigens, such as ZEBRA and MTA23 or lysates from EBV-infected B cells.18 Several dozen antigens that are expressed during the EBV lytic cycle have not been tested because most of these antigens have not been identified and characterized. HLA class II epitope maps are not yet available for the few well-studied EBV lytic cycle antigens. These limitations are evident in the study by Gudgeon et al45 in which none of the LCL-reactivated CD4+ T-cell clones that could kill autologous LCL could be mapped to known EBV antigens. Our experiments have circumvented some of these difficulties using LCLs that process and present antigens via both endogenous and exogenous pathways for recognition by CD4+ T cells.
In healthy EBV-seropositive patients, up to one-third of γ-δ T cells recognized autologous LCL, contributing to an average of approximately 13% of responding PBMCs (Figure 2D). In other studies, γ-δ T cells formed a significant fraction of LCL-reactivated cytotoxic T lymphocyte (CTL) lines that were used in adoptive immunotherapy.46,47 To our knowledge, no study has compared EBV-LCL-directed γ-δ T-cell frequencies in peripheral blood of healthy EBV-seropositive and -seronegative patients. In our study, the fraction of PBMCs that were positive for γ-δ TCR and produced IFNγ was 6-fold higher in EBV-seropositive than in EBV-seronegative patients (Figure 2). Therefore, prior exposure to EBV may influence responses by γ-δ T cells. Similarly, the fraction of PBMCs that were NK cells and produced IFNγ was 5-fold higher in EBV-seropositive than in EBV-seronegative patients (Figure 2). This enhanced response by NK cells in seropositive patients may be suggestive of NK-cell memory or may be a result of rapid activation of NK cells by cytokines produced from reactivated T cells. Although they are components of the innate arm of the immune system, NK cells may retain immune memory as has been observed in mice.48
The ability to detect decline in EBV-specific T-cell surveillance would lead to early identification of immunosuppressed patients at risk for development of EBV-LPD. Measurements of EBV-specific T-cell responses are not routinely performed because of the lack of a test that can be used in the general population. That CTL reactivated by EBV-LCL can provide effective tumor killing in vitro and in vivo49 suggested that EBV-LCL reactivated T-cell responses in peripheral blood should be ideal for evaluating EBV-specific T-cell immune responses. Using autologous EBV-LCL to reactivate peripheral T-cell responses, we were able to distinguish between healthy EBV-seropositive patients and healthy EBV-seronegative patients (Figure 1A,B). Although earlier reports of T-cell responses in EBV-seropositive patients to EBV antigens50 imply that healthy seropositive patients would generate a rapid ex vivo response to autologous LCL, to our knowledge, there is no study that has systematically compared the magnitude, repertoire, and variation of such responses between healthy EBV-seropositive and -seronegative patients.
The antigen specificity of responding T cells in healthy EBV-seropositive patients was not defined in our experiments; however, studies by other investigators would suggest responses to be directed at least against known HLA class II immunodominant antigens, such as EBNA1, EBNA3C, EBNA3B, ZEBRA, and MTA, and known HLA class I immunodominant antigens, such as EBNA3A, EBNA3B, EBNA3C, ZEBRA, RTA, MTA, and EA-D.7,8 Responses directed against B95-8–derived LCLs and endogenous virus-derived LCLs were comparable (Figure 6A-C), emphasizing the feasibility of this assay without the need for generation of LCLs from endogenous virus in all patients. Evaluation of patients for T-cell responses to EBV should now be possible using this LCL-based assay. It would be of interest to compare the LCL-based assay alongside an existing assay such as EBV viral load in patients at risk for development of EBV disease. Because HLA typing is routine in patients receiving transplants and tetramers are easily available for MHC class I epitopes, the LCL-based assay could be used in conjunction with tetramer assays. However, the LCL-based assay offers potential advantages of being a functional assay and being able to detect CD8+ and CD4+ T-cell responses to latent and lytic antigens simultaneously. Clearly, further investigation is necessary to compare the LCL-based assay to assays measuring T-cell responses to EBV antigens. Although virus-specific synthetic peptide mixtures are now readily available, using EBV-LCL as antigen presenting cells provides the additional benefit of presenting both exogenously and endogenously processed viral proteins.51
In conclusion, experiments presented in this study should have far-reaching implications for early identification of immunosuppressed patients at risk for development of EBV-LPD. Responses to autologous EBV-LCL by immune cells may be a marker for overall function of the cellular arm of the immune system given the coevolution of EBV and the human immune system. Examination of immunosuppressed populations will allow evaluation of this assay for its potential to assess the overall function of the cellular immune system. Detection of almost equal CD4+ and CD8+ T-cell contributions toward EBV-specific responses and the presence of nearly equal CD4+ and CD8+ T-cell reactivities directed against EBV emphasize the importance of an effector role of CD4+ T cells in EBV-specific immunosurveillance. Thus, adoptive immunotherapy using EBV-specific CTL is likely to benefit from the presence of CD4+ CTL in addition to CD8+ CTL. Alternative immunotherapy using populations of γ-δ TCR+ CTL generated in vitro after a short exposure to LCLs may be useful in the interval between diagnosis of EBV-LPD and generation of EBV-specific CTL. This approach may be especially useful in patients with EBV-LPD after solid organ transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Profs Nancy Ruddle, Jordan Pober, Joseph Craft, Eugene Shapiro, Samuel Silverstein, and Cliona Rooney for providing valuable comments about this study.
This study was supported by National Institutes of Health grants K08 AI062732, K12 HD001401, CA-12055, CA-16038, T32-CA-09159, and T32 AI07210.
National Institutes of Health
Authorship
Contribution: S.B.-M. and G.M. designed the research; S.B.-M. and M.J.R. performed the experiments; S.B.-M. analyzed the data; S.B.-M. and G.M. wrote the manuscript; and M.R. and B.G. provided EBV-seronegative subjects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sumita Bhaduri-McIntosh, Yale University School of Medicine, Division of Infectious Disease, Department of Pediatrics, Rm 419 LSOG, 333 Cedar St, New Haven, CT 06520; e-mail: sumita.bhaduri-mcintosh@yale.edu.