Abstract
Human cytomegalovirus (CMV) infection has been linked to inflammatory diseases that involve vascular endothelial damage, including vascular disease and chronic transplant rejection. We previously reported that the host CD4+ T-cell response to CMV antigen presented by endothelial cells can produce interferon-γ and tumor necrosis factor-α at levels sufficient to drive induction of fractalkine, a key marker of inflammation, in endothelial cells. In this work, we report that donors with high frequencies of antigen-specific T cells to CMV (high responders) induce higher levels of activation-associated chemokines such as fractalkine, RANTES (regulated on activation, normal T cell expressed and secreted), and macrophage inflammatory protein-1β, together with cell-adhesion markers in endothelial cells compared with donors with low levels of CMV-specific T cells (low responders). High-responder cultures had higher levels of leukocyte recruitment and adherence to the endothelial monolayers associated with progressive damage and loss of the endothelial cells. These processes that led to endothelial destruction only required viral antigen and did not require infectious virus. Our findings further support that CMV may represent one member of a class of persistent pathogens in which a high antigen-specific T-cell response defines an important risk factor for development of chronic inflammation and endothelial cell injury.
Introduction
Human cytomegalovirus (CMV), also known as human herpesvirus 5 (HHV5), is a member of the beta herpesvirus family (reviewed by Mocarski and Courcelle1 ). The virus establishes a latent infection in the host and can periodically reactivate and undergo productive infection in a series of recurrent episodes. As an opportunistic pathogen, reactivation of latent CMV can cause significant morbidity and mortality in immunocompromised populations including transplant recipients, developing fetuses, and HIV-infected patients.2,3 There is also increasing evidence to associate CMV infection with chronic inflammatory-related diseases including vascular diseases.4–10 The mechanisms through which CMV can affect the pathogenesis of these inflammatory diseases are, for the most part, unknown. The virus may contribute to inflammatory-disease processes through direct infection effects or through the host immune response. Although lytic viral infection can damage host cells and tissues directly, cell destruction can result also from the inflammatory response of the host immune response to the virus.6,9,10
Vascular complications and transplant loss have been linked with CMV infection. Specific examples of vascular-related inflammatory disorders in which CMV may play a role include coronary artery disease, restenosis after angioplasty procedures, transplant vascular sclerosis (TVS) in chronic graft rejection, and CMV-associated systemic sclerosis.7,10–14 Prior infection with CMV has been shown to be a strong independent risk factor for restenosis.10,13 CMV has also been found in atherosclerotic lesions, and encodes several gene products to modulate the immune cell responses and vascular cell activities.4,15 An experimental animal model that used rat CMV clearly demonstrated accelerated rates of progression in atherosclerotic lesions in TVS, which led to graft failure.8,14,16 Several studies have indicated an important role for CMV in the posttransplantation vasculopathy (arteriosclerosis) process during graft rejection. Ganciclovir therapy to target CMV replication can eliminate virus-induced TVS and also prolongs graft survival in these animal-model systems.8,9 In addition, ganciclovir has been shown to delay graft rejection after heart transplantation when CMV is present in the donor or recipient.14,17 There are also specific reports that have shown that the immune response contributes to vascular damage with CMV infection. Consistent with a role for CMV infection in restenosis and atherogenesis, CMV infection in the rat model has been shown to increase the neointimal response to vascular injury.18 This CMV-induced response occurred even in the absence of virus from the vascular wall, which suggests that host inflammatory and immune responses to infection of nonvascular tissues, even at relatively distant sites, may contribute to the vascular response to injury. Immunosuppression can also significantly reduce immune activation and allograft arteriosclerosis in CMV-infected rat aortic allografts.19 These results further suggest that in the rat model, CMV-enhanced allograft arteriosclerosis may be an immunopathologic condition that is linked to the host immune response toward the graft and/or the virus rather than a direct virus-induced phenomenon.19
