Chronic myelogenous leukemia (CML) is a clonal, multistep, multilineage myeloproliferative disorder. It is initiated and propagated by a rare population of CML stem cells that have acquired a BCR-ABL fusion gene. The BCR-ABL fusion gene encodes a chimeric oncoprotein that displays constitutively activated tyrosine kinase activity and drives CML pathogenesis. During the chronic phase (CP) of the disease, leukemia stem cells (LSCs) possess many properties typical of normal hematopoietic stem cells (HSCs), including an ability to differentiate into almost all blood-cell types. At the molecular genetics level, the BCR-ABL gene is usually the sole genetic abnormality seen during the CP. The final blast crisis of CML resembles acute myeloid leukemia or acute lymphoblastic leukemia, is characterized by the appearance of a rapidly expanding population of undifferentiated blasts, and is generally unresponsive to treatment. Genetic analysis indicates thatthese blasts represent evolved subclones with additional mutations, but the exact underlying molecular mechanisms that cause disease progression are not yet known. Recently, imatinib (IM) therapy has revolutionized the treatment of CML. IM treatment causes remission in a majority of patients with CML, but relapses are an increasing problem. The presence of BCR-ABL kinase domain mutations in relapsing cells suggests that development of these mutations in CML stem cells may be a major contributor to treatment failure.
LSCs from CML patients have been difficult to study because of their rarity and phenotypic overlap with normal HSCs. Therefore, it has been a great challenge to define the unique properties of these LSCs, and to identify biologically and clinically relevant biomarkers of BCR-ABL+ stem cells that explain their unusual biology and that may help design or predict improved treatment for CML patients.
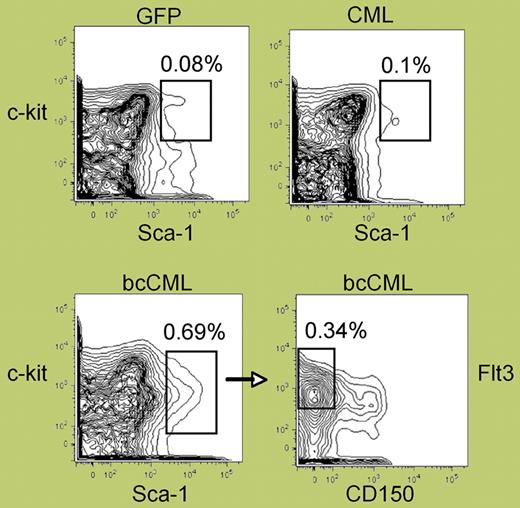
The elegant study by Neering and colleagues in this issue of Blood demonstrates that a BCR-ABL– and Nup98/HoxA9 transduced, LSC-enriched population contains a distinct population (Lin−Flt-3+Sca-1+CD34+ CD1-50−Kit+/−) with significantly high purity (see figure) and leukemia-initiating potential in mouse blast-crisis CML (bcCML). This finding enables phenotypic distinction of blast-crisis LSCs from normal HSCs and characterization of their specific properties in vivo. In particular, BCR-ABL+ LSCs display several unique features that recapitulate physiological responses inferred to occur in human CML, including reduced response to IM, deregulated cell-cycle control, and resistance to apoptosis and radiation-induced DNA damage in vivo. These findings are of particular interest in light of recent evidence suggesting that human LSCs are not only innately resistant to IM and other drugs that target the BCR-ABL oncoprotein, but are also genetically unstable, as demonstrated by rapid accumulation of BCR-ABL mutations in vitro.1,,,–5 It isexpected that LSCs and their progeny identified in the bcCML model would be highly genetically unstable and acquire many BCR-ABL mutations (particularly in the tyrosine kinase domain),4 Nup98/HoxA9 mutations, or other newly acquired chromosomal abnormalities contributing to disease progression. If this model is confirmed by subsequent studies, it may be possible to identify new molecular targets by high-throughput genome arrays of LSCs and their progeny. Indeed, this in vivo model provides a powerful tool for characterizing the unique properties of LSCs and for optimizing and developing more effective therapeutics to eradicate LSCs.
Phenotypic analysis of normal versus leukemic stem cell populations. See the complete figure in the article beginning on page 2578.
Phenotypic analysis of normal versus leukemic stem cell populations. See the complete figure in the article beginning on page 2578.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■