To the editor:
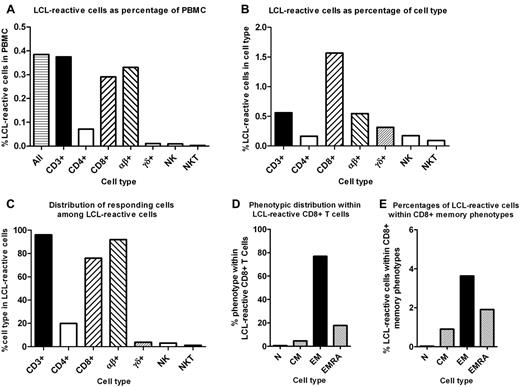
Infection with EBV is normally kept in check by cellular immunity,1,2 which, if disrupted, may contribute to the important role of EBV in the pathogenesis of certain malignancies3 and possibly chronic autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus.4 It is therefore essential that the aggregate T-cell response to EBV can be measured accurately to identify deficiencies in T-cell populations that might predispose to EBV-associated diseases and to devise strategies to correct these deficiencies. A recent study measuring the frequencies of PBMCs reactive to EBV-derived autologous lymphoblastoid cell lines (LCLs) reported that CD4+ and CD8+ T cells made nearly equal contributions toward the EBV-specific response in healthy immune subjects.5 Using a similar assay, we report that the CD8+ T-cell response far outweighed the CD4+ T-cell response to EBV-infected autologous LCLs in 37 healthy immune subjects (Figure 1A-C). Our study was approved by the Royal Brisbane and Women's Hospital Human Research Ethics Committee and The University of Queensland Medical Research Ethics Committee, and informed consent was obtained according to the Declaration of Helsinki. The frequency of LCL-reactive T cells in the CD8+ population was 9.6-fold higher than in the CD4+ population in our study (Figure 1B) compared with 1.7-fold higher in the previous study.5 Of the phenotypically identifiable LCL-reactive cells, 76.0% were CD8+ T cells, 19.9% were CD4+ T cells, 3.9% were γδ T cells, and 3.0% were natural killer (NK) cells in our study (Figure 1C), compared with 43.3%, 33.0%, 12.9%, and 10.8%, respectively, in the previous study.5 We found that LCL-reactive CD8+ T cells were predominantly of the effector memory type and less frequently of the CD45RA+ effector memory (CD45RA+CCR7−) type (Figure 1D-E), in accordance with the previous study.5 The specificity of the T-cell response to EBV was demonstrated by the fact that the mean frequencies of LCL-reactive CD4+ T cells, CD8+ T cells, γδ T cells, and NK cells in PBMC, were 0.00%, 0.00%, 0.00%, and 0.00%, respectively, in 6 EBV-seronegative subjects, compared with 0.07%, 0.29%, 0.01%, and 0.01%, respectively, in EBV-seropositive subjects (Figure 1A). Another important difference between our findings and those of the previous study is that the proportions of LCL-reactive cells in the γδ T-cell and NK-cell populations were 0.3% and 0.2%, respectively, in our study (Figure 1B), compared with 21.7% and 38.9%, respectively, in the previous study.5
Reactivity to autologous LCLs in the PBMCs of EBV-seropositive healthy subjects. The mean reactivity to autologous LCLs was determined in 37 EBV-seropositive healthy subjects between the ages of 26 and 63 years (mean, 46 years) with flow cytometry and intracellular IFN-γ staining using a Gallios flow cytometer (Beckman Coulter) with 9-color acquisition. PBMCs were separated by density centrifugation and cryopreserved. LCLs were generated and grown for 3 months to ensure homogeneity as described previously.7 Each LCL was checked for CD3 and intracellular IFN-γ expression to ensure that there was no evidence of T-cell contamination. Cryopreserved PBMC samples were thawed and cultured for 24 hours before use to allow the cells to rest and reexpress cell-surface molecules. Cultures of 106 PBMCs were stimulated with 5 × 105 autologous LCLs for 6 hours in the presence of brefeldin A. Nonstimulated PBMC cultures were used to measure background IFN-γ expression. Cells were stained with Abs to cell-surface markers and then fixed and permeabilized before intracellular IFN-γ staining. Two Ab panels were used for each subject. Single labeled tubes for each Ab, isotype-matched control Abs, fluorescence-minus-one controls, dead-cell exclusion dyes, and doublet discrimination were used during panel development to ensure accurate positive cutoff values and compensation matrices and to validate cell phenotype detection sensitivity and resolution. The first Ab panel assessed the frequencies and memory phenotypes of LCL-reactive CD4+ and CD8+ T cells and consisted of the following fluorochrome-conjugated Abs: anti–IFN-γ-FITC (Beckman Coulter), anti–CCR7-peridinin chlorophyll protein (PerCP)–Cy5.5 (BD Biosciences), anti–CD45RA-PE-Cy7 (BD Biosciences), anti–CD3-allophycocyanin (APC; Beckman Coulter), anti–CD8-APC-A700 (Beckman Coulter), anti–CD62L-APC-Cy7 (BioLegend), anti–CD4-V450 (BD Biosciences), and Aqua Live/Dead cell exclusion dye (Invitrogen). For the first Ab panel tubes, 400 000 events were collected to enable accurate estimation of LCL-reactive T-cell frequencies. The numbers of background IFN-γ–expressing cells were subtracted from measures of LCL-reactive cells. The second Ab panel assessed the LCL reactivity of αβ T cells, γδ T cells, and NK cells and consisted of: anti–IFN-γ-FITC (Beckman Coulter), anti–TCR-γδ-PE (BioLegend), anti–TCRαβ-PE-Cy7 (BioLegend), anti–CD3-APC (Beckman Coulter), anti–CD19-APC-Cy7 (BD Biosciences), anti–CD16-V450 (BD Biosciences), anti–CD56-V450 (BD Biosciences), and Aqua Live/Dead cell exclusion dye. (A) LCL-reactive cells as percentages of PBMCs. (B) LCL-reactive cells as percentages of cell type. (C) Percentage contribution of cell types among the phenotypically identifiable LCL-reactive cells. (D) Percentage contribution of different memory phenotypes to CD8+ LCL reactivity; N indicates naive (CD45RA+CCR7+); CM, central memory (CD45RA−CCR7+); EM, effector memory (CD45RA−CCR7−); EMRA, CD45RA+EM (CD45RA+CCR7−). (E) Percentages of LCL-reactive cells within different CD8+ T-cell memory phenotypes.
Reactivity to autologous LCLs in the PBMCs of EBV-seropositive healthy subjects. The mean reactivity to autologous LCLs was determined in 37 EBV-seropositive healthy subjects between the ages of 26 and 63 years (mean, 46 years) with flow cytometry and intracellular IFN-γ staining using a Gallios flow cytometer (Beckman Coulter) with 9-color acquisition. PBMCs were separated by density centrifugation and cryopreserved. LCLs were generated and grown for 3 months to ensure homogeneity as described previously.7 Each LCL was checked for CD3 and intracellular IFN-γ expression to ensure that there was no evidence of T-cell contamination. Cryopreserved PBMC samples were thawed and cultured for 24 hours before use to allow the cells to rest and reexpress cell-surface molecules. Cultures of 106 PBMCs were stimulated with 5 × 105 autologous LCLs for 6 hours in the presence of brefeldin A. Nonstimulated PBMC cultures were used to measure background IFN-γ expression. Cells were stained with Abs to cell-surface markers and then fixed and permeabilized before intracellular IFN-γ staining. Two Ab panels were used for each subject. Single labeled tubes for each Ab, isotype-matched control Abs, fluorescence-minus-one controls, dead-cell exclusion dyes, and doublet discrimination were used during panel development to ensure accurate positive cutoff values and compensation matrices and to validate cell phenotype detection sensitivity and resolution. The first Ab panel assessed the frequencies and memory phenotypes of LCL-reactive CD4+ and CD8+ T cells and consisted of the following fluorochrome-conjugated Abs: anti–IFN-γ-FITC (Beckman Coulter), anti–CCR7-peridinin chlorophyll protein (PerCP)–Cy5.5 (BD Biosciences), anti–CD45RA-PE-Cy7 (BD Biosciences), anti–CD3-allophycocyanin (APC; Beckman Coulter), anti–CD8-APC-A700 (Beckman Coulter), anti–CD62L-APC-Cy7 (BioLegend), anti–CD4-V450 (BD Biosciences), and Aqua Live/Dead cell exclusion dye (Invitrogen). For the first Ab panel tubes, 400 000 events were collected to enable accurate estimation of LCL-reactive T-cell frequencies. The numbers of background IFN-γ–expressing cells were subtracted from measures of LCL-reactive cells. The second Ab panel assessed the LCL reactivity of αβ T cells, γδ T cells, and NK cells and consisted of: anti–IFN-γ-FITC (Beckman Coulter), anti–TCR-γδ-PE (BioLegend), anti–TCRαβ-PE-Cy7 (BioLegend), anti–CD3-APC (Beckman Coulter), anti–CD19-APC-Cy7 (BD Biosciences), anti–CD16-V450 (BD Biosciences), anti–CD56-V450 (BD Biosciences), and Aqua Live/Dead cell exclusion dye. (A) LCL-reactive cells as percentages of PBMCs. (B) LCL-reactive cells as percentages of cell type. (C) Percentage contribution of cell types among the phenotypically identifiable LCL-reactive cells. (D) Percentage contribution of different memory phenotypes to CD8+ LCL reactivity; N indicates naive (CD45RA+CCR7+); CM, central memory (CD45RA−CCR7+); EM, effector memory (CD45RA−CCR7−); EMRA, CD45RA+EM (CD45RA+CCR7−). (E) Percentages of LCL-reactive cells within different CD8+ T-cell memory phenotypes.
