Enzyme replacement therapy (ERT) with imiglucerase reduces hepatosplenomegaly and improves hematologic parameters in Gaucher disease type 1 within 6-24 months. Miglustat reduces organomegaly, improves hematologic parameters, and reverses bone marrow infiltration. This trial evaluates miglustat in patients clinically stable on ERT. Tolerability of miglustat and imiglucerase, alone and in combination, pharmacokinetic profile, organ reduction, and chitotriosidase activity were assessed. Thirty-six patients stable on imiglucerase were randomized into this phase II, open-label trial. Statistically significant changes from baseline were assessed (paired t test) on primary objectives with secondary analyses on biochemical and safety parameters. Liver and spleen volume were unchanged in switched patients. No significant differences were seen between groups regarding mean change in hemoglobin. Mean change in platelet counts was only significant between miglustat and imiglucerase groups (P = .035). Chitotriosidase activity remained stable. In trial extension, clinical endpoints were generally maintained. Miglustat was well tolerated alone or in combination. Miglustat's safety profile was consistent with previous trials; moreover, no new cases of peripheral neuropathy were observed. Gaucher disease type 1 (GD1) parameters were stable in most switched patients. Combination therapy did not show benefit. Findings suggest miglustat could be an effective maintenance therapy in stabilized patients with GD1.
Introduction
Gaucher disease, the most common sphingolipid storage disorder, was the first lysosomal storage disease to be effectively treated with enzyme replacement therapy.1 Both the placenta-derived and the human recombinant enzymes (alglucerase [Ceredase] and imiglucerase [Cerezyme], respectively; Genzyme Therapeutics, Cambridge, MA) induce reduction of organomegaly, improve hematologic and biochemical parameters of the disease, and ameliorate bone pain, growth retardation, and some pulmonary complications.2 However, enzyme replacement is administered intravenously, which is inherently inconvenient; it is a large molecule that cannot cross the blood-brain barrier, limiting its effectiveness in the neuronopathic forms (types II and III). Its high cost precludes use in many countries. In addition, despite experience with more than 4000 patients worldwide, and appreciation of the plateau in response by the major hematologic and visceral outcome measures,3 no studies have addressed the issue of maintenance regimens
In 1996, Radin,4 in referring to enzyme therapy, suggested that this “… mode of treatment should be replaceable with a suitable enzyme inhibitor that simply slows formation of the lipid, and matches the rate of synthesis with the rate of the defective, slowly working β-glucosidase.” In April 2000, the results of the first clinical trial with miglustat, an oral substrate inhibitor, as monotherapy in 28 adult patients with non-neuronopathic (type I) Gaucher disease were reported.5 The iminosugar N-butyldeoxynojirimycin (miglustat [Zavesca]; Actelion Pharmaceuticals Ltd, Allschwil, Switzerland), is a small molecule with specific physicochemical properties. It is a reversible inhibitor of the ceramide-specific glucosyltransferase.6 The drug was well tolerated and effective in ameliorating key clinical features of type I Gaucher disease.
Based on these results and recognizing the nontrivial concerns about side effects, the European Agency for the Evaluation of Medicinal Products and the US Food and Drug Administration approved miglustat for marketing in November 2002 and July 2003, respectively, but limited its use to adult patients with mild-to-moderate type I Gaucher disease for whom enzyme replacement, the first-line standard therapy, was not a therapeutic option7 ; the need for long-term surveillance was underscored. The latter requirement relates primarily to safety concerns and emergence of adverse events such as peripheral neuropathy, tremor, and cognitive dysfunction, despite a concurrent safety study that showed no increased incidence of these symptoms in patients exposed to miglustat.8
The present report includes the seminal 6-month switch-over study designed to assess the tolerability and pharmacokinetic profile of miglustat in patients with type I Gaucher disease previously treated with enzyme replacement and to compare their results to those patients who were taking either medication alone or in combination. Follow-up safety data are given on patients who continued in the extension protocol up to 24 months.
Patients and methods
Study patients
Adult patients with type I Gaucher disease receiving enzyme replacement therapy (imiglucerase) for a minimum of 2 years were recruited. None of these patients had ever been exposed to miglustat. These patients fulfilled the Israeli Ministry of Health criteria for enzyme therapy (ie, significant and symptomatic disease at presentation),9 and were stable and receiving a constant dose for at least 6 months before enrollment. Diagnosis was confirmed by biochemical assay of β-glucocerebrosidase and mutation analysis.
Exclusion criteria were as in the seminal study,5 including inability to use adequate contraception, clinically significant diarrhea over the previous 6 months, positive HIV or hepatitis B surface antigen test; and/or a history of, or predisposition to, cataracts (the latter a finding in preclinical animal studies).
Study design
The study was a randomized, open-label, parallel-group, phase II study at a single center. A minimization algorithm with a random component assigned 36 patients to treatment groups according to clinical severity (spleen status and presence/absence of avascular necrosis of a large joint), age (18-30 years, 31-45 years, over 45 years), sex, and years on enzyme replacement (0-5 years, 6-7.5 years, over 7.5 years).10 Starting dosage for miglustat was one 100-mg capsule 3 times a day, whereas the enzyme replacement regimen was as had been prescribed before inclusion in the trial (30 units/kg of body weight/month in 33 patients, 60 units/kg/month in 3 patients). Total study period was 6 months, during which miglustat dosage adjustment was permitted in case of adverse effects, whereas alterations in dosage or frequency of enzyme replacement were disallowed. After 6 months of the trial, patients could enter an extension protocol. Patients could choose to receive either miglustat monotherapy or in combination with imiglucerase. In practice, all patients received miglustat monotherapy.
The protocol was approved by the Institutional Review Board of the Shaare Zedek Medical Center as well as by the Supreme Ethical Committee of the Israeli Ministry of Health.11 All patients provided informed consent in accordance with the Declaration of Helsinki.
Clinical evaluation
Evaluation of spleen and liver volumes by noncontrast computed tomography was done at baseline and after 6 months12 ; patient interviews for safety and compliance, physical examination, and routine laboratory tests were done monthly. Quality of life (QoL) was evaluated using a questionnaire that included the SF-36,13 a modified Medical Outcomes Study Health Distress for assessing the frustration, distress, and anxiety of patients with GD1, and 2 surveys assessing symptoms and treatment-related issues.
The QoL questionnaire was completed at baseline and at months 3 and 6 of the randomized therapy. Six patients from each study group provided pharmacokinetic profiles at month 1. Patients who entered the extension protocol were monitored every 3 months for an additional 18 months. Organ volumes were measured at baseline and at months 12 and 24.
Statistical analysis
Two populations were evaluated: a safety population (patients receiving at least 1 dose of miglustat) and an efficacy population (patients with a baseline evaluation and at least 1 follow-up assessment for organ volume measurement and blood test).
Between-group comparisons were made using an analysis of covariance model, including effects for baseline levels and minimization factors. Age was treated as a continuous factor. Pair-wise treatment comparisons were performed using a step-down procedure. The 5% level of significance was used.
Excess organ volume factor16 was calculated separately for liver and spleen at screening and at month 6 and was presented as mean (SD) volume. Because most patients entered the trial with stable or near-normal values in most disease parameters, calculation of remaining disease was attempted using the following formula: remaining disease = [total possible change − actual change] / total possible change, where the following specific endpoints were taken as acceptable therapeutic goals: liver volume, 1.25 × normal (2.5% BW); spleen volume, 5 × normal (0.2% BW); hemoglobin, men, 12 g/dL; women, 11 g/dL; platelet counts. 120 × 109/mm3; chitotriosidase, 1000 nmol/mL/hr (maintained, ≤ 5% change from baseline value; improved, ≥ 5% change toward normal values; worsened, > 5% change away from normal values).17 Pharmacokinetic parameters were derived by non-compartmental analysis using WinNonlin Version 2.1 (Pharsight, Palo Alto, CA).
Results
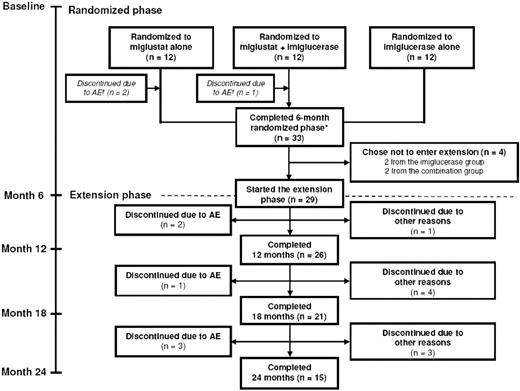
Thirty-six patients entered the study, 12 in each treatment arm. Figure 1 summarizes the disposition of the patients during the 6-month randomized trial and the 18-month extension period. Baseline patient demographics and disease characteristics at the start of initial randomized phase are presented in Table 1. None of the patients developed cataracts during the study period or in the extension.
Patient disposition during 6-month randomized phase and 18-month extension phase. *Refers to stage of the original study; subjects who switched from imiglucerase alone to miglustat alone at month 6 received a maximum of only 18 months of miglustat treatment by month 24.
Patient disposition during 6-month randomized phase and 18-month extension phase. *Refers to stage of the original study; subjects who switched from imiglucerase alone to miglustat alone at month 6 received a maximum of only 18 months of miglustat treatment by month 24.
Efficacy
Six-month randomized phase.
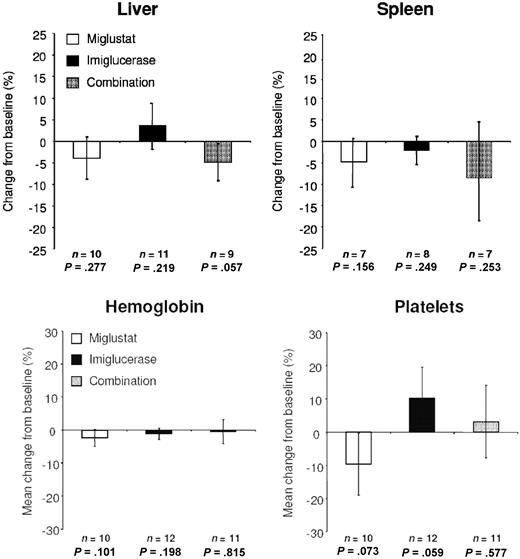
The results of key disease parameters after 6 months of randomized therapy are presented in Figure 2. Pair-wise comparison of mean changes from baseline in organ volume indicated no statistically significant treatment differences, other than the statistically significant reduction in liver volume of the combination group versus the imiglucerase group (P = .047). Liver and spleen volume changes after treatment did not always occur simultaneously or in the same direction.
Organ volume after 6 months of randomized therapy. P values represent findings from pair-wise statistical testing for 6 months versus baseline. Error bars are SD.
Organ volume after 6 months of randomized therapy. P values represent findings from pair-wise statistical testing for 6 months versus baseline. Error bars are SD.
No statistically significant differences in mean change in hemoglobin level were seen between groups. Inter-group comparisons of mean changes from baseline in platelet counts indicated statistically significant differences between the miglustat and imiglucerase groups (P = .035), but no significant differences with combination treatment.
Chitotriosidase activity was characterized at baseline by considerable between-patient variability in all groups; range at presentation in each group was approximately 1000-15 000 nmol/mL/hr. Patients in whom chitotriosidase activity did not decrease during the trial and the extension were patients who had high values at baseline (> 9000 nmol/mL/hr). Comparison of results of other disease parameters between patients with baseline chitotriosidase values < 10 000 nmol/mL/hr versus > 10 000 nmol/mL/hr showed no statistically significant difference (data not shown).
Eighteen-month extension phase.
Table 2 presents results of change in each key disease parameter from baseline in all patients who entered the extension phase based on therapeutic goals for therapy.17 All clinical endpoints indicated disease stabilization in most patients, as calculated using the “Remaining disease” formula described above under “Statistical analysis.”
Safety
All adverse events recorded over the entire 24-month period (6-month randomized plus 18-month extension treatment) are presented in Table 3. None of the patients developed severe adverse effects during the course of the study. Electromyography and nerve conduction velocity showed abnormalities in 8 subjects (3 receiving miglustat; 2 on enzyme therapy; 3 on combination) but these were not all associated with symptoms, and in the absence of a baseline evaluation, clinical significance was unclear. Five subjects experienced adverse events that were classed as serious (one case each of multiple myeloma, tonsillectomy, accidental overdosing with phosphates, prostatic hypertrophy, and pneumonia), but none was considered related to study treatment. These latter 5 events occurred between 15 and 27 months after advent of the trial. There were no deaths during the course of this 24-month study.
Quality of life
At month 6, there was a significant treatment difference in mean changes from baseline in SF-36 Mental Health between patients receiving miglustat (who improved) compared with those receiving imiglucerase or combination (who deteriorated); mean changes were + 8.7% versus − 8.5% versus − 8.1%, respectively (P = .057). In addition, the miglustat group reported greater treatment convenience compared with the imiglucerase and the combination groups (77.8% versus 33.3% versus 30.0%, respectively; P = .028 by Fisher exact test), and greater overall treatment satisfaction compared with enzyme replacement (77.8% versus 33.3% versus 30.0%, respectively, P = .053). Remaining items were not significantly different either between groups or versus baseline (data not shown).
Pharmacokinetics
The combination of miglustat and enzyme replacement did not affect the pharmacokinetic profile of enzyme (data not shown). Mean area under the curve of plasma miglustat concentration versus time was ∼16% greater when miglustat was administered alone than in combination, and the Cmax was ∼29% higher.
Discussion
This clinical trial, the largest randomized study in patients with type I Gaucher disease, is also the first to investigate a new maintenance modality for patients who had been stabilized on enzyme replacement. The advantages of this approach are directly related to (1) the oral administration, which increases quality of life and obviates infusion-related complications, and (2) the lack of immunoreactivity, which allows treatment in those very few patients who experience severe allergic reactions or neutralizing antibodies to the enzyme18 among others. Likewise, this modality may be an alternative for patients with serious side effects or incomplete response to enzyme therapy.19 Inclusion of a combination arm was predicated on the theoretical possibility of a synergistic or additive effect because of different modes of action. In the comparable animal model of knock-out Sandhoff mice, a combination regimen of bone marrow transplantation (to provide enzyme) and miglustat showed better effect than either treatment alone.20 A second reason for the combination arm was to ascertain the safety of the coadministration of enzyme and miglustat. Concerns had been raised of an inhibitory effect by miglustat on endogenous and exogenous glucocerebrosidase.21
The pharmacokinetic data confirmed the lack of negative interactions between the 2 drugs. The combination with imiglucerase did not provide any substantial benefit in stable patients. Nonetheless, although combination does not reduce the risks or disadvantages of either, one may consider combination therapy for patients with neuronopathic disease or for patients with severe disease who have not achieved prescribed therapeutic goals with enzyme alone.17,22
As in all previous clinical trials in patients with type I Gaucher disease, this study has no placebo-controlled arm. This is particularly unfortunate given our own observation in adult patients with symptomatic Gaucher disease, who withdrew from enzyme therapy for various reasons and for variable periods of time, but who did not revert to baseline.23 Indeed, some disease parameters continued to improve after withdrawal. However, a placebo arm was unacceptable to our Ethics Committee.
As underscored by data from an international registry, most patients, regardless of dosing regimen, reach a plateau in response (often near normalization) to enzyme therapy within 2 or 3 years.3 Hence, expectations of a dramatic therapeutic response in those patients who had already received enzyme replacement for 2 years (and several > 8 years), are unrealistic for achieving equivalent responses in all disease parameters as seen in naive symptomatic patients. Thus, the fact that virtually all patients remained stable in all key parameters and in chitotriosidase levels (Table 3) is of crucial importance with regard to maintenance options. Improvement during the trial in some parameters in some patients on miglustat provides evidence that the response plateau, resulting from successful enzyme therapy (partially explained as having been caused by disease-related fibrosis in the severely affected liver and/or spleen)24 can, in fact, be overridden.
It may be conjectured that normalization of the persistent thrombocytopenia is an unrealistic expectation: this was highlighted in the international registry (ICGG) regardless of dosage regimens.3,25 The use of the formula to individually quantify total possible change expected in each parameter and in each patient underscores this point.
