Patients with autoimmune lymphoproliferative syndrome (ALPS) and systemic lupus erythematosis (SLE) have T-cell dysregulation and produce abnormal, activated T lymphocytes and an atypical peripheral T-cell population, termed double negative T cells (DNTs). T-cell functions, including DNT transition in T-cell development and T-cell activation, are critically dependent on Notch signaling. We hypothesized that inhibiting Notch signaling would be effective in ALPS and SLE by reducing the production of abnormal DNTs and by blocking aberrant T-cell activation. We tested this hypothesis using murine models of ALPS and SLE. Mice were randomized to treatment with the notch pathway inhibitor (gamma-secretase inhibitor), N-S-phenyl-glycine-t-butyl ester (DAPT), or vehicle control. Response to treatment was assessed by measurement of DNTs in blood and lymphoid tissue, by monitoring lymph node and spleen size with ultrasound, by quantifying cytokines by bead-array, by ELISA for total IgG and anti–double-stranded DNA (dsDNA) specific antibodies, and by histopathologic assessment for nephritis. We found a profound and statistically significant decrease in all disease parameters, comparing DAPT-treated mice to controls. Using a novel dosing schema, we avoided the reported toxicities of gamma-secretase inhibitors. Inhibiting the Notch signaling pathway may thus present an effective, novel, and well-tolerated treatment for autoimmune and lymphoproliferative diseases.
Introduction
The pathophysiology of a number of autoimmune and lymphoproliferative diseases is related to T-cell dysregulation. Patients and mice with 2 of these diseases, autoimmune lymphoproliferative syndrome (ALPS) and systemic lupus erythematosis (SLE), produce both abnormal, activated T lymphocytes and a marked expansion of a usually very small T-cell population, double negative T cells (DNTs; cell phenotype: CD3+, CD4−, CD8−, T-cell receptor [TCR]αβ+).1,–3 These abnormalities in T-cell regulation are caused in part by defects in the Fas apoptotic pathway, leading to abnormal lymphocyte survival with subsequent autoimmunity.4,5
The essential role of the Fas apoptotic pathway in lymphocyte homeostasis was first elucidated in studies in Fas-deficient MRL-lpr mice.6 Mice homozygous for Fas mutations develop hypergammaglobulinemia, glomerulonephritis, massive lymphadenopathy, and expansion of DNTs.6,7 This discovery provided insights into the pathophysiology of a similar syndrome observed in humans, ALPS. Patients with ALPS usually present at a young age with lymphadenopathy and splenomegaly.8 A high percentage of patients develop autoimmunity, most commonly with autoimmune cytopenias.9 ALPS is often associated with heterozygous mutations in the genes encoding the Fas protein, TNFRSF6 (tumor necrosis factor receptor superfamily 6), and related proteins that regulate lymphocyte survival.10 These mutations are usually inherited in an autosomal dominant fashion with variable penetrance.10
In contrast to ALPS, the defective apoptosis in SLE is caused by factors extrinsic to the Fas pathway that interfere with its activation, including excess soluble Fas protein.4,11 Systemic manifestations in SLE patients are heterogeneous, frequently including autoimmunity, vasculitis, arthritis, and glomerulonephritis.12
While many patients with ALPS and SLE respond to conventional therapies, some patients are refractory and new treatments are needed. The majority of treatment modalities for patients with ALPS and SLE focus on use of nonspecific immunosuppressants, frequently with significant toxicity profiles and limited efficacy. Because these treatment modalities are nonspecific, development of target-specific treatment approaches may be beneficial.
One candidate for targeted treatment is the Notch signaling pathway, because it has several critical roles in T-cell function, including DNT transition in T-cell development and T-cell activation.13 Notch signaling is mediated through a pathway of 4 mammalian transmembrane Notch receptors (Notch1-4).13 After ligand binding, 2 cleavages of the Notch receptors occur, first by a metalloprotease and subsequently by gamma-secretase, releasing the intracellular domain of Notch1 (ICN) that translocates to the nucleus and binds to the transcription factor CSL (RBP-Jk), activating transcription of a number of key intracellular proteins.13 Gamma-secretase inhibitors (GSIs) block the second cleavage, preventing the release of ICN and transcriptional activation. We hypothesized that inhibiting Notch signaling would be effective in reducing symptoms and treating the disease in patients with ALPS and SLE both by reducing the production of abnormal DNTs and by blocking aberrant T cell activation. We tested this hypothesis using 2 murine models of defective lymphocyte apoptosis, CBA-lprcg and MRL-lpr.14
CBA-lprcg has a phenotype similar to human ALPS, as these mice develop massive lymphadenopathy and splenomegaly with DNT infiltration of these organs. While there are a number of mouse models that mimic human ALPS, CBA-lprcg mice have been shown to be the most similar to the majority of ALPS patients.11 While the MRL-lpr mouse model has been used to study ALPS, this model has phenotypic features very similar to human SLE: these mice develop autoantibodies, glomerulonephritis, and a vasculitic dermatitis. The MRL-lpr mouse model is a well-studied model of SLE and is used frequently for preclinical testing of new agents for this disease.15,,–18
GSIs are in clinical development for treatment of other disorders, including Alzheimer disease and T-cell leukemia. We tested one of these inhibitors, N-S-phenyl-glycine-t-butyl ester (DAPT), in the 2 mouse models.19,20 Early reports using GSIs in other disease models delivered drugs at high doses for a short-duration and mice developed thymic hypoplasia and intestinal goblet cell hyperplasia within 7 days.21,22 These effects were clearly dose and time dependent.23,24 To reduce toxicity, we used a lower potency GSI and treated mice daily for 5 days a week. Using this approach, we found that inhibiting the Notch pathway was effective and safe in both mouse models, suggesting that titration of GSI potency may be critical to future clinical trials with GSIs. In addition, GSIs may become a novel therapeutic approach for a wide range of autoimmune diseases.
Methods
Animals and DAPT treatment schedule
CBA-lprg and MRL-lprr mice were obtained from The Jackson Laboratories, Bar Harbor, ME and were maintained in pathogen-free housing. Mice aged 5 to 6 months were randomized to treatment with DAPT (Calbiochem, San Diego, CA) or vehicle control by gavage. DAPT was given at a dose of 5mg/kg per day, 5 days per week. DAPT was prepared by reconstituting powder with 100% ethanol and stored in aliquots as a stock solution at −20°C. Each day fresh stock solution was diluted into a mixture of corn oil and ethanol (EtOH) to a final concentration of 5% EtOH and 95% corn oil.25 All experiments were performed under an Institutional Animal Care and Use Committee approved protocol. All experiments were repeated at least once for validation. For murine drug-efficacy experiments, mice were treated in cohorts and response data were pooled after completion of all studies. A total of 35 CBA-lprcg mice (15 treatments and 20 controls) and 37 MRL-lprr mice (21 treatments and 16 controls) were used for these experiments. Unless otherwise noted, CBA-lprcg mice were treated for 12 weeks and MRL-lpr mice were treated 6 to 8 weeks, and different cohorts of mice were used to assess the different disease parameters. MRL-lpr mice were killed at an earlier time point based on the limited survival of untreated with advanced disease. Mouse deaths from disease or drug treatment are detailed in the results section, specific for that experiment.