Because a proportion of cells within LCLs are lytically infected, the T-cell response to LCLs comprises responses to both lytic and latent EBV antigens. In our study, the mean frequency of lytically infected cells in 11 LCLs was 2.1%, as determined by expression of the immediate early protein BZLF1 measured with the BZ1 mAb (Santa Cruz Biotechnology). This is higher than the 0.6% level of lytic infection found by the earlier study.5 To reduce the possible effect of variability in the level of lytic infection on the T-cell response to LCLs, we used a PBMC/LCL ratio of 2:1, compared with 10:1 used in the previous study.5 Varying the PBMC/LCL ratio from 2:1 to 10:1 did not alter the LCL-specific frequencies in the CD4+ and CD8+ T-cell populations, but increasing the ratio to 20:1 decreased the frequencies. The differences between our results and those of Bhaduri-McIntosh et al may be due to technical differences in the assays used, such as a shorter incubation time of 6 hours compared with 20 hours and a longer LCL culture time of 3 months compared with 3-4 weeks, respectively.5 Our longer LCL culture time was used to avoid contamination of the LCL by EBV-specific T cells, which could falsely increase the recorded frequency of LCL-reactive cells in the tested populations. Furthermore, we studied nearly 4 times as many EBV-exposed subjects as the earlier study.5
Our finding that the frequency of EBV-specific T cells was much higher in the CD8+ T-cell population than the CD4+ T-cell population is consistent with previous studies using other assays.6-8 Studies using the IFN-γ ELISPOT assay to measure the response to autologous LCLs in fractionated T-cell populations of immune subjects reported that the frequency of LCL-specific T cells was 5-7 times higher in the CD8+ population than in the CD4+ population.6,7 Another study found that the frequency of CD4+ T cells responding to EBV-infected B-cell lysates in healthy EBV carriers8 was much lower than the frequency of CD8+ T cells specific for EBV epitopes.2 Our finding that LCL-reactive cells constituted 1.6% of the CD8+ population in healthy immune subjects (Figure 1B) is similar to the 1.3% found by Yang et al using an IFN-γ ELISPOT assay.6 The great preponderance of CD8+ T cells over CD4+ T cells in the immune response to EBV in healthy EBV carriers is consistent with the massive expansion of CD8+ T cells, but not CD4+ T cells, during infectious mononucleosis9 and the role of EBV-specific CD8+ T cells as the principal effectors mediating regression of LCL outgrowth in healthy EBV carriers.10
Authorship
Acknowledgments: The authors thank Dr Stefan Blum, Kaye Hooper, and Bernie Gazzard for assistance in the collection of blood samples. This work was supported by a project grant from Multiple Sclerosis Research Australia.
Contribution: M.P.P. planned the study, helped analyze the results, and wrote the manuscript; P.A.C. performed the laboratory studies and analyzed the data; C.M.M.P. assisted with the laboratory studies; S.R.B. helped plan the study and analyze the data; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Michael P. Pender, Level 9, Health Sciences Building, Royal Brisbane and Women's Hospital, Brisbane, Queensland 4029, Australia; e-mail: m.pender@uq.edu.au; or Prof Scott R. Burrows, Cellular Immunology Laboratory, Queensland Institute of Medical Research, 300 Herston Road, Brisbane, Queensland 4029, Australia; e-mail: scott.burrows@qimr.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal