Collagen binding to glycoprotein VI (GPVI) induces signals critical for platelet activation in thrombosis. Both ligand-induced GPVI signaling through its coassociated Fc-receptor γ-chain (FcRγ) immunoreceptor tyrosine-activation motif (ITAM) and the calmodulin inhibitor, W7, dissociate calmodulin from GPVI and induce metalloproteinase-mediated GPVI ectodomain shedding. We investigated whether signaling by another ITAM-bearing receptor on platelets, FcγRIIa, also down-regulates GPVI expression. Agonists that signal through FcγRIIa, the mAbs VM58 or 14A2, potently induced GPVI shedding, inhibitable by the metalloproteinase inhibitor, GM6001. Unexpectedly, FcγRIIa also underwent rapid proteolysis in platelets treated with agonists for FcγRIIa (VM58/14A2) or GPVI/FcRγ (the snake toxin, convulxin), generating an approximate 30-kDa fragment. Immunoprecipitation/pull-down experiments showed that FcγRIIa also bound calmodulin and W7 induced FcγRIIa cleavage. However, unlike GPVI, the approximate 30-kDa FcγRIIa fragment remained platelet associated, and proteolysis was unaffected by GM6001 but was inhibited by a membrane-permeable calpain inhibitor, E64d; consistent with this, μ-calpain cleaved an FcγRIIa tail-fusion protein at 222Lys/223Ala and 230Gly/231Arg, upstream of the ITAM domain. These findings suggest simultaneous activation of distinct extracellular (metalloproteinase-mediated) and intracellular (calpain-mediated) proteolytic pathways irreversibly inactivating platelet GPVI/FcRγ and FcγRIIa, respectively. Activation of both pathways was observed with immunoglobulin from patients with heparin-induced thrombocytopenia (HIT), suggesting novel mechanisms for platelet dysfunction by FcγRIIa after immunologic insult.
Introduction
Platelets play a leading role in hemostasis and thrombotic diseases such as heart attack or stroke. At sites of blood vessel injury, platelets roll, adhere, and then firmly attach to exposed subendothelial matrix by membrane receptors. At high shear stress, thrombus formation is initiated primarily by glycoprotein VI (GPVI) that binds collagen and GPIb-IX-V that binds von Willebrand factor (VWF).1,–3 GPVI and GPIb-IX-V are physically and functionally co-associated on the platelet surface, forming a unique adhesion/signaling complex.4 Occupancy of these receptors triggers intracellular signaling, leading to secretion of secondary mediators of platelet activation, such as thromboxane A2 and ADP, and activation of platelet integrins, primarily αIIbβ3 (GPIIb-IIIa) that binds fibrinogen or VWF and mediates platelet aggregation. GPVI is a member of the immunoglobulin (Ig) receptor family with 2 extracellular Ig domains, a transmembrane domain and a cytoplasmic tail. It forms a noncovalent complex with the common Fc receptor γ-chain (FcRγ) dimer required for GPVI surface expression.3,5,6 In response to ligand binding to GPVI, an immunoreceptor tyrosine-activation motif (ITAM) within the cytoplasmic portion of FcRγ is phosphorylated by (GPVI-associated) Src family kinases, Fyn and Lyn, allowing assembly of Syk.7,8 Although one consequence of GPVI signaling is activation of αIIbβ3, GPVI ligand-induced signaling also results in dissociation of calmodulin from a positively charged membrane-proximal sequence within the cytoplasmic tail of the receptor and leads to metalloproteinase-mediated shedding of a soluble approximate 55-kDa GPVI ectodomain fragment.9,,–12 This implies that activation of extracellular proteolytic pathways is a key mechanism for regulating the surface expression and function of GPVI/FcRγ
A second ITAM-containing receptor on platelets is FcγRIIa (CD32), an approximate 40-kDa low-affinity IgG receptor, also expressed on monocytes, neutrophils, and macrophages.13,14 Like GPVI, FcγRIIa contains 2 extracellular Ig domains and signals using an ITAM-dependent pathway. However, unlike GPVI, the ITAM is contained within the cytoplasmic tail.15,16 FcγRIIa therefore resembles a hybrid of GPVI (extracellular region) and FcRγ (cytoplasmic domain). FcγRIIa on platelets is co-associated with GPIb-IX-V17 and can be activated by VWF, or after stimulation of G-protein–coupled receptors.18 FcγRIIa also mediates platelet activation in response to immune complexes and is implicated in platelet dysfunction seen in patients with heparin-induced thrombocytopenia (HIT).19 In this case, immune complexes made up of autoantibodies bound to platelet factor 4 (PF-4)/heparin complexes activate platelets by FcγRIIa, which in turn can lead to arterial occlusion, venous thromboembolism, and thrombocytopenia.20 Antiplatelet monoclonal antibodies, including VM58 against GPIV21 and 14A2 against CD151,22,23 can also induce platelet activation through the interaction of their Fc region with FcγRIIa; VM58- or 14A2-induced activation is blocked by the anti–FcγRIIa monoclonal antibody, IV.3.
In this study, we investigated whether ITAM-dependent signaling by FcγRIIa would, like GPVI/FcRγ signaling, also induce metalloproteinase-mediated ectodomain shedding of GPVI. Our analysis of the effects of FcγRIIa ligands, VM58 and 14A2, on GPVI cleavage showed that FcγRIIa also underwent rapid proteolysis when platelets were treated with agonists acting at either FcγRIIa (VM58 or 14A2) or GPVI/FcRγ (the snake toxin, convulxin). However, we found that FcγRIIa cleavage involved intracellular calpain-dependent deletion of the ITAM domain rather than ectodomain shedding. Together, these findings (1) identify dual extracellular (metalloproteinase-dependent) and intracellular (calpain-dependent) proteolytic pathways activated as a consequence of ITAM-dependent signaling, (2) provide a mechanism for irreversible inactivation of both GPVI/FcRγ and FcγRIIa on platelets, and (3) suggest potential novel mechanisms for dampening clinical sequelae associated with ITAM-dependent signaling in patients with HIT.
Methods
All experiments described in this study were carried out with the approval of Monash University Standing Committee on Ethics in Research Involving Humans (SCERH), and informed consent was obtained in accordance with the Declaration of Helsinki.
Heparin and thrombin were purchased from Sigma, (St Louis, MO). N-ethylmaleimide, μ-calpain, GM6001 (a broad-range hydroxamic acid-based metalloproteinase inhibitor), piceatannol, PP2, staurosporine, calmodulin inhibitor, W7 (N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide), and calpain inhibitor E64d (L-3-carboxy-trans-2,3-epoxypropionyl-L-leucylamido-(3-methyl) butane) were from Calbiochem (La Jolla, CA). Protease inhibitor cocktail, Complete, was from Roche Diagnostic (Mannheim, Germany). The GPVI agonist, convulxin,24 from the venom of the tropical rattlesnake Crotalus durissus terrificus was a gift from Dr Kenneth Clemetson (Berne, Switzerland). The synthetic peptides, GRGDSP, SFLLRN (thrombin receptor agonist peptide, TRAP), and leupeptin were from Auspep (Melbourne, Australia). VWF from human factor VIII concentrates and botrocetin from Bothrops jararaca were purified as previously described.25,26
Antibodies
Affinity-purified monoclonal antibodies (mAbs), WM23 against the extracellular sialomucin domain of GPIbα,27 14A2 against CD151,22,23 VM58 against GPIV (CD36),21,28 CRC54 against β3 integrin,29 and CRC64 Fab′ fragments against αIIbβ330 have been previously described. Anti–calmodulin mAb was from Upstate (New York, NY). The anti–GPVI mAb (hybridoma medium 6B-12)31 was used for immunoblotting at a dilution of 1:150 as previously described.9 A murine anti–GPVI mAb, 1G5, was raised and affinity-purified using a recombinant extracellular fragment of human GPVI; 1G5 recognized a single platelet protein of approximately 62 kDa in Western blots of platelet lysates. The anti–FcγRIIa mAb, IV.3,32 was purified from hybridoma medium on protein A-Sepharose (Amersham, Amersham, United Kingdom). Antiserum raised in rabbits against the human FcγRIIa extracellular domain and affinity-purified rabbit IgG against glycocalicin (the isolated ectodomain of GPIbα) have been previously described.33,34 Rabbit antiserum against the recombinant cytoplasmic tail of human FcγRIIa 212Arg-284Asn (a kind gift from Dr Bruce Wines, the Burnet Institute, Melbourne, Australia), and purified polyclonal IgG against recombinant GPVI cytoplasmic tail were raised and purified as previously described.33,35 Polyclonal anti–peptide antibodies were raised against synthetic peptides, CYSGHSL or CKIGQLFRKLIRERALG (containing an N-terminal Cys for conjugation), corresponding to cytoplasmic sequences of human GPIbα605-610 or GPV545-560, respectively, by published methods.36,37 Anti–peptide IgG was affinity-purified using the immunizing peptide coupled to a 1:1 mixture of Affigel 10/15 (BioRad, Hercules, CA).36,–38 Nonimmune mouse IgG was prepared from mouse serum essentially as described in “Purification of IgG from human serum” for human Ig. Horseradish peroxidase (HRP)–conjugated sheep anti–mouse or anti–rabbit antibodies were from Chemicon (Melbourne, Australia).