Assessment of response to DAPT treatment: ultrasound measurement of lymph node and spleen size
We followed lymph node and spleen size in the mice using a Vevo 660 small animal ultrasound (VisualSonics, Toronto, ON). We have previously reported and validated this ultrasound model using these mouse strains, and detailed methods are included in our prior published work.26
Mice were treated with DAPT or vehicle after they developed clinically identifiable lymphadenopathy, defined as having at least one lymph node of 100 mm3 in volume as assessed by ultrasound. We followed the largest lymph node at initiation of treatment in each mouse. On study entry and every 2 weeks until sacrifice, one lymph node was serially measured for volume by three-dimensional imaging, and splenic length and height were measured by B-mode imaging. Comparisons were made between treated and untreated animals by 2-sided t test. All data analysis and ultrasound measurements were performed blinded to treatment group.
Assessment of response to DAPT treatment: peripheral blood, lymph nodes, and spleen for DNTs
We followed blood counts and DNTs in the mice by retro-orbital bleed at initiation of treatment and serially every other week. Comparisons were made between treated and untreated animals by 2-sided t test. Complete blood count (CBC) analysis was performed on a HemeVet 850FS hematology analyzer (CDC Technologies, Oxford, CT) and flow cytometric analysis for DNTs was performed using anti-TCRβ chain-FITC, anti-CD4-PE, anti-CD8a (Ly-2)-PerCP-CY5.5, and anti-CD3ϵ-APC-Cy7. T-cell activation was measured using anti-CD44-PerCP-CY5.5. All antibodies were purchased from BD Pharmingen (Franklin, NJ). We monitored absolute DNT cell counts, defined as white blood cells (WBCs; measured by HemeVet hematology analyzer) times the percentage of lymphocytes (calculated by HemeVet hematology analyzer and confirmed by FSC/SSC on flow cytometry) times the percentage of DNTs (calculated by flow cytometry).
Multicompartment analysis was performed to determine the effect of gamma-secretase inhibitors on different splenic cell populations at killing after 3 months of treatment. Flow cytometry using the aforementioned antibodies was used to determine the effect of gamma-secretase inhibitors on DNTs, CD3+CD4+ T cells, CD3+CD8+ T cells, and non–T cells.
Assessment response to DAPT treatment
MRL-lprr mice were randomized to treatment with DAPT or vehicle for 6 to 8 weeks to assess the impact of GSI on autoantibodies and nephritis.
Autoantibodies.
Serum was obtained from mice by retro-orbital bleed at initiation of treatment and serially every other week. Serum samples were frozen at the time of collection and all samples were analyzed at the same time for mouse anti-ds DNA IgG specific antibodies and total mouse IgG2a specific antibodies using 96-well quantitative enzyme-linked immunosorbent assay (ELISA) kits from Alpha Diagnostic International (San Antonio, TX). IgG2a is the dominant isotype produced by MRL-lpr animals.27 All samples were run in duplicate.
Nephritis.
After completion of the treatment period, mice were killed and kidneys were harvested and fixed in formalin. Kidney sections were stained with hematoxylin and eosin (H&E), and histopathology was assessed by a pathologist blinded to treatment group. A nephritis index based on prior published methodology was used to score kidney disease.28 Kidneys were scored on 4 measures: (1) degree of medullary lymphocytic infiltrate (lymphoid proliferation); (2) degree of cortical lymphocytic infiltrate; (3) degree of glomerulonephritis; and (4) degree of nephropathy (multifocal tubular epithelial cell hypertrophy with or without tubular dilatation or casts). All 4 measures were scored 0 to 5 with zero representing no abnormality and 1 to 5 representing minimal, mild, moderate, marked, and severe, respectively. A cumulative score of 0 to 20 could be determined by a combination of the 4 separate measures.
Real-time PCR quantitation of Notch-activated transcripts
We performed quantitative real time polymerase chain reaction (RT-PCR) to quantify RNA transcripts of Hes1 and Deltex1. Total RNA was isolated from harvested lymph nodes using the RNeasy Mini Kit (Qiagen, Valencia, CA). One microgram of total cellular RNA was reverse transcribed using Invitrogen Reverse Transcription reagents (Carlsbad, CA) and oligo dT. Gene expression was analyzed using real time PCR on an ABI7500 SDS system (Applied Biosystems, Foster City, CA). One hundred nanograms of cDNA were used in each quantitative PCR assay. Probes and primers for Deltex1, Hes1, and VEGF were synthesized by Applied Biosystems. All reactions were performed in triplicate for 40 cycles, per the manufactuer's recommendation. Samples were normalized using the geometric mean of beta-5 tubulin (Applied Biosystems), and data are reported as the ratio of treated cells to untreated control cells.
Immunoblotting for activated notch protein
We performed immunoblots to assess quantity of activated cleaved Notch 1 protein. For these experiments, we used our published immunoblot methods, except that cells were lysed in RIPA lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA), and for each sample 80 μg total cell lysate was loaded on a 10% Tris-HCl precast gel for sodium dodecyl sulfate-polyacrylamide gel electrophoresis.29 Bands were outlined and quantitated densitometrically using Quantity-One software (BioRad, Hercules, CA).
T-cell functional assays
Lymph node cells, splenocytes, and thymocytes were collected from mice treated with DAPT or control. T lymphocytes from mice were sustained in vitro using published techniques.30 Cells were mitogen-stimulated with phytohemagglutinin (3 μg/mL) (Sigma-Aldrich, St Louis, MO) in triplicate wells. After 24 hours, supernatants were collected and frozen at −80°C until analyzed. Cell proliferation was assayed after 48 hours, using methyl-thiazole-tetrazolin (MTT) (Sigma-Aldrich) as previously described.31
Frozen supernatants were thawed and mouse inflammation cytometric bead array (CBA) kit (BD Pharmingen) was used to quantify tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL)-6, -10, and -12, and monocyte chemoattractant protein-1 (MCP-1), following the manufacturer's recommended protocol. Fas-mediated apoptosis was assessed using our published techniques.5,32
Results
Notch inhibition decreases lymphoproliferation in CBA-lpr mice
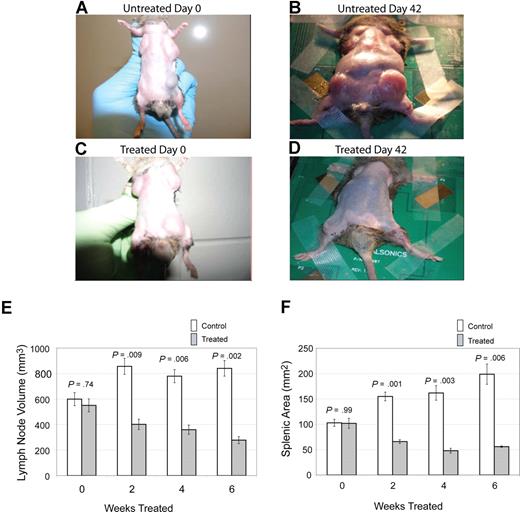
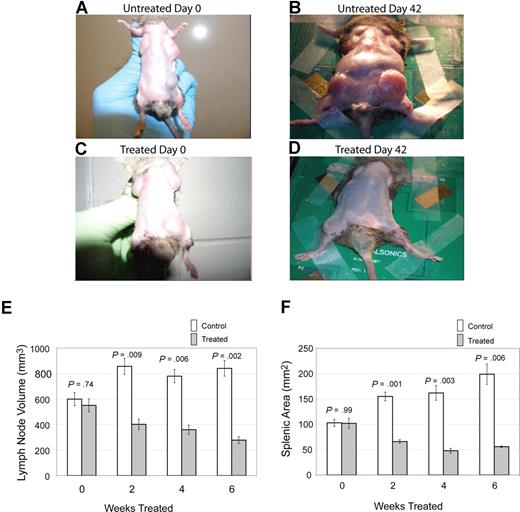
The primary clinical manifestation in patients with ALPS is lymphoproliferation, with marked lymphadenopathy and splenomegaly.9 To assess the effect of notch inhibition on lymphoproliferation, we treated CBA-lprcg mice daily, 5 days a week, with the GSI DAPT or control, and we followed lymph node and spleen size by small animal ultrasound. We found lymph node volumes correlated with cell counts (r = 0.9041; P < .001 by ANOVA) and spleen size correlated with cell counts (r = 0.9625; P < .001). Lymph nodes and spleens were measured in 6 treated and 9 control animals. Mice were followed for 3 months and then killed. We found a marked decrease in lymphoproliferation in CBA-lprcg mice treated with DAPT compared with control animals (Figure 1A-D). At initiation of treatment, average size of lymph nodes and spleens were not statistically different between treated and control mice (Figure 1E,F). Treated mice showed a statistically significant decrease in lymph node (P = .009, Figure 1E) and spleen size (P = .001, Figure 1F) after only 2 weeks of treatment (P = .009) (Figure 1E). These results demonstrate that DAPT is an effective agent for alleviating murine lymphoproliferation.
Notch inhibition decreases lymphoproliferation. CBA-lprcg mice were randomized to treatment with DAPT 5mg/kg per day versus vehicle control (6 treated, 9 control). After 6 weeks of treatment, a decrease in disease burden is visually apparent. Shown here are a control animal at (A) initiation and (B) after 42 days of vehicle, and a treated mouse at (C) initiation of DAPT treatment and (D) after 42 days of treatment. Serial ultrasounds were performed every 2 weeks to document lymph-node volume (mm3) and splenic area in (mm2). Treated mice showed a statistically significant (P = .009) decrease in lymph-node volume after 2 weeks of treatment compared with control mice (E). Treated mice also showed a statistically significant (P = .001) decrease in splenic area by 2 weeks of treatment compared with control mice (F). No statistical difference existed between groups at initiation of treatment. Bars represent mean lymph node volume (E) or splenic area (F) from mice at each time point, and error bars represent SEM.
Notch inhibition decreases lymphoproliferation. CBA-lprcg mice were randomized to treatment with DAPT 5mg/kg per day versus vehicle control (6 treated, 9 control). After 6 weeks of treatment, a decrease in disease burden is visually apparent. Shown here are a control animal at (A) initiation and (B) after 42 days of vehicle, and a treated mouse at (C) initiation of DAPT treatment and (D) after 42 days of treatment. Serial ultrasounds were performed every 2 weeks to document lymph-node volume (mm3) and splenic area in (mm2). Treated mice showed a statistically significant (P = .009) decrease in lymph-node volume after 2 weeks of treatment compared with control mice (E). Treated mice also showed a statistically significant (P = .001) decrease in splenic area by 2 weeks of treatment compared with control mice (F). No statistical difference existed between groups at initiation of treatment. Bars represent mean lymph node volume (E) or splenic area (F) from mice at each time point, and error bars represent SEM.
Notch inhibition decreases DNTs in CBA-lprcg and MRL-lpr mice and DNTs are more sensitive to DAPT than other cell populations
Patients and mice with ALPS and SLE have increased numbers of an atypical T-cell population, DNTs (T-cell phenotype CD3+, CD4−, CD8−, and TCRαβ+), that represents less than 1% of the peripheral T-cell population in healthy people.1 DNTs are abnormally elevated in peripheral blood, lymph nodes, and spleens of CBA-lprcg mice as well as MRL-lpr mice.33,34
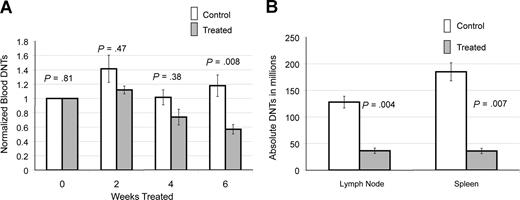
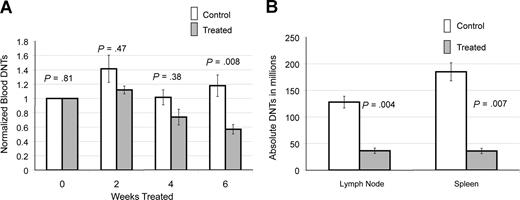
We followed peripheral blood DNTs in 6 treated and 13 control CBA-lprcg mice by serial retro-orbital bleeds. These mice were also used for the ultrasound experiments. At initiation of treatment, the average peripheral blood absolute DNTs (defined as WBC in mm3 × % CD3+/CD4−/CD8−/TCRαβ+cells) were similar for treated and control animals (treated average = 16.4×109/L [16 413/mm3] and control average = 14×109/L [14 019/mm3]; P = .81). Marked heterogeneity was observed in peripheral blood absolute DNTs at initiation of study, ranging from 1.5 to 67.3×109/L (1521 to 67 281/mm3). To make comparisons between groups, serial DNT measurements for each mouse were normalized to the untreated baseline of the individual mouse. We found a statistically significant decrease in absolute DNTs, comparing treated and control animals by 6 weeks of treatment (P = .008; Figure 2A).
Notch inhibition decreases DNTs. (A) Response of DNTs in peripheral blood to GSI in 6 treated and 13 contorl CBA-lprcg mice. Retro-orbital bleeds were performed every 2 weeks to assess absolute DNTs/mm3, comparing mice treated with DAPT and vehicle control (absolute DNTs: WBC in mm3 × % CD3+/TCRα/β+/CD4−/CD8− cells) Serial absolute DNT measurements were normalized to the untreated baseline for each mouse. Treated mice showed a statistically significant decrease in absolute DNTs compared with controls by 6 weeks of treatment. Bars represent normalized mean absolute double negative T-cell counts from mice at each time point, and error bars represent SEM. (B) Average number of DNTs in lymph nodes and spleens (defined as total cell count × % CD3+/TCRα/β+/CD4−/CD8− cells), comparing GSI-treated and control animals, showing a statistically significant decrease in both. Error bars represent SEM.