In our approach to studying chronic inflammation in the setting of the CMV-infected host environment, we have focused on the contributing role of the host immune response to vascular inflammation and damage. We are working from the hypothesis that CMV-associated chronic endothelial-cell inflammation and damage is mediated through induction of chemokines produced by activated endothelium, where endothelial cells are activated by the cytokine response from CMV-antigen–specific T-cell stimulation. We have previously shown that the host CD4+ T-cell response to CMV antigen can produce interferon (IFN)–γ and tumor necrosis factor (TNF)–α at levels that are sufficient to drive induction of fractalkine, a key marker of inflammation, in endothelial cells cocultured with peripheral blood mononuclear-cell (PBMC) populations.9,20,21 In this report, repeated analyses of a CMV-seropositive donor group demonstrated that the CD4+ populations from some donors induced approximately 10-fold higher fractalkine levels than others in response to CMV-antigen stimulation. Because the induction of fractalkine and other chemokines is an antigen-specific T-cell–activation process that varies considerably among different donors, this variability provided an opportunity to test whether the CMV-specific T-cell frequency also affected the extent of chemokine-mediated attraction and attachment of inflammatory cells to endothelial cells with subsequent endothelial damage. Our findings indicate that PBMC populations from donors with high frequencies of antigen-specific T cells to CMV, compared with donors with low T-cell frequencies, induce higher levels of fractalkine and other chemokines that are associated with increased chemoattraction of inflammatory cells and higher levels of cell-adhesion markers, which leads to extensive endothelial damage. These findings further support that CMV may represent one member of a class of pathogens in which a high antigen-specific T-cell response may define an important risk factor for development of chronic inflammation and endothelial-cell injury.
Materials and methods
Blood was obtained from volunteer donors through the University of California San Diego Center for AIDS Research Virology Core; this protocol was approved by the University of California San Diego Human Research Protocol Program Project 050307, and informed consent was obtained in accordance with the Declaration of Helsinki.
Cells
Human PBMCs were isolated from heparinized blood of healthy donors using density centrifugation over Ficoll-Hypaque (Pharmacia-Biotech, Piscataway, NJ). Donor CMV serostatus was determined by latex agglutination (Becton Dickinson, Sparks, MD). After 2 washes with phosphate-buffered saline (PBS), PBMCs were suspended at a concentration of 106 cells/mL in RPMI 1640 with glutamine, 10% fetal bovine serum, and penicillin/streptomycin (100 U and 100 μg/mL of medium, respectively). CD4+ T cells were isolated from PBMCs according to manufacturer instructions using a negative-selection MidiMACs system and LS+ immunomagnetic columns (Miltenyi Biotech, Auburn, CA).
Primary human foreskin fibroblast cultures were maintained in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal calf serum (FCS; US certified from Invitrogen to be endotoxin and mycoplasma free) and penicillin/streptomycin (100 U and 100 μg/mL of medium, respectively).
Primary human aortic endothelial cells (AECs) and culture medium were purchased from Clonetics (San Diego, CA). Each cell lot was from a single donor. All experiments were repeated with at least 2 different donor AECs. Cocultures were set up with resting confluent primary AEC monolayers and overlaid with 106 PBMCs or CD4+ cells as specified. Cocultured cells were then stimulated with heat-inactivated CMV strain AD169 and incubated in a humidified incubator for 72 hours at 37°C in 5% CO2. Cocultures were maintained in a 50:50 mix of RPMI 1640/10% FCS and endothelial growth media-2/2% FCS (Clonetics) to support both cell types.
Transwell migration assays
For differential chemoattraction assays, the transwell inserts used were 6.5 mm in diameter and had 5-μm pore-size polycarbonate membranes in 24-well polystyrene plates available from CoStar/Corning (Corning, NY). Coculture supernatants or control medium samples were loaded into lower well chambers using a volume of 600 μL. The upper-well insert was then placed into the well, and 4 × 106 PBMCs in a 150-μL volume of control medium were placed into the upper-chamber well inserts of 24-well transwell migration assay plates. Transwell assays were placed in a 5% CO2/37°C humidified incubator for a cell-migration time period of 3 hours. For each assay, 3 replicate wells were set up, and the transmigrated populations were counted from each well independently. Results are expressed as migrated cells minus the background number of migrated cells.
Enzyme-linked immunosorbent assay
Soluble fractalkine (CX3CL1) was detected in culture supernatants with an enzyme-linked immunosorbent assay (ELISA) detection kit (DY365 DuoSet system for human fractalkine/CX3CL1; R&D Systems, Minneapolis, MN). For fractalkine detection, specific antibodies were obtained from R&D Systems. Each well of a 96-well Immulon plate (Fisher, Tustin, CA) was coated overnight at room temperature with 100 μL of 0.4 μg of primary antibody in PBS (pH 7.2). Plates were washed in 0.05% Tween 20 in PBS and then blocked for 2 hours in 1% bovine serum albumin/5% sucrose in PBS. Standards or culture-sample supernatants (100 μL) were added and incubated overnight at room temperature. Peroxidase-conjugated detection antibody (R&D Systems) at a dilution of 1:200 (100 μL) was added and incubated at room temperature for 30 minutes. Color was then developed by adding 100 μL of a solution of hydrogen peroxide mixed with tetramethylbenzidine (R&D Systems). Optical density was read at a wavelength of 450 nm, with subtraction of 630 nm in a Bio-Tek kinetics reader (Bio-Tek Instruments, Winooski, VT).
For detection of RANTES (regulated on activation, normal T cell expressed and secreted) and macrophage inflammatory protein (MIP)–1β, human cytokine kits were obtained from R&D Systems, and assays were performed in duplicate per protocol.
IFN-γ enzyme-linked immunospot assay
Millipore (Billerica, MA) multiscreen IP 96-well microtiter plates were coated overnight with 2.5 μg/mL mouse antihuman IFN-γ capture antibody (clone 1-DIK; Diapharma/Mabtech, Franklin, OH) at 4°C in PBS. Plates were then blocked with complete culture medium and incubated for 2 hours at 37°C. Stimulant (100 μL) was added to the wells followed by PBMCs. PBMCs were prepared as outlined above and added to the plates in triplicate at concentrations of 100 000 and 200 000 cells per well. Plates were then incubated for 20 hours at 37°C in a fully humidified atmosphere of 5% CO2 in air. At the conclusion of culture, the wells were washed 3 times with PBS/0.05% Tween 20 followed by 3 washes in PBS alone. The secondary biotin-conjugated antihuman IFN-γ (clone 7-B6–1; Diapharma/Mabtech) was then added to the wells at 1 μg/mL dilution for 120 minutes at 37°C. The wells were washed 3 times with PBS/0.05% Tween 20 followed by 3 washes in PBS alone. Immediately after incubation with biotinylated detection antibody, the plates were washed 3 times with PBS/0.05% Tween 20 followed by 3 washes in PBS alone. Avidin-Peroxidase-Complex (APC; Vector Labs, Burlingame, CA) complex was then added to each well, and the plate was incubated for 60 minutes at room temperature. Spots that corresponded to individual cytokine-secreting cells were enumerated using an ImmunoSpot reader (Cellular Technologies, Cleveland, OH) with a sensitivity set at 150. The enzyme-linked immunospot (ELISPOT) ranges for frequencies of CMV-specific responding T cells were consistent with flow-cytometry analyses that were performed on the same samples, in which intracellular IFN-γ+ CD4+ T cells activated in response to CMV antigen were detected (data not shown).
Adherence assays
For these assays, endothelial monolayers were exposed to coculture supernatants prepared from high- or low-responder donors, and the resulting activated endothelial monolayers were compared for ability to bind PBMCs. Coculture supernatants were prepared from high- and low-responder donors using CD4+ T cells from each donor stimulated with CMV antigen and cultured with endothelial monolayers. On day 3, the coculture supernatants that resulted from the activated endothelial/CD4+ T-cell cultures were collected and separated from nonadherent cells and cell debris by filtration through a 0.22-μm filter. These cell-free supernatants were placed on new, resting-state endothelial-cell cultures and maintained for 24 hours. Culture supernatants were removed, and monolayers were rinsed briefly in RPMI 1640/10% FCS medium. PBMCs (105) in a volume of 500 μL of RPMI 1640/10% FCS medium were added to coculture-conditioned medium-treated endothelial-cell monolayers in 24-well plates. PBMCs were incubated with endothelial cultures for 30 minutes at 37°C in a CO2 culture incubator. PBMCs and media were then removed, and monolayers were washed 3 times with 1× PBS. Monolayers were then compared for numbers of adherent PBMCs as a level of comparison for adherence ability between high- and low-responder donors. For each well sample, 3 different microscope fields were counted. The adherent PBMC counts were then presented as the mean and standard deviation (SD) for graphing.
Combined differential adherence and endothelial damage assay
Confluent primary AECs in 48-well tissue-culture plates were activated for 24 hours by either high- or low-responder supernatants (500 μL per well). PBMCs (2 × 106 per well) were then added to these differentially activated endothelial cultures and allowed to adhere overnight. Nonadherent cells were then removed, and each well was washed 3 times. The original nonadherent cells plus medium were centrifuged for 5 minutes at 300g to pellet cells; cell-free supernatant was then removed and added to same wells of endothelial monolayers. The resulting cocultures were monitored for endothelial damage by microscopy over the course of 5 days. Cells were fixed in BD Cytofix (BD Biosciences, Franklin Lakes, NJ) on day 5. Images were collected with a Nikon (Melville, NY) digital microscopy system (DS-L1 unit with DS camera head DS-5M) using a Nikon Diaphot phase-contrast microscope. For quantitation, cells were counted using a ×20 objective lens under phase-contrast conditions.
Flow cytometry
Cells were labeled using antibodies specific for CD45+ (CyChrome conjugated; BD Biosciences), CD16+/CD56+ (phycoerythrin [PE] conjugated; BD Biosciences), CD14+ (APC conjugated; BD Biosciences), CD3+ (PE conjugated; BD Biosciences), CD8+ (PE conjugated; BD Biosciences), and CD4+ (APC conjugated; BD Biosciences). Nonspecific binding was blocked using 2% FCS plus 1% bovine serum albumin in 1× PBS for 10 minutes in a volume of 100 μL containing 105 to 106 cells. This was followed by the addition of fluorescent-tagged antibodies for 30 minutes at 4°C in the dark at concentrations according to manufacturer instructions. Cells were then washed by resuspending them in 2 mL of blocking buffer and pelleting at 300g for 10 minutes. Supernatant was removed, and each cell pellet was resuspended in 500 μL of 4% paraformaldehyde. The cells were analyzed on a FACSCalibur (BD Biosciences) flow cytometer using CellQuest 3.1 software (BD Biosciences).
Results
Chemokine induction differs among donors in response to CMV antigen
Previously, we had demonstrated that the CD4+ T-cell response to CMV antigen can drive induction of fractalkine in endothelial cells and that the cytokines, TNF-α and IFN-γ, produced by the T-cell response are critical to fractalkine induction.20 No fractalkine induction was detected in the endothelial cells when there was no antigen source to stimulate the CD4+ T cells and produce IFN-γ and TNF-α. In addition, CD4+ T cells from CMV-seronegative donors consistently failed to induce fractalkine in endothelial cells, whereas nonspecific stimulation with phytohemagglutinin resulted in endothelial cell activation and fractalkine induction in CD4+ T cells in all donors regardless of CMV serostatus.20 In these experiments, we focused on using CMV-seropositive samples, because only in the setting where T-cell activation occurs as a memory response to CMV have we observed fractalkine induction with endothelial activation; thus, CMV-seronegative samples do not induce fractalkine in this system.
Although all CMV-seropositive donors screened could induce fractalkine in endothelial cells, they did not all respond by inducing the same levels of fractalkine. For these experiments, PBMCs were collected from CMV-seropositive donors and added as suspension cell cultures to primary AEC monolayer cultures. The cocultures were then treated with heat-inactivated CMV antigen to stimulate CMV-antigen–specific CD4+ T cells. On day 3 of coculture, the fractalkine levels secreted into the culture supernatants were measured by ELISA. Fractalkine-induction levels during coculture were measured for each donor using PBMC samples obtained from 2 separate blood draws; the second blood specimen was collected 2 to 4 months after the original sample. PBMC populations were prepared on the same day as the sample was collected for coculture with endothelial cells and CMV-antigen stimulation. Repeated analyses of a CMV-seropositive donor group demonstrated that the CD4+ populations of PBMCs from some donors induced 10-fold higher fractalkine levels than some low responders in response to CMV-antigen stimulation (Table 1). Donors could be divided into 2 main groups: the mean for high fractalkine-induction donors was 210 (± 21.5) ng/mL of fractalkine by day 3 in the coculture system compared with 27.8 (± 8.17) ng/mL for low responders.
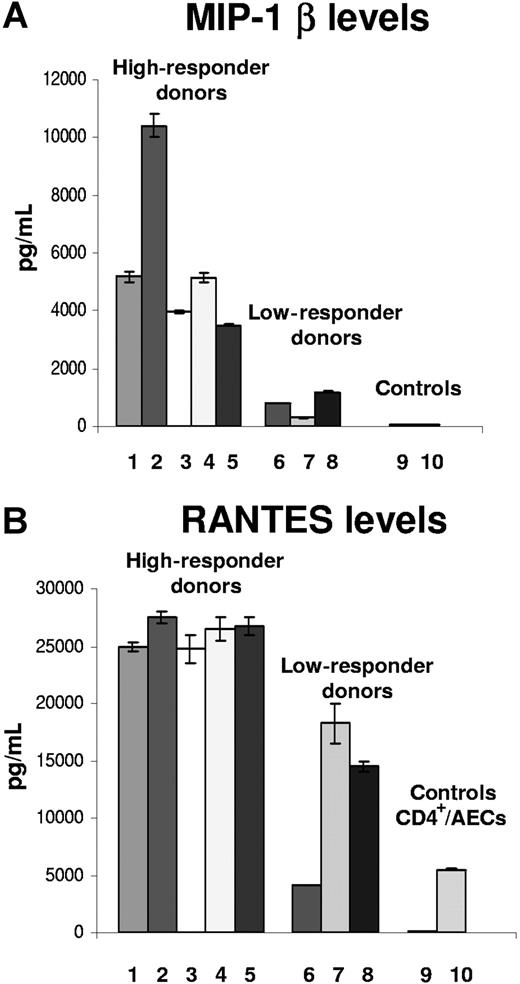
The same high- or low-responder donors with respect to fractalkine induction could also be divided into 2 main groups on the basis of their production of MIP-1β in response to CMV antigen (Figure 1A). The mean MIP-1β level for high responders was 5500 pg/mL and for low responders was 758 pg/mL. However, both responder groups showed high levels of induced factor compared with the negative, resting-state controls (mean: ∼30 pg/mL). After activation of the endothelial cells, the low-responder group had a mean increase of approximately 25-fold compared with the high-responder group, which increased a mean of approximately 180-fold.
MIP-1β and RANTES levels (mean± SD) in CD4+ T cells and AECs after CMV-antigen stimulation at 3 days for each donor. Samples 1 through 8 demonstrate ELISA results from individual donors. Sample 9 represents a negative control from resting-state endothelial cells alone. Sample 10 is an additional control that shows results from endothelial cells cocultured with CD4+ T cells and no viral antigen stimulation. (A) MIP-1β. (B) RANTES.
MIP-1β and RANTES levels (mean± SD) in CD4+ T cells and AECs after CMV-antigen stimulation at 3 days for each donor. Samples 1 through 8 demonstrate ELISA results from individual donors. Sample 9 represents a negative control from resting-state endothelial cells alone. Sample 10 is an additional control that shows results from endothelial cells cocultured with CD4+ T cells and no viral antigen stimulation. (A) MIP-1β. (B) RANTES.
RANTES levels for the same donors could also be divided into the same groups of low (mean: ∼13 000 pg/mL) and high responders (mean: ∼28 000 pg/mL) (Figure 1B). These same high- or low-responder donors were also consistently high or low for fractalkine induction in endothelial cells when maintained as cocultures in the presence of CMV antigen. Although the differences were not as marked, the low-responder levels of RANTES were consistently lower by more than 50% compared with the high-responder levels. Background levels of RANTES were also relatively high (mean: ∼5300 pg/mL) in supernatants of CD4+ T cells from each donor cocultured with AECs in the absence of viral antigen stimulation (data not shown). For the high-responder group, the levels of RANTES produced by the activated endothelial cells ranged from 25 000 to 30 000 pg/mL. Again, CMV-seronegative donor PBMCs failed to induce RANTES in the presence of CMV antigen. These results also confirm the presence of RANTES and MIP-1β in addition to fractalkine in these activated endothelial/CD4+ T-cell cocultures.