Purification of IgG from human serum
A 2-step method was used to purify IgG from serum.39 IgG from patients with HIT or control serum was fractionated by 0% to 40% ammonium sulfate precipitation, resuspended in 20mM KH2PO4, 50mM NaCl, pH 8.0, and dialyzed into the same buffer at 4°C. To remove albumin, dialyzed antibody was loaded onto a 10 × 1-cm column of DEAE-Affigel Blue (BioRad), and unbound fractions were pooled and dialyzed into TS buffer (0.01 M Tris-HCl, 0.15 M NaCl, pH 7.4). IgG concentration was calculated from the A280 and an extinction coefficient of 1.4 (mg/mL)−1cm−1.
FcγRIIa and calmodulin fusion proteins
cDNA encoding 212Arg-284Asn of the cytoplasmic sequence of human mature FcγRIIa14 was subcloned into pPROEX HTa plasmid (Life Technologies, Rockville, MD) using unique BamH1 and Xba1 restriction sites. An N-terminal hexaHis-tagged-FcγRIIa fusion protein was expressed in Escherichia coli DH5α MCR grown in LB medium containing 1mM isopropyl-β-D-thiogalactopyranoside for 4 hours at 37°C and purified with Ni-NTA-Superflow resin (Qiagen, Valencia, CA) according to the manufacturer's instructions. Eluted protein was dialyzed into 0.5 M Tris-HCl, 0.5 mM EDTA, pH 8.0. The His-tag was removed by treatment with recombinant tobacco etch virus (rTEV) protease (Life Technologies) in the presence of 1mM dithiothreitol for 5 hours at room temperature, followed by a second passage over Ni-NTA resin. Analysis by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining confirmed a single band. Calmodulin-glutathione S-transferase (GST) fusion protein and GST alone were prepared as previously described.9
Proteolysis of platelet receptors
Assays used for measuring shedding of GPVI from human platelets were performed essentially as previously described.9 Human washed platelets (5 × 108/mL) were resuspended in Tyrode buffer either alone or in the presence of metalloproteinase inhibitors, GM6001 (100 μM), or EDTA (10 mM), the αIIbβ3-blocking peptide GRGDSP, inhibitors of Src kinase (PP2; 10 μM), Syk (piceatannol, 30 μg/mL), or kinases in general (staurosporine, 10 μM), the calpain inhibitors E64d or leupeptin (10-100 μM), or the anti-FcγRIIa mAb, IV.3 (10 μg/mL) (all final concentrations). Samples were then treated with either convulxin (0.5 μg/mL), NEM (2 mM), W7 (150 μM), TRAP (10 μM), VWF/botrocetin (both 10 μg/mL), WM23 (2 μg/mL), 14A2 (2 μg/mL), VM58 (2 μg/mL), or human IgG (100 μg/mL) from normal or HIT patient serum. Heparin (0.5 U/mL) was added to platelet suspensions containing human IgG. Samples were incubated at room temperature for indicated times, then 10mM EDTA (final concentration) was added, and the platelets were separated from the supernatant by centrifugation. The pellet was lysed on ice for 30 minutes in TS buffer containing 1% (wt/vol) Triton X-100 and Complete protease inhibitor. Samples were analyzed by electrophoresis on SDS 5% to 20% polyacrylamide gels, and Western blotting with anti–GPVI mAb (6B-12), or anti–FcγRIIa tail antiserum. In some experiments, nitrocellulose membranes were also probed with 1 μg/mL anti–cytoplasmic tail peptide antibodies against GPVI, GPIbα, or GPV or with CRC54 against the β3 subunit of αIIbβ3. Blots were visualized using HRP-conjugated secondary antibodies and enhanced chemiluminescence (Amersham).
Flow cytometry
Levels of GPVI or FcγRIIa expressed on untreated or W7-treated (150 μM, final concentration) platelets, prepared as described in “Proteolysis of platelet receptors,” were assessed by flow cytometry. Platelets were fixed in TS buffer containing 2% (wt/vol) paraformaldehyde for 15 minutes at room temperature then pelleted and resuspended in 0.1 mL TS buffer containing 0.1% (wt/vol) BSA and 10 μg/mL IV.3, anti–GPVI mAb, 1G5, or nonimmune mouse IgG. Tubes were placed on ice for 30 minutes, centrifuged, and resuspended in 10 μg/mL FITC-conjugated anti–mouse IgG. After a further 30 minutes on ice, bound antibody was measured in a FACStar flow cytometer, and analysis was performed using CellQuest software (Becton Dickinson, San Jose, CA).
Association of calmodulin with the cytoplasmic tail of FcγRIIa
Platelet lysate (2 mg) prepared as described in “Proteolysis of platelet receptors” was mixed with 20 μg of GST alone or GST-calmodulin in a total of 0.4 mL. Aliquots (0.1 mL) of a 1:1 mixture of glutathione-Sepharose 4B were added, and samples were rocked for 2 hours at room temperature. Beads were pelleted and washed 3 times with 1.0-mL aliquots of 0.02 M Tris-HCl, 0.15 M NaCl, pH 7.4, and bound protein was solubilized in SDS-PAGE loading buffer and analyzed by Western blotting with anti–FcγRIIa tail antiserum. The association of calmodulin with FcγRIIa in platelet lysates was also measured by immunoprecipitation, using an antibody against the ectodomain of FcγRIIa and blotting with anti–calmodulin mAb, using previously described methods.4
Cleavage of recombinant FcγRIIa by calpain
Low calcium-requirement μ-calpain was dialyzed against 20 mM imidazole, 5 mM β-mercaptoethanol, 1 mM CaCl2, pH 7.0. Aliquots (10 μg) of recombinant FcγRIIa 212Arg-284Asn in 30 μL of the same buffer were mixed with 0.1 U of calpain for 4 to 24 hours at 37°C. Matrix-assisted laser desorption/ionization–time of flight (MALDI-TOF) analysis was performed with an Applied Biosystems (Foster City, CA) Voyager-DE STR BioSpectrometry Workstation operated in positive polarity and linear mode using 10 mg/mL α-cyano-4-hydroxycinnamic acid matrix (Agilent Technologies, Melbourne, Australia). Matrix (1 μL) was spotted on the target plate and air dried; control or calpain-treated samples (1 μL) diluted in acetonitrile/water (1:1) containing 0.1% (vol/vol) formic acid were spotted onto dried matrix and allowed to dry. Data were collected from 500 laser shots (337 nm nitrogen laser), and the signal was averaged and processed with Data Explorer software (version 4.6; Applied Biosystems). Assignment of amino acid sequences was performed using the Compute Mw/pI tool at Expasy (http://kr.expasy.org) and the database sequence of FcγRIIa (accession no. P12318).
Results
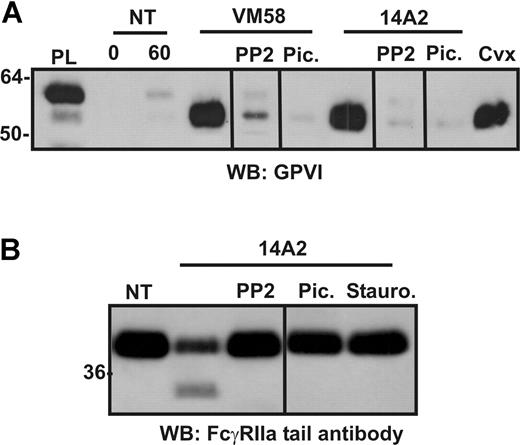
Activation of a second ITAM-containing receptor on platelets causes receptor cleavage
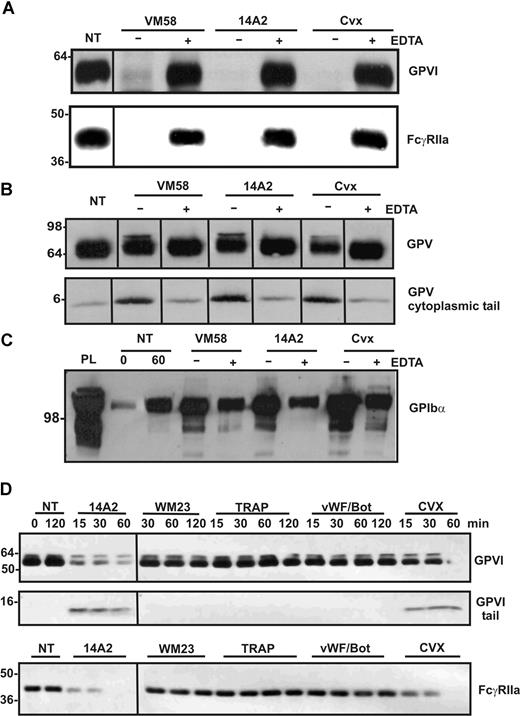
We have previously shown that ligation of GPVI/FcRγ and consequent ITAM-dependent signaling results in activation of metalloproteinase-dependent shedding of the GPVI ectodomain.9 Because FcγRIIa shares several structural and functional properties with GPVI/FcRγ, including the presence of an ITAM, we investigated whether ligation of FcγRIIa could also activate proteolytic pathways in platelets and induce shedding of GPVI. The mAbs, 14A2 against CD151 and VM58 against CD36, have both been previously shown to induce platelet aggregation through activation of FcγRIIa.23,28 We verified that washed platelets aggregated when mixed with 2 μg/mL of either 14A2 or VM58 and that this aggregation was blocked by pretreatment with either 3 μg/mL IV.3 (functional blocking antibody against FcγRIIa) or Fab′ fragments of an anti–αIIbβ3 antibody CRC64 (functional blocking antibody against the platelet aggregation receptor, αIIbβ3) (data not shown). Treatment of washed platelets for 1 hour with VM58 or 14A2 or the GPVI agonist, convulxin, resulted in the loss of GPVI from the platelet surface (Figure 1A) and concomitant release of an approximate 55-kDa fragment of GPVI into the supernatant (data not shown) as detected by Western blotting with the anti–GPVI ectodomain mAb, 6B-12. Unexpectedly, however, under each of these activation conditions using agonists to either GPVI or FcγRIIa, intact FcγRIIa was also completely lost from the platelets (Figure 1A) as determined by Western blotting with antiserum against the cytoplasmic tail of the receptor. This loss of both GPVI and FcγRIIa was blocked by EDTA. Loss of FcγRIIa was not simply due to nonselective degradation in activated platelets, because levels of GPV associated with the platelet pellet (Figure 1B) and GPIbα released into the supernatant (Figure 1C) showed only minor differences during this time frame in response to VM58, 14A2, or convulxin, as detected by blotting with antibodies against the cytoplasmic tail of GPV or glycocalicin (the ectodomain of GPIbα). The timecourse for cleavage induced by 14A2 or convulxin is shown in Figure 1D with the loss of full-length GPVI and FcγRIIa detected within 15 minutes and the appearance of a platelet-associated fragment of GPVI. In contrast, control IgG (WM23) or agonists that do not use ITAM-based signaling, thrombin receptor agonist peptide, or the GPIbα ligand VWF/botrocetin did not cause cleavage of GPVI or FcγRIIa after 2 hours of incubation (Figure 1D).
Ligands of ITAM-containing receptors, FcγRIIa or GPVI, induce shedding of these receptors from platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with FcγRIIa ligands, VM58 or 14A2 (2 μg/mL, final concentration), or with the GPVI ligand, convulxin (Cvx; 0.5 μg/mL, final concentration) for 1 hour, in the presence (+) or absence (−) of EDTA (10 mM, final concentration). Platelets were isolated by centrifugation and lysed in Triton X-100 buffer as described in “Proteolysis of platelet receptors.” Immunoblots of (A) full-length GPVI (top) and FcγRIIa (bottom) detected using anti-GPVI mAb (6B-12) or anti-FcγRIIa tail antiserum, respectively; (B) full-length and cleaved GPV in platelet lysates detected by using anti-GPV tail IgG; and (C) soluble GPIbα present in supernatant fractions detected by using anti-glycocalicin IgG. (D) Timecourse analysis of levels of platelet proteins in response to treatment by 14A2, WM23, TRAP, VWF/botrocetin, or convulxin, using antibodies directed against the cytoplasmic tail of GPVI and FcγRIIa. Blots were visualized using HRP-conjugated secondary antibodies and ECL. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Ligands of ITAM-containing receptors, FcγRIIa or GPVI, induce shedding of these receptors from platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with FcγRIIa ligands, VM58 or 14A2 (2 μg/mL, final concentration), or with the GPVI ligand, convulxin (Cvx; 0.5 μg/mL, final concentration) for 1 hour, in the presence (+) or absence (−) of EDTA (10 mM, final concentration). Platelets were isolated by centrifugation and lysed in Triton X-100 buffer as described in “Proteolysis of platelet receptors.” Immunoblots of (A) full-length GPVI (top) and FcγRIIa (bottom) detected using anti-GPVI mAb (6B-12) or anti-FcγRIIa tail antiserum, respectively; (B) full-length and cleaved GPV in platelet lysates detected by using anti-GPV tail IgG; and (C) soluble GPIbα present in supernatant fractions detected by using anti-glycocalicin IgG. (D) Timecourse analysis of levels of platelet proteins in response to treatment by 14A2, WM23, TRAP, VWF/botrocetin, or convulxin, using antibodies directed against the cytoplasmic tail of GPVI and FcγRIIa. Blots were visualized using HRP-conjugated secondary antibodies and ECL. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
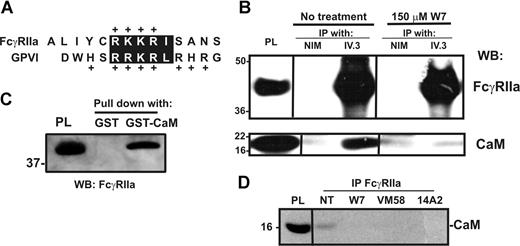
Calmodulin is associated with the cytoplasmic tail of FcγRIIa on platelets
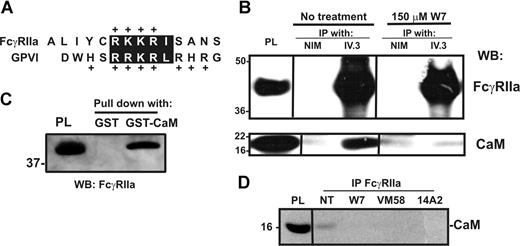
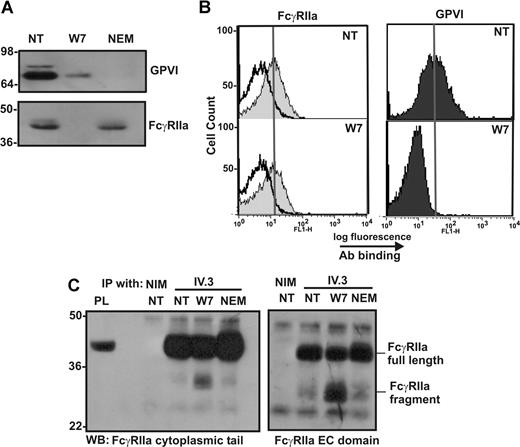
Calmodulin associates with a positively charged, membrane-proximal sequence within the cytoplasmic tail of GPVI in resting platelets.40,41 This sequence is analogous to calmodulin-binding sites in other receptors that also undergo ectodomain shedding42,43 ; the calmodulin inhibitor, W7, both disrupts calmodulin binding to GPVI and induces metalloproteinase-mediated shedding.9 Because human FcγRIIa contains a positively charged, membrane-proximal sequence analogous to that of GPVI (Figure 2A), we investigated whether calmodulin also bound to FcγRIIa, and whether the loss of intact FcγRIIa was also calmodulin regulated. First, GST-calmodulin, but not GST alone, specifically pulled-down FcγRIIa from platelet lysates (Figure 2B). This association was divalent cation dependent and inhibitable by EDTA (data not shown). Conversely, calmodulin was coimmunoprecipitated from platelet lysates by the anti–FcγRIIa mAb, IV.3, but not by nonimmune mouse IgG (Figure 2C top). Pretreatment of platelets with W7 or FcγRIIa ligands 14A2 or VM58 disrupted the association of calmodulin with FcγRIIa (Figure 2C bottom and Figure 2D). Second, compared with untreated platelets in which there was no observable proteolysis of GPVI or FcγRIIa, treating platelets with W7 not only resulted in the loss of full-length GPVI but also the loss of intact FcγRIIa (Figure 3A,B). W7-induced loss of intact FcγRIIa was associated with the appearance of an approximately 30-kDa digestion fragment of FcγRIIa that remained associated with the platelet pellet and was detected weakly by anti–FcγRIIa tail antiserum. Levels of the approximately 30-kDa fragment were variable between experiments for times up to 2 hours (not shown); the anti–FcγRIIa tail antiserum is presumably less sensitive at detecting cleaved fragment relative to intact FcγRIIa. W7-mediated loss of both FcγRIIa and GPVI was blocked by EDTA (Figure 3A,B lane 4).
Calmodulin associates with the cytoplasmic tail of FcγRIIa. (A) The cytoplasmic tail of human FcγRIIa contains a membrane-proximal, positively charged amino acid sequence analogous to the calmodulin-binding sequence in human GPVI. Identical residues or conservative substitutions are highlighted. (B) Pull-down from human platelet lysates with GST alone or GST-calmodulin (GST-CaM) in the presence of 1mM Ca2+. Proteins were captured with glutathione-Sepharose and immunoblotted by using an anti–FcγRIIa tail antiserum. Platelet lysate (PL) was run as a control lane. (C) Washed platelets were untreated or treated with W7 (150 μM, final concentration) for 1 hour in the presence of EDTA, then platelets were lysed and immunoprecipitated with nonimmune mouse (NIM) IgG or anti-FcγRIIa mAb, IV.3. Immunoprecipitates were captured on protein A/G-Sepharose and analyzed by Western blotting with anti–FcγRIIa tail antiserum (top panel) or anti–calmodulin mAb (bottom). (D) Levels of calmodulin associated with FcγRIIa after treatment of washed platelets with W7, 14A2, or VM58 were assessed by immunoprecipitation using a polyclonal antibody against the FcγRIIa extracellular domain, followed by Western blot with an anti–calmodulin antibody. All Western blots were visualized with HRP-conjugated secondary antibodies and ECL. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Calmodulin associates with the cytoplasmic tail of FcγRIIa. (A) The cytoplasmic tail of human FcγRIIa contains a membrane-proximal, positively charged amino acid sequence analogous to the calmodulin-binding sequence in human GPVI. Identical residues or conservative substitutions are highlighted. (B) Pull-down from human platelet lysates with GST alone or GST-calmodulin (GST-CaM) in the presence of 1mM Ca2+. Proteins were captured with glutathione-Sepharose and immunoblotted by using an anti–FcγRIIa tail antiserum. Platelet lysate (PL) was run as a control lane. (C) Washed platelets were untreated or treated with W7 (150 μM, final concentration) for 1 hour in the presence of EDTA, then platelets were lysed and immunoprecipitated with nonimmune mouse (NIM) IgG or anti-FcγRIIa mAb, IV.3. Immunoprecipitates were captured on protein A/G-Sepharose and analyzed by Western blotting with anti–FcγRIIa tail antiserum (top panel) or anti–calmodulin mAb (bottom). (D) Levels of calmodulin associated with FcγRIIa after treatment of washed platelets with W7, 14A2, or VM58 were assessed by immunoprecipitation using a polyclonal antibody against the FcγRIIa extracellular domain, followed by Western blot with an anti–calmodulin antibody. All Western blots were visualized with HRP-conjugated secondary antibodies and ECL. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
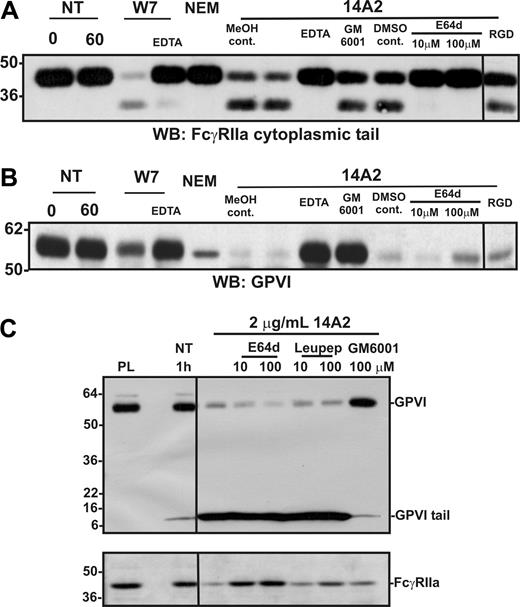
Ligand-mediated activation of FcγRIIa leads to activation of both a metalloproteinase and a calpain-like protease in platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with W7 (150 μM, final concentration), NEM (2 mM), or 14A2 (2 μg/mL) for 1 hour. Some samples also contained EDTA (10 mM), GM6001 (100 μM), E64d (10-100 μM), GRGDSP peptide (RGD; 1 mM), or methanol (MeOH) or DMSO vehicle controls. Levels of (A) GPVI and (B) FcγRIIa in platelet lysates were assessed by Western blotting with anti-GPVI (6B-12) or anti–FcγRIIa tail antiserum. Blots were visualized using HRP-conjugated secondary antibodies and ECL. (C) Platelets were treated with 14A2 in the presence of either membrane-permeable (E64d) or membrane-impermeable (leupeptin) inhibitors of calpain, or GM6001 (100 μM, final concentration). Levels of full-length FcγRIIa and full-length and cytoplasmic tail remnant of GPVI were assessed by Western blotting with anti–FcγRIIa tail antiserum or anti–GPVI tail IgG. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Ligand-mediated activation of FcγRIIa leads to activation of both a metalloproteinase and a calpain-like protease in platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with W7 (150 μM, final concentration), NEM (2 mM), or 14A2 (2 μg/mL) for 1 hour. Some samples also contained EDTA (10 mM), GM6001 (100 μM), E64d (10-100 μM), GRGDSP peptide (RGD; 1 mM), or methanol (MeOH) or DMSO vehicle controls. Levels of (A) GPVI and (B) FcγRIIa in platelet lysates were assessed by Western blotting with anti-GPVI (6B-12) or anti–FcγRIIa tail antiserum. Blots were visualized using HRP-conjugated secondary antibodies and ECL. (C) Platelets were treated with 14A2 in the presence of either membrane-permeable (E64d) or membrane-impermeable (leupeptin) inhibitors of calpain, or GM6001 (100 μM, final concentration). Levels of full-length FcγRIIa and full-length and cytoplasmic tail remnant of GPVI were assessed by Western blotting with anti–FcγRIIa tail antiserum or anti–GPVI tail IgG. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
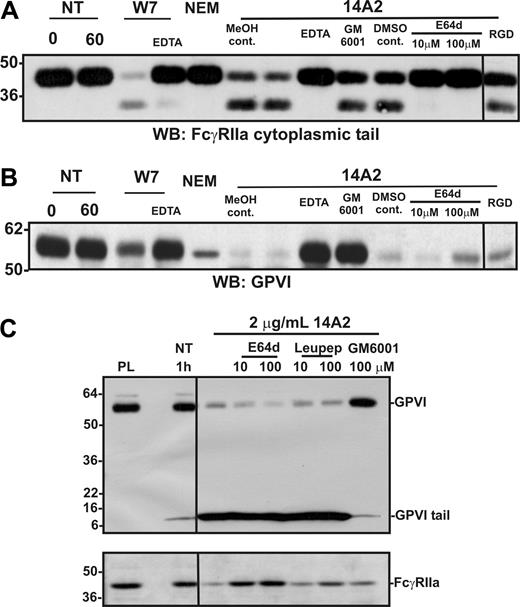
Proteolysis of FcγRIIa is independent of metalloproteinase activity
A possible role for metalloproteinases, in particular A Disintegrin And Metalloproteinase (ADAM) family sheddases, in the proteolysis of FcγRIIa was investigated in 2 ways. First, platelet suspensions were treated with the thiol-modifying reagent NEM, which causes metalloproteinase-dependent shedding of GPVI on platelets,4 presumably by activation of the extracellular cysteine switch commonly found in ADAM prodomains.44 NEM treatment, however, resulted in selective loss only of GPVI, whereas FcγRIIa remained intact under the same conditions for up to 1 hour (Figure 3A lane 5 cf 3B). This suggests that activation of platelet ADAMs caused cleavage of GPVI but not FcγRIIa. Second, the broad-spectrum metalloproteinase inhibitor, GM6001, only inhibited 14A2-induced cleavage of GPVI but not FcγRIIa (Figure 3A lane 9 cf 3B). Cleavage of both FcγRIIa and GPVI was inhibitable by EDTA (Figure 3A,B lane 8).
Role of calpain in proteolysis of FcγRIIa
The lack of inhibition of FcγRIIa proteolysis by GM6001 and the failure of NEM to induce cleavage (unlike NEM-induced shedding of GPVI) raised the possibility that FcγRIIa proteolysis was intracellular. In this regard, calpain has been implicated in intracellular cleavage of PECAM-1.45 To examine a possible role for intracellular calpain in the proteolysis of FcγRIIa, platelets were incubated with a membrane-permeable inhibitor of calpain, E64d. Under these conditions, 14A2-induced proteolysis of FcγRIIa was markedly reduced (Figure 3A lanes 11,12); however, E64d had no effect on cleavage of GPVI (Figure 3B lanes 11,12). In Figure 3C, an anti–GPVI tail antibody, which detects both full-length (∼ 62 kDa) and cleaved remnant (∼ 10 kDa) forms of platelet-associated GPVI, was used to probe lysates of platelets treated with 14A2; levels of FcγRIIa associated with the same platelet pellet were also evaluated by Western blot using the anti–FcγRIIa tail antiserum. Cleavage of FcγRIIa was inhibited by either 10 μM or 100 μM E64d but not by a membrane-impermeable calpain inhibitor, leupeptin. In contrast, 14A2-induced shedding of GPVI was not blocked by either calpain inhibitor (but was selectively blocked by GM6001). EDTA inhibited both metalloproteinase-mediated GPVI shedding and calpain-mediated FcγRIIa cleavage (Figure 3A,B). The explanation for the latter result was not further investigated, but it has been reported that activation of intracellular calpain-mediated cleavage events requires Ca2+ flux.46 Nonetheless, it is evident that divalent cation dependency is common to both ITAM-linked proteolytic pathways. Neither 14A2-induced cleavage of FcγRIIa nor GPVI was affected by an RGD-containing peptide, indicating αIIbβ3-mediated signaling was not regulating proteolysis of either receptor (Figure 3A,B last lane), although the cytoplasmic tail of the β3 subunit of αIIbβ3 is also a substrate for calpain.47 Taken together, the combined results suggest that intracellular signaling pathways activated by FcγRIIa ligation lead to activation of a platelet metalloproteinase that targets GPVI, as well as intracellular calpain (or calpain-like protease) that cleaves FcγRIIa.
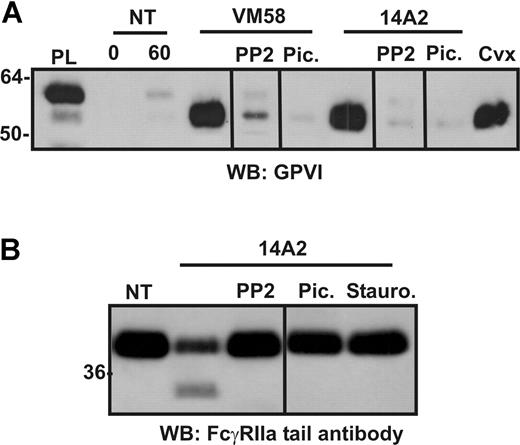
Proteolysis is a consequence of activation of ITAM-harboring receptors
We have previously shown that ligand-induced GPVI shedding depends on early ITAM-dependent signaling events. To characterize a role for ITAM-based signaling events triggered by FcγRIIa ligation in proteolysis of GPVI and FcγRIIa, washed platelets were treated with either 14A2 or VM58, or 0.5 μg/mL convulxin, in the presence of the Src family kinase inhibitor, PP2, the Syk kinase inhibitor, piceatannol, or the broad-spectrum kinase inhibitor, staurosporine. Similar to GPVI shedding induced by GPVI ligation,9 VM58- or 14A2-induced GPVI shedding (Figure 4A) or 14A2-induced cleavage of FcγRIIa on platelets (Figure 4B) was blocked by inhibitors of Src and Syk kinases, indicating a role for these intracellular tyrosine kinases in activation of FcγRIIa-mediated receptor proteolysis. Staurosporine also inhibited 14A2-dependent proteolysis under these conditions (Figure 4B).
Inhibition of ITAM-associated signaling molecules or cytoplasmic calpain blocks loss of FcγRIIa from platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with 2 μg/mL (final concentration) of either VM58 or 14A2, or 0.1 μg/mL convulxin (Cvx) for 1 hour. Some samples also contained PP2 (10μM), piceatannol (30 μg/mL), or staurosporine (10 μM) as indicated. Levels of (A) GPVI fragment in supernatants or (B) FcγRIIa in platelet lysates was assessed by SDS-PAGE and Western blotting using anti–GPVI mAb (6B-12) or anti–FcγRIIa tail antiserum, respectively. PL indicates platelet lysate, containing full-length GPVI for reference. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Inhibition of ITAM-associated signaling molecules or cytoplasmic calpain blocks loss of FcγRIIa from platelets. Washed human platelets were resuspended in Tyrode buffer and either not treated (NT) or treated with 2 μg/mL (final concentration) of either VM58 or 14A2, or 0.1 μg/mL convulxin (Cvx) for 1 hour. Some samples also contained PP2 (10μM), piceatannol (30 μg/mL), or staurosporine (10 μM) as indicated. Levels of (A) GPVI fragment in supernatants or (B) FcγRIIa in platelet lysates was assessed by SDS-PAGE and Western blotting using anti–GPVI mAb (6B-12) or anti–FcγRIIa tail antiserum, respectively. PL indicates platelet lysate, containing full-length GPVI for reference. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
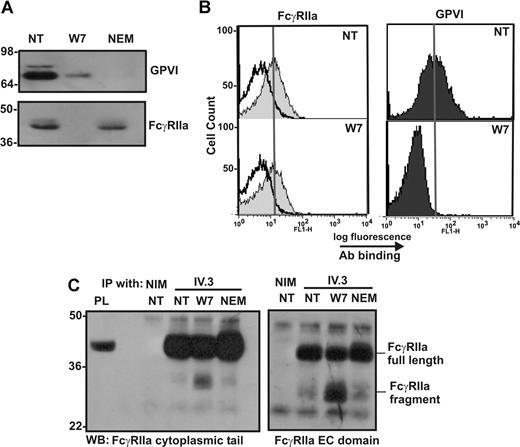
Extracellular portion of cleaved FcγRIIa remains on the platelet surface
To further characterize the nature of the agonist-dependent proteolysis of FcγRIIa, washed platelets were treated with 150 μM W7 or 10 mM NEM, and levels of FcγRIIa associated with platelets were assessed by Western blotting or flow cytometry. The levels of both GPVI and FcγRIIa are reduced in platelets treated with W7, as assessed by Western blotting with an anti–GPVI mAb or anti–FcγRIIa cytoplasmic tail antiserum (Figure 5A). When the same platelet samples were analyzed by flow cytometry using the anti–FcγRIIa mAb, IV.3, or anti-GPVI mAb, 1G5 (Figure 5B), the levels of FcγRIIa detectable on the platelet surface were essentially unchanged in nontreated and W7-treated platelets, suggesting that the extracellular IV.3 epitope remained intact. The maintenance of the IV.3 epitope in W7-treated platelets and intracellular cleavage was confirmed by immunoprecipitation of FcγRIIa from lysates of W7-treated platelets by IV.3 and detection by antisera raised against either the cytoplasmic tail or the extracellular region of FcγRIIa (Figure 5C). Both full-length FcγRIIa and the approximate 30-kDa fragment of FcγRIIa were detected by 2 different anti–FcγRIIa antisera in Western blots of immunoprecipitated proteins from lysates of W7-treated platelets, but only full-length FcγRIIa was detected in nontreated or NEM-treated platelets. These data indicate that the extracellular IV.3 epitope was intact in the platelet-associated approximate 30-kDa FcγRIIa fragment, and, based on the relative molecular weights of intact FcγRIIa (∼ 40 kDa) and the digestion fragment (∼ 30 kDa), that cleavage most probably occurs within the cytoplasmic tail of FcγRIIa.
The cleaved form of FcγRIIa is detectable by immunoprecipitation and Western blot but not by flow cytometry. Washed human platelets were left untreated (NT) or treated with (final concentrations) 150 μM W7 or 10 mM NEM for 2 hours. Platelets were fixed and (A) lysed then analyzed for levels of GPVI and FcγRIIa by Western blot, or (B) stained with nonimmune mouse IgG (empty histogram in FcγRIIa panel), anti–FcγRIIa antibody IV.3, or anti–GPVI mAb, 1G5, and secondary FITC-labeled antibodies were then analyzed by fluorescence-activated cell sorting (FACS). The vertical line in each histogram indicates the mean fluorescence intensity in untreated platelets stained with each antibody. (C) Non-treated (NT) platelets or platelets treated with 150 μM W7 or 10 mM NEM for 2 hours were pelleted and lysed then mixed with 10 μg of either nonimmune mouse IgG (NIM) or IV.3. Immune complexes were captured and assessed by SDS-PAGE and Western blotting with anti–FcγRIIa tail antiserum or anti–FcγRIIa extracellular (EC) domain IgG.
The cleaved form of FcγRIIa is detectable by immunoprecipitation and Western blot but not by flow cytometry. Washed human platelets were left untreated (NT) or treated with (final concentrations) 150 μM W7 or 10 mM NEM for 2 hours. Platelets were fixed and (A) lysed then analyzed for levels of GPVI and FcγRIIa by Western blot, or (B) stained with nonimmune mouse IgG (empty histogram in FcγRIIa panel), anti–FcγRIIa antibody IV.3, or anti–GPVI mAb, 1G5, and secondary FITC-labeled antibodies were then analyzed by fluorescence-activated cell sorting (FACS). The vertical line in each histogram indicates the mean fluorescence intensity in untreated platelets stained with each antibody. (C) Non-treated (NT) platelets or platelets treated with 150 μM W7 or 10 mM NEM for 2 hours were pelleted and lysed then mixed with 10 μg of either nonimmune mouse IgG (NIM) or IV.3. Immune complexes were captured and assessed by SDS-PAGE and Western blotting with anti–FcγRIIa tail antiserum or anti–FcγRIIa extracellular (EC) domain IgG.
Recombinant FcγRIIa cytoplasmic tail fragment is cleaved by calpain
A soluble fusion protein encoding the cytoplasmic tail sequence 212Arg-284Asn of FcγRIIa was analyzed by mass spectrometry (Table 1) and was found to have a molecular mass of 8204 Da. When recombinant FcγRIIa cytoplasmic tail (10 μg) was incubated at 37°C in the presence of purified calpain, the resultant digest analyzed by mass spectrometry (Table 1) showed new spectral peaks not found in samples of calpain alone or untreated 212Arg-284Asn fragment. At least 5 new peaks representing 5 fragments of FcγRIIa indicate 3 potential cleavage sites (Table 1). Two closely spaced calpain-cleavage sites at 222Lys/223Ala and 230Gly/231Arg were identifiable after 4 hours of calpain treatment, with these earliest cleavages consistent with the generation of the approximate 30-kDa fragment of FcγRIIa detected after 1 hour in VM58-, 14A2- or convulxin-treated platelets. Additional proteolysis occurred at longer times, including at 235Ala/236Ile after 24 hours. These data indicate that the FcγRIIa cytoplasmic tail is a novel substrate for calpain, and, importantly, that calpain activity at the most favorable sites (222Lys/223Ala and 230Gly/231Arg) would irreversibly disable FcγRIIa by liberating the ITAM-containing region from FcγRIIa.
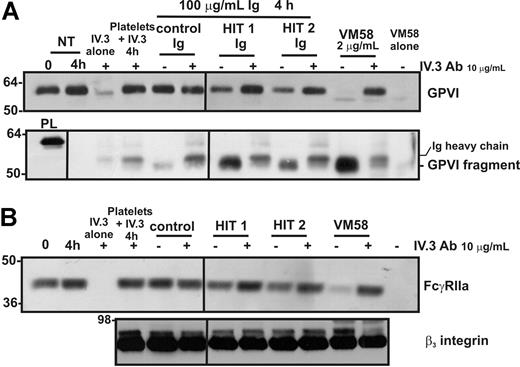
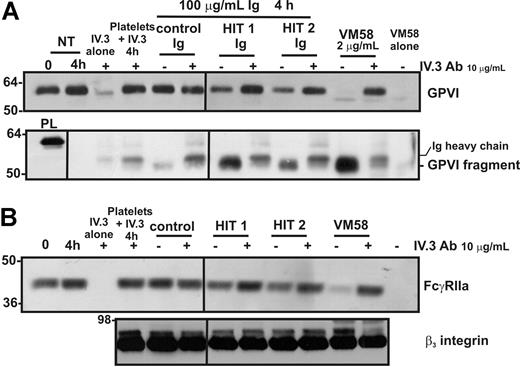
Purified Ig fraction from the sera of 2 patients with HIT induces FcγRIIa-dependent shedding of platelet GPVI and intracellular proteolysis of FcγRIIa
Patients with HIT have circulating autoantibodies directed against a complex of heparin and PF-4 (and/or other platelet-associated proteins); these immune complexes can activate platelets by ligation of FcγRIIa.19,20 To test whether proteolysis of platelet FcγRIIa and GPVI may be associated with platelet abnormalities in patients with HIT, normal platelets were incubated with isolated IgG from patients with HIT in the presence of heparin (required for heparin-dependent epitope expression). Levels of GPVI (Figure 6A) and FcγRIIa (Figure 6B) were reduced in platelets incubated with HIT patient IgG but not control IgG. The potency of HIT IgG (total IgG fraction) is relatively weak compared with affinity-purified mAb VM58 (although it should be noted that these results were obtained in the absence of added PF-4), but the same digestion products of both GPVI and FcγRIIa are observed. As a control, levels of the β3 integrin remained stable under all treatment conditions (Figure 6B). Levels of the approximate 55-kDa soluble GPVI fragment were also increased in the supernatants of platelets treated with IgG from patients with HIT (Figure 6A). The loss of both intact FcγRIIa and GPVI from the platelet surface and the appearance of the GPVI fragment in the supernatant were ablated by the FcγRIIa-blocking mAb, IV.3, suggesting that activation leading to GPVI and FcγRIIa proteolysis was mediated specifically by FcγRIIa.
Shedding of either GPVI or FcγRIIa induced by Ig from patients with HIT is blocked by an anti–Fc receptor antibody. Washed platelets were incubated with either VM58 or Ig from control or HIT patient serum. Some samples also contained the blocking anti–FcγRIIa mAb, IV.3 (10 μg/mL, final concentration). Levels of (A) GPVI on platelets (top) or GPVI fragment in supernatant (bottom) or (B) FcγRIIa (top) and β3 integrin (bottom) on platelets were assessed by Western blotting with anti–GPVI mAb (6B-12), anti–FcγRIIa tail antiserum, or the anti-β3 mAb (CRC54) as indicated. Equivalent amounts of VM58 and IV.3 alone or mixed with platelets in the absence of agonist, were run in parallel lanes to distinguish bands corresponding to Ig heavy chains. PL indicates platelet lysate, containing full-length GPVI for reference. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Shedding of either GPVI or FcγRIIa induced by Ig from patients with HIT is blocked by an anti–Fc receptor antibody. Washed platelets were incubated with either VM58 or Ig from control or HIT patient serum. Some samples also contained the blocking anti–FcγRIIa mAb, IV.3 (10 μg/mL, final concentration). Levels of (A) GPVI on platelets (top) or GPVI fragment in supernatant (bottom) or (B) FcγRIIa (top) and β3 integrin (bottom) on platelets were assessed by Western blotting with anti–GPVI mAb (6B-12), anti–FcγRIIa tail antiserum, or the anti-β3 mAb (CRC54) as indicated. Equivalent amounts of VM58 and IV.3 alone or mixed with platelets in the absence of agonist, were run in parallel lanes to distinguish bands corresponding to Ig heavy chains. PL indicates platelet lysate, containing full-length GPVI for reference. Vertical lines have been inserted to indicate a repositioned gel lane. All lanes within each figure came from the same experiment and the same gel/Western blot.
Discussion
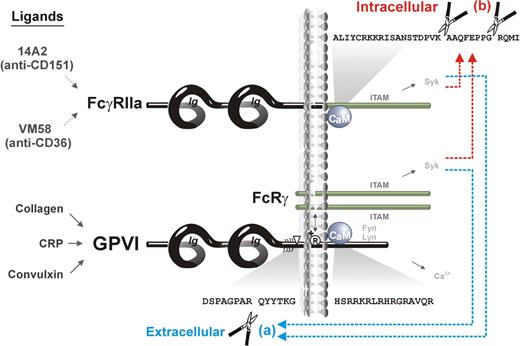
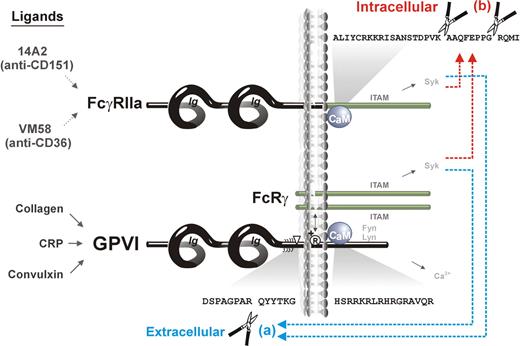
In this study, we show that (1) activation of ITAM-mediated signaling in human platelets activates both extracellular (metalloproteinase) and intracellular (calpain) proteolytic pathways that regulate the function of ITAM-using receptors, GPVI/FcRγ and FcγRIIa, respectively (Figure 7). In the case of GPVI, there is extracellular metalloproteinase-mediated cleavage leading to loss of the ligand-binding ectodomain, whereas in the case of FcγRIIa, there is intracellular calpain-mediated cleavage leading to loss of the ITAM-containing cytoplasmic tail. (2) There is “trans-inhibition” induced by receptor-specific ligands, ie, a ligand (convulxin) acting at GPVI induces both ectodomain cleavage of GPVI and intracellular cleavage of FcγRIIa, whereas ligands acting at FcγRIIa (mAbs VM58 or 14A2) induce the same extracellular and intracellular cleavages. (3) Calmodulin plays a central role in regulating both ITAM-mediated proteolytic pathways, because calmodulin binds directly to cytoplasmic tails of both GPVI9,40,41 and FcγRIIa (this study), and proteolysis of both receptors is induced by the calmodulin inhibitor, W7. Together, these findings are significant for the potential regulation and function of both receptors. GPVI-initiated inactivation of FcγRIIa would provide a mechanism for down-regulating FcγRIIa-dependent platelet activation after adhesion, possibly that involving GPIb-IX-V co-associated with both receptors.4,16 FcγRIIa-initiated shedding of GPVI would, in turn, provide a mechanism for down-regulating platelet reactivity relevant to platelet dysfunction associated with HIT autoantibodies or other immunologic insult.
Scheme showing ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors. Binding of ligands to either FcγRIIa (14A2 or VM58) or GPVI/FcRγ (collagen, CRP, or convulxin) activates both (a) extracellular metalloproteinase-mediated ectodomain shedding of GPVI,12 and (b) intracellular calpain-mediated cleavage of FcγRIIa, resulting in deletion of the ITAM domain. Both pathways are also induced by the calmodulin inhibitor, W7, which dissociates calmodulin from the cytoplasmic domain of GPVI and FcγRIIa.
Scheme showing ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors. Binding of ligands to either FcγRIIa (14A2 or VM58) or GPVI/FcRγ (collagen, CRP, or convulxin) activates both (a) extracellular metalloproteinase-mediated ectodomain shedding of GPVI,12 and (b) intracellular calpain-mediated cleavage of FcγRIIa, resulting in deletion of the ITAM domain. Both pathways are also induced by the calmodulin inhibitor, W7, which dissociates calmodulin from the cytoplasmic domain of GPVI and FcγRIIa.