Notch inhibition decreases DNTs. (A) Response of DNTs in peripheral blood to GSI in 6 treated and 13 contorl CBA-lprcg mice. Retro-orbital bleeds were performed every 2 weeks to assess absolute DNTs/mm3, comparing mice treated with DAPT and vehicle control (absolute DNTs: WBC in mm3 × % CD3+/TCRα/β+/CD4−/CD8− cells) Serial absolute DNT measurements were normalized to the untreated baseline for each mouse. Treated mice showed a statistically significant decrease in absolute DNTs compared with controls by 6 weeks of treatment. Bars represent normalized mean absolute double negative T-cell counts from mice at each time point, and error bars represent SEM. (B) Average number of DNTs in lymph nodes and spleens (defined as total cell count × % CD3+/TCRα/β+/CD4−/CD8− cells), comparing GSI-treated and control animals, showing a statistically significant decrease in both. Error bars represent SEM.
We also analyzed the lymph nodes and spleens of 5 treated and 7 control CBA-lprcg mice at sacrifice. One treated mouse died from complications of gavage, and 6 control animals died from disease prior to the 3 month time-point. We found a decrease in DNTs in lymph nodes and spleens in the treated mice compared with control mice at sacrifice. We assessed the DNTs from the largest lymph node (the lymph node serially followed by ultrasound) in the animals. Average numbers of DNTs in lymph nodes (defined as total cell count × % CD3+/CD4−/CD8−/TCRαβ+ cells) from treated mice was 36 × 106 cells (range: 2–70 × 106), and average number in control animals was 128 × 106 (range: 60-212 × 106; P = .004; Figure 2B). Average numbers of DNTs in spleen from treated mice was 36 × 106 cells (range: 14-61 × 106), and average number in control animals was 185 × 106 cells (range: 75-353 × 106; P = .007; Figure 2B).
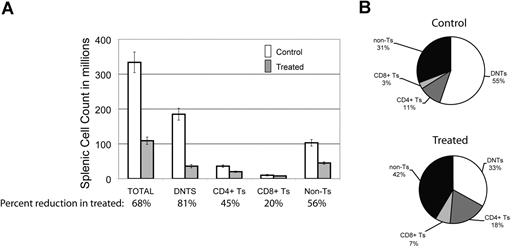
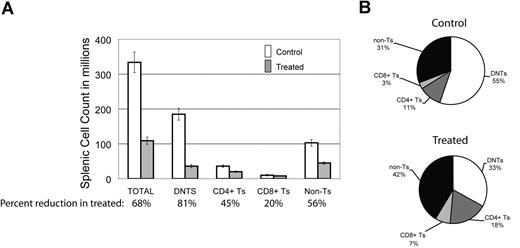
To look for the relative impact of GSI on various peripheral lymphocyte populations, we assessed the effect of DAPT on different splenocyte populations in the mice. We found there was a 68% decrease in the average number of total splenocytes comparing treated animals to control (Figure 3A). Each cell type was reduced in absolute number in the spleens of treated animals: 45% reduction in CD3+/CD4+ T cells, 20% reduction in CD3+/CD8+ T cells, and a 56% reduction in non–T cells (CD3−) cells. This reduction was most marked in DNTs, where we observed an 81% reduction (Figure 3A). In terms of percentage of each cell type in mouse spleens, we found a decreased percentage of DNTs in treated animals but increased percentages of the other cell fractions (Figure 3B). We found similar results in 3 treated and 2 control MRL-lpr mice (data not shown). These results suggest that Notch inhibition decreases DNTs, a surrogate marker of disease in murine ALPS, and that the DNT population is more sensitive to Notch inhibition than other lymphocyte populations in the mice.
DNTs are more sensitive to Notch inhibition than other cell populations. The effect of DAPT was assessed on different cell populations in mouse spleens at sacrifice. (A) Absolute number of cells in different lymphocyte populations (absolute cell count in spleen × % of lymphocyte subset by flow cytometry), comparing 5 treated to 7 control animals. Percentages listed on X-axis represent mean percentage reduction of cells in treated animals compared with control. Error bars represent SEM. There was a 68% decrease in the average number of total cells comparing treated to control animals. A profound decrease (81%, P = .007) was found in the DNT cell compartment. A less significant decrease was found in CD4+ (45%), CD8+ (20%), and non–T-cell (CD3−) (59%) lymphocytes. (B) Relative percentage of each lymphocyte population in mouse spleens comparing treated to control animals.
DNTs are more sensitive to Notch inhibition than other cell populations. The effect of DAPT was assessed on different cell populations in mouse spleens at sacrifice. (A) Absolute number of cells in different lymphocyte populations (absolute cell count in spleen × % of lymphocyte subset by flow cytometry), comparing 5 treated to 7 control animals. Percentages listed on X-axis represent mean percentage reduction of cells in treated animals compared with control. Error bars represent SEM. There was a 68% decrease in the average number of total cells comparing treated to control animals. A profound decrease (81%, P = .007) was found in the DNT cell compartment. A less significant decrease was found in CD4+ (45%), CD8+ (20%), and non–T-cell (CD3−) (59%) lymphocytes. (B) Relative percentage of each lymphocyte population in mouse spleens comparing treated to control animals.
Notch Inhibition decreases autoimmunity in MRL-lpr mice
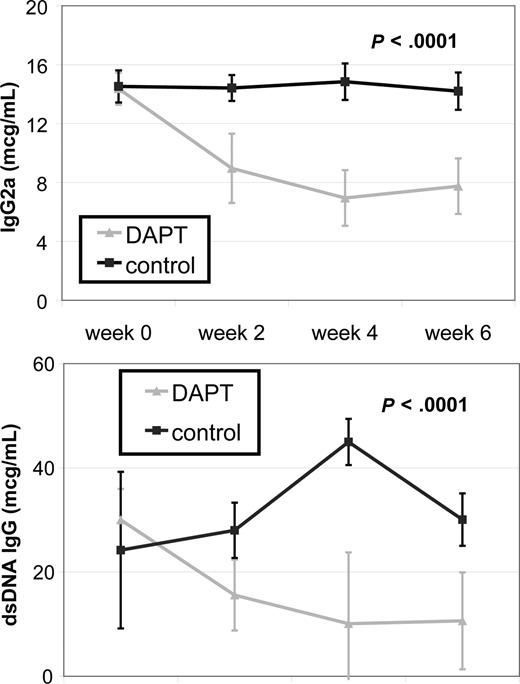
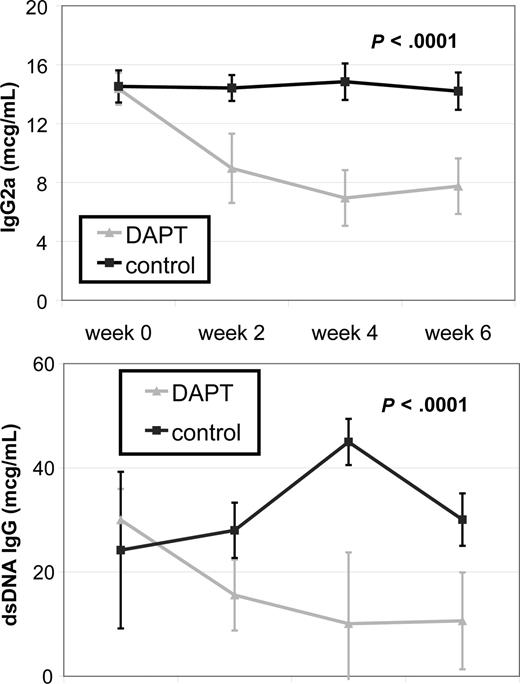
Systemic autoimmunity causes some of the most challenging to treat and potentially life-threatening disease manifestations in patients with ALPS and SLE. To determine the effect of Notch inhibition on autoimmunity, we treated autoimmunity-prone MRL-lpr mice with DAPT and measured 2 known biomarkers of autoimmunity, total IgG2a and anti-dsDNA, by quantitative ELISA. We compared antibody titers from 4 treated and 4 control mice. For both antibodies, there was no statistical difference in average titers of treatment groups at study initiation of treatment. Mice treated with DAPT had a statistically significant decrease in average antibody levels compared with control for both biomarkers (P < .001) as determined by ANOVA (Figure 4). These results demonstrate inhibiting the Notch pathway effectively reduces the autoimmune markers in these mice.
Notch inhibition decreases autoantibody production. MRL-lpr mice were randomized to treatment with DAPT or vehicle (4 treated, 4 control). Retro-orbital bleeds were obtained every 2 weeks to measure mouse total IgG (anti-IgG2a) and mouse anti-dsDNA IgG specific antibodies in sera by quantitative ELISA. Mice treated with DAPT had a statistically significant decrease in average titer levels compared with control for both antibodies (P < .001) as determined by ANOVA. Error bars represent SD.
Notch inhibition decreases autoantibody production. MRL-lpr mice were randomized to treatment with DAPT or vehicle (4 treated, 4 control). Retro-orbital bleeds were obtained every 2 weeks to measure mouse total IgG (anti-IgG2a) and mouse anti-dsDNA IgG specific antibodies in sera by quantitative ELISA. Mice treated with DAPT had a statistically significant decrease in average titer levels compared with control for both antibodies (P < .001) as determined by ANOVA. Error bars represent SD.
Notch inhibition improves nephritis in MRL-lpr mice
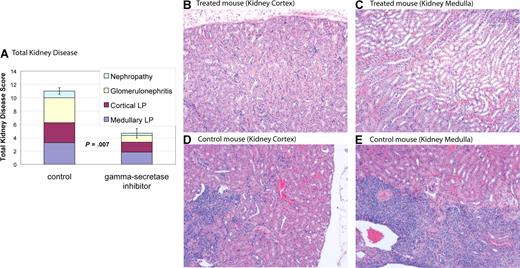
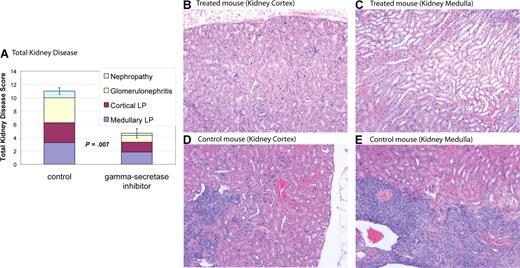
MRL-lpr mice develop nephritis that is similar to humans with SLE.28 MRL-lpr mice were treated with DAPT or vehicle control. Kidneys from 6 treated mice and 4 control mice were used for comparisons. Eight weeks after initiation of treatment mice were killed, and kidney disease was assessed by a pathologist blinded to treatment group. Kidney disease was scored by a nephritis index, including measurement of 4 disease parameters (see “Nephritis”). Average indices for treated mice demonstrated a reduction in all 4 disease parameters compared with control and a statistically significant reduction in total kidney disease (P = .007) (Figure 5 and Table 1). Four of 6 treated mice had marked improvement in kidney disease (nephritis index < 50% of index of control animal with least disease), one of 6 treated mice had modest improvement in kidney disease (nephritis index 30% lower than control animal with least disease), and one of the 6 treated mice had no improvement, with an index similar to control animals. These results demonstrate Notch inhibition is effective at improving kidney disease in murine SLE.
Gamma-secretase inhibitors improve nephropathy. MRL-lpr mice were randomized to treatment with DAPT or vehicle. Eight weeks after starting therapy, mice were killed and kidneys were assessed for disease. Kidney disease burden was scored 0 to 5 on 4 measures: (1) nephropathy; (2) glomerulonephritis; (3) cortical lymphoid proliferation (LP); and (4) medullary lymphoid proliferation (LP). Higher scores represent more severe disease. (A) Average total cumulative score for 4 control and 6 treated animals. Colored portions of the bars depict the individual disease measures. Treated mice demonstrated a reduction in all 4 disease parameters compared with control and a statistically significant reduction in total kidney disease (P = .007). Error bars depict SD of total kidney disease score. (B-E) Kidney pathology by H&E stain. Treated animals have healthy kidneys with limited disease (B,C). In contrast, control animals have pronounced lymphocytic infiltration in cortex (D) and medulla (E). In addition, control animals have damaged glomeruli and proteinaceous material in renal tubules. Images were captured using a Zeiss Axiovert 40C light microscope (Carl Zeiss, Thornwood, NJ) equipped with an apochromatic 10×/0.25 NA objective lens and a Nikon 995 camera (Nikon, Melville, NY).
Gamma-secretase inhibitors improve nephropathy. MRL-lpr mice were randomized to treatment with DAPT or vehicle. Eight weeks after starting therapy, mice were killed and kidneys were assessed for disease. Kidney disease burden was scored 0 to 5 on 4 measures: (1) nephropathy; (2) glomerulonephritis; (3) cortical lymphoid proliferation (LP); and (4) medullary lymphoid proliferation (LP). Higher scores represent more severe disease. (A) Average total cumulative score for 4 control and 6 treated animals. Colored portions of the bars depict the individual disease measures. Treated mice demonstrated a reduction in all 4 disease parameters compared with control and a statistically significant reduction in total kidney disease (P = .007). Error bars depict SD of total kidney disease score. (B-E) Kidney pathology by H&E stain. Treated animals have healthy kidneys with limited disease (B,C). In contrast, control animals have pronounced lymphocytic infiltration in cortex (D) and medulla (E). In addition, control animals have damaged glomeruli and proteinaceous material in renal tubules. Images were captured using a Zeiss Axiovert 40C light microscope (Carl Zeiss, Thornwood, NJ) equipped with an apochromatic 10×/0.25 NA objective lens and a Nikon 995 camera (Nikon, Melville, NY).
DAPT down-regulates Notch pathway murine CBA-lprcg and MRL-lpr mice
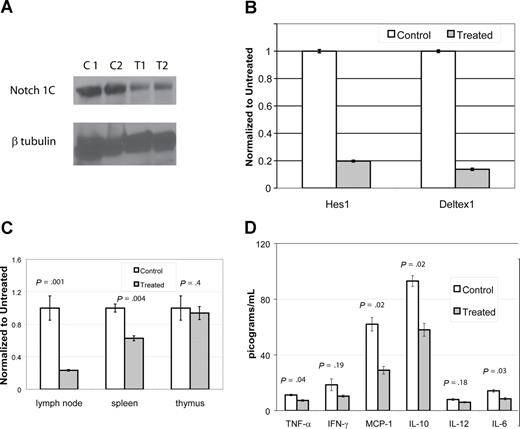
To assess if DAPT was blocking gamma-secretase and to demonstrate reduction in the activation of a relevant Notch receptor (Notch1), we performed immunoblots using an antibody specific for the second cleavage site of ICN. We compared lymph node cells from mice treated with DAPT for 96 hours at 5 mg/kg per day to cells from control animals. We performed immunoblots on cells from 4 CBA-lprcg mice (2 treatment, 2 control) and 10 MRL-lpr mice (6 treatment, 4 control). DAPT significantly reduced activated intracellular Notch1 (ICN1) levels in the treated mice compared with controls (Figure 6A and data not shown). In the CBA-lprcg mice, ICN1 was decreased 65% to 70% compared with control animals (treated average intensity/mm2: 4.2 [range: 3.4-5]; control average: 11 [range: 10.9-11]; P = .04). In the MRL-lpr mice, ICN1 was decreased 30% to 65% compared with control animals in 5 of the 6 treated animals tested (treated average intensity/mm2: 2.5 [range: 1.6-3.2]; control average: 4.6 [range: 4.2-4.9]; P = .01). One treated MRL-lpr mouse did not have reduction in ICN after 96 hours of treatment.
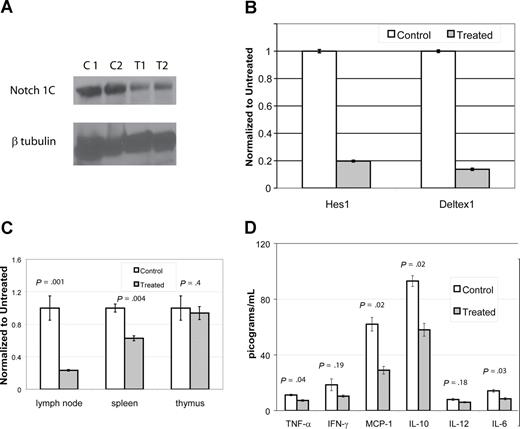
DAPT decreases activated Notch protein, transcription of downstream effectors, T cell proliferation, and cytokines. (A,B) Lymph node cells were harvested from mice after 4 days of treatment with DAPT or control. Panel A depicts immunoblot of activated Notch protein (Notch 1C) showing significantly decreased activated protein in 2 representative treated animals compared with 2 controls. In addition, RNA was isolated to assess down-stream targets of Notch1C activated transcription, including Hes1 and Deltex1. (B-tubulin was used as internal control.) Panel B depicts RT-PCR of Hes1 and Deltex1, comparing a representative control and treated animal. Error bars represent SD. (C,D) CBA-lprcg mice were treated with DAPT or control for 3 months (5 treated; 3 control). At killing, lymph node cells, splenocytes, and thymocytes were harvested, supported in vitro, and stimulated with mitogen. After 24 hours, supernatants were collected and cytokines assessed by cytometric bead array. After 48 hours, proliferation was measured by MTT. Panel C depicts proliferation, comparing treated to control. Bars represent mean of the treated/control (untreated) cells (relative absorbance of triplicate cultures, as described in “T-cell functional assays”); error bars represent SD. Panel D depicts results of cytokine bead array, demonstrating elevated levels of IL-10 and MCP-1 and a marked and statistically significant decrease in treated animal. Error bars depict SEM. Similar results were found with MRL mice.
DAPT decreases activated Notch protein, transcription of downstream effectors, T cell proliferation, and cytokines. (A,B) Lymph node cells were harvested from mice after 4 days of treatment with DAPT or control. Panel A depicts immunoblot of activated Notch protein (Notch 1C) showing significantly decreased activated protein in 2 representative treated animals compared with 2 controls. In addition, RNA was isolated to assess down-stream targets of Notch1C activated transcription, including Hes1 and Deltex1. (B-tubulin was used as internal control.) Panel B depicts RT-PCR of Hes1 and Deltex1, comparing a representative control and treated animal. Error bars represent SD. (C,D) CBA-lprcg mice were treated with DAPT or control for 3 months (5 treated; 3 control). At killing, lymph node cells, splenocytes, and thymocytes were harvested, supported in vitro, and stimulated with mitogen. After 24 hours, supernatants were collected and cytokines assessed by cytometric bead array. After 48 hours, proliferation was measured by MTT. Panel C depicts proliferation, comparing treated to control. Bars represent mean of the treated/control (untreated) cells (relative absorbance of triplicate cultures, as described in “T-cell functional assays”); error bars represent SD. Panel D depicts results of cytokine bead array, demonstrating elevated levels of IL-10 and MCP-1 and a marked and statistically significant decrease in treated animal. Error bars depict SEM. Similar results were found with MRL mice.
To determine whether DAPT resulted in decreased transcriptional activation by activated Notch receptors, we performed quantitative, real time PCR to detect transcription of downstream targets of Notch signaling. We assessed transcript levels of 2 downstream targets, Hes1 and Deltex1,13 using lymph node cells obtained from 2 treated and 2 control from each mouse strain. These mice were treated with DAPT for 96 hours at 5mg/kg per day. We found more than a 70% decrease in both Notch-activated transcripts in treated mice compared with control (Figure 6B). We quantitated VEGF (vascular endothelial growth factor) RNA transcripts as a control for a gene not involved in Notch1C activated transcription and found it was not decreased in gamma-secretase treated animals (data not shown).
We performed similar experiments to assess the Notch pathway, comparing thymocytes and splenocytes in treated and untreated animals. Unlike the lymph node cells, we did not find reduction in ICN1 by immunoblot or Notch activated transcripts by RT-PCR in thymocytes (data not shown). It was difficult to separate mouse thymuses from neck lymph nodes and there was potentially a small amount of contamination between these 2 structures. We found expression of ICN1 and levels of Notch activated transcripts from mouse splenocytes to be very heterogeneous. We found marked differences in ICN1 and RT-PCR transcripts between untreated animals, making it impossible to perform meaningful comparisons with treated animals. This is probably reflective of the differences in expression of Notch in different cell types and the marked heterogeneity of cells in murine spleens.
Notch inhibition inhibits T-cell proliferation and reduces IL-10 and MCP-1
To determine whether DAPT treatment had any effects on T-cell function, we (1) assessed T-cell proliferation from cells harvested from lymph nodes, spleens, and thymuses from treated and control animals after mitogen stimulation (2); assessed cytokine production from stimulated T cells (3); assessed T-cell activation by measuring CD44 expression; and (4) determined if there is any change in the underlying apoptotic defect after DAPT-treatment. We treated CBA-lprcg mice for 3 months (5 treated, 3 control) and MRL-lpr mice for 8 weeks (2 treated, 2 control).
We found a substantial decrease in the proliferative capacity of lymph node cells and splenocytes harvested from treated mice as compared with control, but no change in the proliferative capacity of thymocytes (Figure 6C). The supernatants from stimulated wells from lymph node cells were assessed for cytokine production. We found elevated levels of IL-10 and MCP-1 in control cells and a marked and statistically significant decrease of both cytokines in treated cells (Figure 6D). We found a statistically significant decreased expression of TNF-alpha and IL-6, comparing treated to control cells, however the baseline levels in control animals was at the lower limit of the sensitivity for the assay, questioning the biologic significance.
As described earlier, we found the total number of CD4+ T cells was decreased in lymph nodes and spleens in treated animals compared with controls. We found CD44+ was universally expressed in these cells (ie, > 95% of CD4+ cells were also CD44+) and the percentage CD44+ was not different, comparing treated to control. Thus, DAPT decreased the absolute number of activated CD4+/CD44+ cells, but did not change the percentage of CD4+/CD44+ cells relative to CD4+/CD44− cells (data not shown). We assessed if DAPT reversed the defect in the Fas apoptotic pathway in lymphocytes. Normally, lymphocytes harvested from both mouse strains do not undergo normal apoptosis when exposed to anti-Fas IgM monoclonal antibody. We found DAPT did not alter this defect (data not shown).
DAPT is well tolerated in CBA-lprcg and MRL-lpr mice
No toxicities were noted in mice treated with 5 mg/kg DAPT on a daily schedule of 5 days per week except for neutropenia after prolonged therapy. Mice did not lose weight or develop diarrhea. Published toxicities for gamma-secretase inhibitors include thymic atrophy and goblet cell hyperplasia with deterioration of intestinal epithelium.24 There was no pathologic evidence of thymic atrophy using our dosing regimen by gross assessment, blinded histopathology, total thymic cell count, or cell distribution by flow cytometry (data not shown). One third of DAPT-treated mice had a subtle, visually appreciable but not statistically significant increase in goblet cell numbers compared with control animals on H&E stain as assessed by a blinded pathologist (data not shown).
Mice had relative sparing of normal T cells (CD3+/CD4+ and CD3+/CD8+) in peripheral blood. As with DNTs, marked heterogeneity was observed in CD3+/CD4+ and CD3+/CD8+ cells at initiation of treatment. To make comparisons, absolute CD4+ counts (defined as WBC × % CD3+/CD4+/CD8−/TCRαβ+cells) and absolute CD8+ counts (defined as WBC × % CD3+/CD4−/CD8+/TCRαβ+ cells) at sacrifice were normalized to the untreated baseline of the individual mouse. There was no statistically significant change in average absolute CD3+/CD4+ (P = .66) and CD3+/CD8+ (P = .92) T cells in mice treated with gamma-secretase inhibitors. There was a reduction in the total absolute lymphocyte count (ALC) in treated mice, however, this reduction was due entirely to decreases in numbers of DNTs (data not shown).
No mouse had a decrease in hemoglobin when treated with DAPT (average hemoglobin at initiation of treatment: 98 g/L [range 69-122 g/L]; average after 3 months of treatment: 117 g/L [range 104-134 g/L]). Mice that were anemic at initiation of gamma-secretase inhibition had improvement of anemia. Twenty-eight percent of treated mice did develop a modest reduction in platelet count after chronic treatment with DAPT, but no mouse developed thrombocytopenia and 42% of control mice developed a similar reduction in platelet count (average platelet count at initiation or DAPT treatment, 736 × 109/L [range 400-1143 × 109/L]; average after 3 months of treatment: 875 × 109/L[range 369-1844 × 109/L]). Mice treated with DAPT did develop neutropenia after prolonged treatment (average absolute neutrophil count [ANC] at initiation of treatment: 4.285 × 109/L [range 0.507-9.878 × 109/L]; average after 3 months of treatment: 1.353 × 109/L [range 0.201-4.126 × 109/L]). Neutropenia has not previously been reported as a side effect of gamma-secretase inhibition. No mouse developed an ANC less than 0.500 × 109/L before 3 months of treatment. Fifty percent of treated animals developed an ANC less than 0.500 × 109/L after 3 months of treatment, but no mouse developed an ANC less than 0.200 × 109/L .
Discussion
Notch signaling plays multiple roles in hematopoietic and lymphoid development. Notch signaling is required for early T-lineage commitment, DNT transition, and TCR-mediated T-cell proliferation and activation.35,–37 Inhibitors of Notch signaling are gaining clinical interest because alterations in the Notch signaling pathway have been shown to be involved in a number of diseases, including T-cell acute lymphoblastic leukemia.20,38 Because Notch signaling is involved in T lineage commitment and T-cell activation, we hypothesized that inhibiting this pathway may be an effective treatment for T cell mediated autoimmune and lymphoproliferative diseases. We chose to investigate Notch inhibition in mouse models of 2 human diseases, ALPS and SLE. We found that inhibiting the Notch pathway with the GSI DAPT was effective in both.
Previously, no link between Notch signaling and SLE or ALPS has been reported. Here we found that gamma secretase inhibition was effective in reducing lymphadenopathy, splenomegaly, and autoantibodies in all mice tested. We found DAPT was effective in treating kidney disease in the majority of mice tested. We have also observed that SLE mice treated with GSI did not develop vasculitic skin lesions (data not shown), however this study was not powered to test this variable manifestation.
Our findings have important biologic implications. We found that GSIs had the most pronounced effect on DNTs among the lymphocyte populations assessed in these mice. Normal DNTs have an absolute requirement for Notch signaling and may not survive under Notch inhibition.36 This raises the possibility that elevated DNTs in SLE patients and ALPS patients may drive and upregulate abnormal B cell activity and its subsequent autoimmunity, and thus may not merely be an epiphenomenon of the disease.39,40 Future studies will focus on whether the improvement in B-cell symptoms in the mice were caused by a direct effect of Notch inhibition on B lymphocytes or as an indirect effect of targeting the DNTs.
There are differences in the DNT elevations, comparing ALPS and SLE patients. Patients with SLE do not develop the profound lymphoproliferation found in ALPS and have a mixture of elevated numbers of both α-β and γ-δ DNTs.41 Patients with ALPS have a virtually pathognomonic elevation of alpha/beta DNTs in the range of several thousands.42 Our finding that the activity of GSI is most pronounced in the DNT compartment may be more clinically meaningful for ALPS patients compared with SLE patients. Future work will evaluate the activity of GSIs in other SLE mouse models, including NZB/W to ensure our findings are relevant to SLE patients.
The origin of peripheral blood DNTs is debated, but the a recent study has suggested they may be a subset of thymic-derived regulatory T (Treg) cells.43 Another proposed theory is that the DNTs are CD8+ T cells that have lost CD8 expression, however, new evidence suggests that may not be the case.44,45 The marked difference in the sensitivity between DNTs and CD8+ T cells to Notch inhibition that we observed in this study does not support the “CD8 loss” hypothesis. Future studies will focus on the effects of low-dose gamma-secretase treatment on specific T-cell subsets.
Our findings have significant potential clinical implications. Treatment for patients with autoimmune diseases can be challenging. Most of the agents used to treat autoimmune diseases are nonspecific immunosuppressants, frequently with high side-effect profiles and, in many cases, limited efficacy. Gamma-secretase inhibitors may offer a more targeted therapeutic option for some autoimmune diseases.
Patients with ALPS can develop a myriad of clinical manifestations that resolve with age in some patients. Autoimmune cytopenias are the clinical manifestation most commonly requiring medical intervention. Occasionally, patients develop lymphoproliferation that compresses end-organs, requiring treatment. Many patients with ALPS who require treatment respond to short courses of immunosuppressive medications, however, some patients are refractory to these therapies and others, who may respond, do not tolerate the side effects of chronic treatment. Targeted medications with tolerable side effect profiles would be beneficial. We have shown gamma-secretase inhibitors were effective in reducing lymphoproliferation and controlling autoantibodies in a murine model of ALPS. Studies in children with ALPS have demonstrated a correlation between a medication-induced decrease in DNTs and improvement in autoimmune manifestations.46,47 We have demonstrated gamma-secretase inhibitors were effective in reducing DNTs in the mice. Circulating levels of IL-10 have been shown to be markedly elevated in the majority of patients with ALPS, and this elevation has been hypothesized to drive the Th-2 response and autoimmune manifestations in ALPS.9 We have demonstrated gamma-secretase inhibitors were effective in reducing production of IL-10 by T lymphocytes. The autoimmunity in human ALPS has differences from the murine models. For example, murine ALPS (unlike human ALPS) is, in part, an immune complex based disease. Nevertheless, collectively these results suggest this class of agents may have activity in human ALPS.
The clinical phenotype of SLE patients is variable and almost any organ system can be affected. Treatments are usually directed at reducing autoimmunity and/or inflammation. We have shown gamma-secretase inhibitors were effective in reducing glomerulonephritis and autoimmune antibodies, including dsDNA in MRL-lpr mice. Studies in SLE patients have shown that a reduction in dsDNA correlates with disease improvement, as dsDNA has been shown to be pathogenic.48,49 Monocyte chemoattractant protein-1 (MCP-1) has been implicated as a driving chemokine in many inflammatory and autoimmune diseases.50 MCP-1 has been shown to be responsible for the autoimmune manifestations and drive the Th-1 response in murine SLE.51,52 Antagonizing MCP-1 has been shown to improve disease in MRL-lpr mice.53 We have shown that gamma-secretase inhibitors were effective in reducing MCP-1 production by lymphocytes. Additional murine models of SLE exist, including NZB/W, which may be potentially more clinically relevant. Efficacy and toxicity in these models needs to be established prior to testing of gamma-secretase inhibitors in human SLE.
Using our intermittent low-dose treatment approach, we found DAPT was well tolerated for months, and the reported side effects of gamma-secretase inhibitors were avoided. The only potential toxicity we noted was neutropenia, which has not been previously described. To date, Notch inhibition has only been tested in one other model of autoimmune disease, multiple sclerosis.54,55 Given the etiologic differences in ALPS, SLE, and multiple sclerosis, we demonstrate the feasibility and effectiveness for Notch inhibition of T-cell function as an approach for a range of T cell–mediated pathologic features.
Patients with a number of autoimmune disorders, including ALPS, are predisposed to develop malignancy.56 Targeting the notch signaling pathway is hypothesized to have efficacy in a number of malignancies, including T-cell leukemias and lymphomas.57 Gamma-secretase inhibitors may have the added benefit of reducing the incidence of secondary cancers in ALPS. We can test this hypothesis in future work using murine models as they have a predisposition to develop plasmacytomas.
Several GSIs have gone through clinical trials.58 Our study demonstrates that intermittent low-dose GSI can be effective and safe in animal models of SLE and ALPS. Before using a similar dosing schema in clinical trials, more extensive preclinical drug toxicity studies must be completed. Our finding of gross morphologic sparing of intestines and thymus, combined with the relative sparing of CD4+ and CD8+ T cells in the spleen, suggest the possibility that with a low dose GSI exposure on an intermittent schedule, some T-cell function may be spared. This implies that the anti-DNT effects may exceed the inhibition of normal T cells.
We anticipate the development of specific therapeutics for each of the Notch proteins. Based on its critical role in T-cell development and activation, we would hypothesize that Notch-1–specific inhibitors may hold the most promise for efficacy with the least potential for toxicity in autoimmune and lymphoproliferative diseases. Nevertheless, Notch-3 has also been shown to be involved in the regulation of peripheral T cells and Treg expansion.59,60 Notch-3 specific inhibitors could have promise in autoimmune diseases.
In summary, we have demonstrated that DAPT, in a novel intermittent low-dose schedule, is an effective approach in preclinical models of SLE and ALPS. These results suggest that inhibiting the Notch pathway may be effective in treating other autoimmune and lymphoproliferative conditions. Further preclinical studies are needed to confirm efficacy and establish safety of GSIs using this dosing schema. Potentially, the results of this work may provide the foundation for future clinical trials testing GSIs in autoimmune and lymphoproliferative diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kathleen E. Sullivan, MD, PhD, for valuable discussions, suggestions, and critical review of this manuscript. We also thank John M. Maris, MD, for use of the small animal ultrasound and Warren S. Pear, MD, PhD, for discussions and suggestions.
This work was supported by grants from the United States Immunodeficiency Network (USIDNET, N01-A1-30 070; D.T.T.), a Foerderer-Murray Award (D.T.T.), the Goldman Philanthropic Partnerships and the Rockefeller Brothers Fund (D.T.T.), and the Sanford Chair and Weinberg Fund (S.A.G.).
Authorship
Contribution: D.T.T. and S.A.G. designed the research and drafted the manuscript. P.Z.W, V.B, G.R. J.F., and J.K.C. also contributed to experimental design. D.T.T., A.E.F, M.B., Y.J.G, J.H, T.R., and C.S performed research. P.Z.W. contributed vital new reagents. D.T.T., S.A.G, P.Z.W, R.M.B., J.K.C, V.B, G.R., and J.F. analyzed and interpreted data. A.E.F, D.T.T, and C.S. performed statistical analysis. All authors were involved in critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David T. Teachey, MD, Divisions of Hematology and Oncology, Children's Hospital of Philadelphia, ARC 902, 3615 Civic Center Boulevard, Philadelphia, PA 19104; e-mail: teacheyd@email.chop.edu.