Identification of high- and low-responder donors on the basis of CMV-specific T-cell frequency
The next set of experiments was designed to determine whether the level of fractalkine induction in response to CMV antigen was directly related to the frequency of CD4+ T cells that can recognize CMV antigen. Representative high- and low-responder donors were screened for T-cell response to CMV antigen. PBMCs were collected from previously identified high and low fractalkine responders, treated with CMV antigen, and analyzed by ELISPOT for levels of IFN-γ+ cells to determine frequency of human CMV responding T cells. Samples were also analyzed for intracellular IFN-γ and activated CD4+ T-cell–specific markers using flow-cytometry analysis (data not shown). The results demonstrate that the mean of the CMV-specific T-cell frequency for high fractalkine responders (frequency of 363 cells per 105 PBMCs from ELISPOT data) is eightfold higher than the mean of CMV-specific T cells found in low fractalkine responders (frequency of 43 cells per 105 PBMCs). The highest responder had a frequency of 5.5% of CD4+ T cells responding to CMV antigen, with the lowest responder having 0.23%. These results indicate that a higher frequency of antigen-specific T cells to CMV can result in the induction of higher levels of fractalkine.
High T-cell frequency is associated with increased chemokine-associated effects, including increased levels of chemoattraction and adherence of PBMC populations
Because a higher CMV-specific T-cell frequency was associated with a greater chemokine-induction response, particularly fractalkine, we wanted to determine whether this also extended to increased activity on chemoattraction and adherence. Coculture supernatants from each donor were prepared from endothelial monolayers and collected as described previously.20 Transwell migration assays were set up to compare the levels of chemoattraction between coculture supernatants generated from a low-responder donor vs. a high-responder donor. The coculture supernatants were diluted before addition of the transwells using RPMI 1640 with 1% FCS for 1:10 and 1:100 dilutions. Diluted coculture supernatants from a high-responder donor, a low-responder donor, or negative control medium were loaded into lower chambers of transwell units. PBMCs were loaded into upper chambers of transwells, and a period of 3 hours was used to determine migrations. Transmigrated PBMC populations were then collected from the lower chambers of 3 transwells and pooled. Replicate pooled samples were used to calculate the mean transmigrated cell number per milliliter (± SD) (Figure 2).
CMV-antigen high responders induce greater migration of PBMCs than CMV low responders. Results are shown as the mean transmigrated cell number per milliliter of 2 pooled sample sets. (A) Cell-migration results are shown from a 1:10 dilution of culture supernatants prepared from high- or low-responder donor CD4+ T-cell and endothelial cocultures. (B) Cell-migration results from a 1:100 dilution of culture supernatants from low and high responders. The highest levels of cell migration are seen with high-responder culture supernatants. For all samples, the background level of migration was subtracted. After subtracting background cell-migration values, results were then graphed as the mean (± SD).
CMV-antigen high responders induce greater migration of PBMCs than CMV low responders. Results are shown as the mean transmigrated cell number per milliliter of 2 pooled sample sets. (A) Cell-migration results are shown from a 1:10 dilution of culture supernatants prepared from high- or low-responder donor CD4+ T-cell and endothelial cocultures. (B) Cell-migration results from a 1:100 dilution of culture supernatants from low and high responders. The highest levels of cell migration are seen with high-responder culture supernatants. For all samples, the background level of migration was subtracted. After subtracting background cell-migration values, results were then graphed as the mean (± SD).
Using culture supernatants prepared from high- or low-responder donor CD4+ T-cell and endothelial cocultures stimulated with CMV antigen, the highest levels of cell migration were observed with the high-responder culture supernatants (Figure 2). Cell migration using the low-responder culture supernatants are at a level similar to the background level for the negative control medium. The highest levels of cell migration were demonstrated with high-responder culture supernatants. Low-responder and negative control samples showed cell-migration levels at background. Again, CMV-seronegative donors failed to respond to CMV-antigen stimulation (data not shown). Results are representative of 3 cell-migration assays with different pairs of high- and low-responder donors.