In previous studies, we showed a requirement for ligand-induced ITAM-mediated signaling in the activation of a platelet metalloproteinase leading to shedding of GPVI.9 The pathophysiologic consequences of depletion of the major collagen-binding receptor on platelet function are an impaired response to collagen in humans48 and in mice.49 Conversely, a direct correlation between levels of platelet GPVI and risk of acute coronary events has been identified in patients at time points when myocardial necrosis markers were still in the normal range,50 suggesting that elevated levels of platelet GPVI may act as an early biomarker of impending acute coronary events. In the present study, several lines of evidence show that activation of the same pathways that lead to metalloproteinase-mediated regulation of platelet GPVI expression, also leads to proteolysis of the ITAM-bearing receptor, FcγRIIa, and that ligand-induced activation of FcγRIIa can trigger the same cleavage and signaling events leading to proteolysis of FcγRIIa and GPVI. First, on ligation of FcγRIIa through the Fc portion of either of 2 mAbs (VM58 or 14A2), FcγRIIa as well as GPVI undergoes proteolysis detectable by Western blot of platelet lysates (Figure 1). This loss of full-length receptor appeared to be specific for the ITAM-associated receptors (GPVI/FcRγ and FcγRIIa) because under the same conditions of activation, shedding of GPV or GPIbα was not similarly accelerated. Proteolysis of both FcγRIIa and GPVI could be triggered by ligation of either receptor within comparable time periods (Figures 1,3,Figure 4–5), and loss of both receptors was blocked by pretreatment with inhibitors of Src family kinases and Syk (Figure 4), indicating that common signaling proteins or pathways were involved in the proteolytic responses. We did not directly assess the consequence of cleavage of FcγRIIa on FcγRIIa function; however, Calverley et al51,52 also suggested human platelets may respond to collagen in a graded fashion, depending on FcγRIIa receptor levels.
Calmodulin associated with the cytoplasmic tail of FcγRIIa is implicated as regulating ligand-induced proteolysis of FcγRIIa, as previously shown for cleavage of other platelet receptors.9,12,53,–55 FcγRIIa contains a juxtamembrane sequence, containing 5 positively charged amino acids, analogous to known calmodulin-binding sequences in GPVI40 as well as other platelet receptors, including GPIbβ and GPV,56 P2Y1,57 and PECAM-1.53 A calmodulin fusion protein pulled down FcγRIIa from platelet lysates, and calmodulin was co-associated with FcγRIIa in resting platelet lysates. Further, this association was disrupted by treatment of platelets with calmodulin inhibitors58,59 (Figure 2C) and resulted in activation of FcγRIIa cleavage (Figures 3,5). The disruption of calmodulin binding and proteolysis of specific receptors was achieved in the past by treatment of leukocytes,42 endothelial cells,60 or platelets9,12,53,54,61 with calmodulin inhibitors such as W7 and trifluoperazine. In platelets, calmodulin regulates the shedding of ectodomains of GPVI and GPV and intracellular proteolysis of PECAM-1. In the case of GPV, members of the ADAM family of cell membrane enzymes control shedding12,54 ; for PECAM-1, calpain mediates intracellular proteolysis (E64d inhibitable).45 Several lines of evidence suggest that the cleavage of FcγRIIa is also mediated by activation of intracellular calpain. First, treatment of platelets with a membrane-permeable calpain inhibitor E64d, but not the membrane-impermeable calpain inhibitor, leupeptin, blocked cleavage of FcγRIIa triggered by ligand binding to the receptor. A recombinant FcγRIIa cytoplasmic tail fragment was also cleaved by purified calpain at 222Lys/223Ala and 230Gly/231Arg, sequences that overlap the calmodulin binding site, with additional proteolysis occurring at longer times. Cleavage within the earliest-targeted region of the cytoplasmic tail, however, would be sufficient to release the ITAM domain and irreversibly disable the signaling function of FcγRIIa. Interestingly, EDTA also blocked intracellular cleavage of FcγRIIa, in response to all agonists and W7 treatment, presumably by chelating extracellular calcium and interfering with Ca2+ flux required for calpain activity.46 Thus, activation of both proteolytic pathways by ITAM-dependent signals (metalloproteinase-mediated GPVI shedding and calpain-mediated FcγRIIa inactivation) is divalent cation-dependent (EDTA inhibitable).
Calpain is a ubiquitous cysteine protease, with the activity of its 3 isoforms regulated by Ca2+ levels.62 Calpain plays a major role in regulating cytoskeletal rearrangements and association with the plasma membrane, and calpain activity is important for cell motility, cell attachment, and cell division.62,63 Intracellular calpain substrates number more than 100,63 and cleavage sites involve elements of primary sequence as well as secondary and tertiary structures.64 Notably, the ability of a protein to bind calmodulin confers a strong likelihood that this protein is a substrate for calpain.65 The μ-calpain isoform is prevalent in platelets66,67 and has been localized to focal adhesions,68 where it regulates shape change, motility, and adhesion mainly through modulation of integrin clustering and function.69,70 Calpain regulates αIIbβ3 on activated platelets by cleavage of the β3 cytoplasmic tail, resulting in the removal of 2 NXXY motifs that are important for ligand binding, bidirectional signaling, and cytoskeletal attachment.47,70 Our data, showing ligand-dependent activation of ITAM-containing platelet receptors induces calpain-dependent removal of the ITAM domain from FcγRIIa, imply that calpain might not only regulate FcγRIIa but also αIIbβ3 downstream of GPVI/FcRγ or FcγRIIa; consistent with this, calpain is notably activated in collagen-adherent platelets.71 The calpain cleavage sites in FcγRIIa (222Lys/223Ala and 230Gly/231Arg) are proximal to 208Cys, which is a palmitoylation site,72 and 214Ser and 217Ser, which are predicted phosphorylation sites for protein kinase A (PKA) and PKC, respectively; analogous palmitoylation sites are found in PECAM-1 and GPIbβ.53 It is intriguing to speculate that calpain-dependent proteolysis of FcγRIIa is regulated, at least in part, by calmodulin binding and/or phosphorylation at 214Ser and 217Ser, as postulated for PKC-dependent modulation of epidermal growth factor receptor activity.43,73
The activation of dual proteolytic pathways may be more broadly relevant to ITAM-dependent regulation in other cells. For example, signaling through ITAM domains is most widely studied in systems investigating immune receptor function on B lymphocytes, T lymphocytes, and natural killer cells,74,–76 culminating in activation of extracellular-regulated kinase, elevated phospholipase Cγ activity, and increased Ca2+ flux.77 More recently, biologic roles for ITAM domains have been expanded to include osteogenesis,78 and cell proliferation,79 in addition to ITAM domain function in GPVI/FcRγ-mediated platelet activation80,81 ; FcγRIIa also regulates immune cell–mediated platelet clearance and thrombocytopenia.82 However, with respect to the clinical importance of platelet dysfunction in patients with HIT, there are at least 2 possibilities emerging from the present findings. First, autoantibodies present in IgG fractions isolated from HIT patient serum in the presence of heparin activate signaling pathways leading to FcγRIIa cleavage, as well as GPVI shedding. Although further work is required to determine both the extent of cleavage of GPVI and associated loss of platelet function in response to HIT patient IgG, it will be of interest to correlate levels of active FcγRIIa and GPVI on platelets of patients with HIT with platelet clearance rates, and to establish whether HIT platelets show impaired responsiveness to collagen. Second, therapeutic proteolytic inactivation of FcγRIIa without accompanying platelet activation, for example by using calmodulin inhibitors or selective-activation of calpain, could provide new ways of treating HIT. Finally, the amount and proteolytic status (intact versus residual inactive fragment) of GPVI or FcγRIIa, in addition to GPIbα,83 should provide useful clinical markers of platelet turnover and clearance in patients predisposed to thrombosis.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Carmen Llerena and Cheryl Berndt for expert laboratory assistance, Shane Reeve for help with mass spectrometry analysis, and Dr Bruce Wines and Halina Trist for provision of the recombinant FcγRIIa cytoplasmic tail protein.
This work was supported by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia.
Authorship
Contribution: E.E.G., D.K., R.K.A., and M.C.B. designed the research, analyzed data, and co-wrote the manuscript; M.L.K., M.S.P., R.I.B., and P.M.H. contributed intellectual input and vital reagents; E.E.G., D.K., J.F.A., and F.M. carried out the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
E.E.G. and D.K. contributed equally to this work.
Correspondence: Michael C. Berndt, Monash University Department of Immunology, Alfred Medical Research and Education Precinct (AMREP), Commercial Rd, Melbourne, Australia; e-mail: michael.berndt@med.monash.edu.au.