Chitotriosidase activity has become a marker of severity of Gaucher disease and of treatment response and hence was an important surrogate measure in the seminal study of miglustat.5 In the current cohort, there was a wide range of activity levels despite prior enzyme therapy. Patients who do not show dramatic decreases in chitotriosidase after 3-8 years of enzyme treatment probably will not show dramatic decreases with other therapies. Why certain patients are unresponsive is unclear, but during the extension, some patients were withdrawn when chitotriosidase levels increased. The context for this is important, because trials with miglustat had been halted at our center because of concern of cognitive decline in one patient.26 Because of this concern, during a 3-month period, all trial patients as well as other patients underwent comprehensive neuropsychologic testing; then there was a further 3-month hiatus by the manufacturer before treatment was reinstated. Because there were no clinically significant changes despite fluctuations in chitotriosidase, some patients were withdrawn because it was not known when the trial would be reinstated. It should be noted that 2 patients experienced bone pain concurrent with increased chitotriosidase levels and were therefore withdrawn from the extension. It is noteworthy that bone pain disappeared in both patients after withdrawal, one of whom elected to restart miglustat and is currently continuing to receive the drug.
Most side effects associated with miglustat were similar to those reported in the seminal study,5 with the exception of tremor. Why these patients are different from the naive patients is unknown. Tremor, however, was reversible in most with dose reduction or cessation of miglustat and did not necessarily motivate patients to withdraw from the trial. Three patients were evaluated at the Developmental and Metabolic Neurology Branch, National Institutes of Health/National Institute of Neurological Disorders and Stroke; the tremor was diagnosed as a physiologic tremor comparable with the effect of coffee on some people.
Contrary to what was observed in the seminal trial, no new cases of peripheral neuropathy were observed in this study. It is noteworthy that a few patients who withdrew from the extension experienced symptoms of peripheral neuropathy after withdrawal; these symptoms eventually dissipated. The reason for these phenomena is unclear, and a study is currently ongoing to evaluate the prevalence and incidence of peripheral neuropathy in patients with type I Gaucher disease.27
Interest in substrate reduction therapy has been heightened because of intimations that small iminosugar molecules may also function as pharmaceutical chaperones.28 Miglustat was shown to increase activity of β-glucocerebrosidase of wild-type and mutated enzymes in Gaucher disease29,30 and of α-galactosidase A in Fabry disease using 1-deoxygalactonojirimycin.31 This field is evolving, but it may be hypothesized that such effects may be concentration-dependent, tissue-specific, and/or disease-specific.
In conclusion, we believe that this trial provides an important and seminal message regarding substrate reduction but equally about maintenance regimens for non-neuronopathic Gaucher disease. This experience will serve as a model for the ongoing clinical trials investigating therapeutic modalities for maintenance therapy in patients with Gaucher disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the Nobel Laureate Prof. Baruch Blumberg (University of Oxford, United Kingdom) for valuable comments on the original draft of the manuscript. We acknowledge the contribution of Andy Garrett (Quintiles, Edinburgh, United Kingdom), who provided the minimization algorithm. We are indebted to the patients who participated in the study and to Irena Kesselman and the nursing staff of the Gaucher Clinic at the Shaare Zedek Medical Center.
The study was supported by Oxford GlycoSciences, the original manufacturer of miglustat (OGT 918).
Authorship
Contribution: D.E. and A.Z. designed the trial. D.E. wrote all drafts of the manuscript. J.F.M.G.A. and S.v.W. performed the laboratory analyses. I.H.H. performed all of the radiologic assessments. A.D., D.A., S.Z., G.A., and A.Z. saw all the patients for clinical evaluations during the trial and its extension, and collected all the data. D.E. devised the new algorithm of remaining disease.
Conflict-of-interest disclosure: D.E. and A.Z. were paid consultants to Oxford GlycoSciences after completion of the trial and until the company was sold. The remaining authors declare no competing financial interests.
Correspondence: Deborah Elstein, Gaucher Clinic, Shaare Zedek Medical Center, P.O. Box 3235, Jerusalem 91031, Israel; e-mail:elstein@szmc.org.il.