Flow-cytometry analysis was used to confirm the identity of the migrating cell types as inflammatory populations. By using high-responder culture supernatants, the majority of the transmigrated populations were identified as CD14+ comprising a mean of 45%, followed by CD4+ at 19.6%, CD16+/56+ at 7.7%, and CD8+ T cells at 7.8%. The higher-fold increases over background of cell migration with the coculture supernatants compared with migration with the negative control medium on the basis of the mean for the CD14+ cells was a 2.9-fold increase, for CD4+ cells an 8.8-fold increase, for CD8+ cells a 4.5-fold increase, and for CD16+/56+ cells a 3.1-fold increase. Overall, these transwell-migration assay results revealed a differential effect in ability to chemoattract or mobilize PBMC populations between CMV high- and low-responding donors and suggested to us that the differential T-cell response can lead to a greater chemokine-based gradient with increased chemoattraction/mobilization and adherence of inflammatory cells to activated endothelial monolayers.
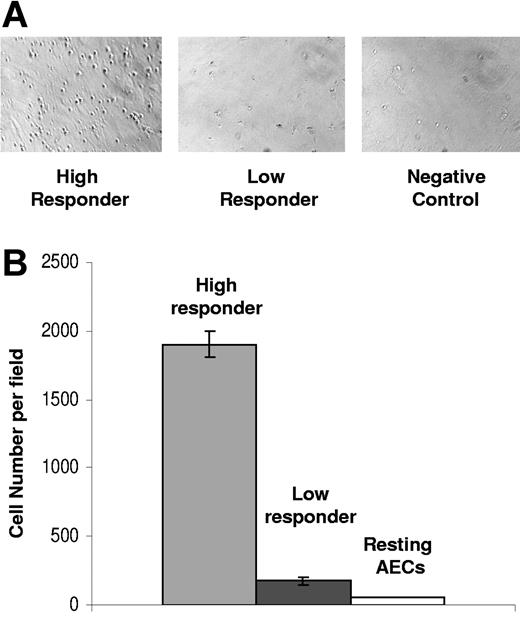
We next examined the quantity of inflammatory cells that adhered to endothelial cells in high- versus low-responding donors. To test for differences in levels of adherence, endothelial monolayers were exposed to the coculture supernatants from high and low responders, and the resulting activated endothelial monolayers were compared for ability to bind PBMCs. In each experiment, the high-responder donor had approximately ninefold greater numbers of PBMCs adhering to endothelial cells compared with those of the low responders (Figure 3). Results are representative of 3 assays from 3 different pairs of high- and low-responder donors.
Adherence of PBMCs is greater in endothelial cells activated by high-responder coculture supernatants in contrast to those from low responders. (A) The micrograph panels created using phase contrast show the different levels of PBMC adherence associated with high- versus low-responder donor conditioned coculture medium. Many PBMCs have bound to the endothelial cells and are visible as small, bright, rounded refractile cells against the endothelial monolayer in the left panel compared with low-responder donor cocultures (center) and negative control (right). For image information, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article. (B) PBMC adherence is higher for endothelial cells activated by high-responder coculture supernatants in contrast to those from low responders and resting AECs. For each sample, 3 microscope fields were counted for the number of bound PBMCs per field and are shown as the mean (± SD).
Adherence of PBMCs is greater in endothelial cells activated by high-responder coculture supernatants in contrast to those from low responders. (A) The micrograph panels created using phase contrast show the different levels of PBMC adherence associated with high- versus low-responder donor conditioned coculture medium. Many PBMCs have bound to the endothelial cells and are visible as small, bright, rounded refractile cells against the endothelial monolayer in the left panel compared with low-responder donor cocultures (center) and negative control (right). For image information, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article. (B) PBMC adherence is higher for endothelial cells activated by high-responder coculture supernatants in contrast to those from low responders and resting AECs. For each sample, 3 microscope fields were counted for the number of bound PBMCs per field and are shown as the mean (± SD).
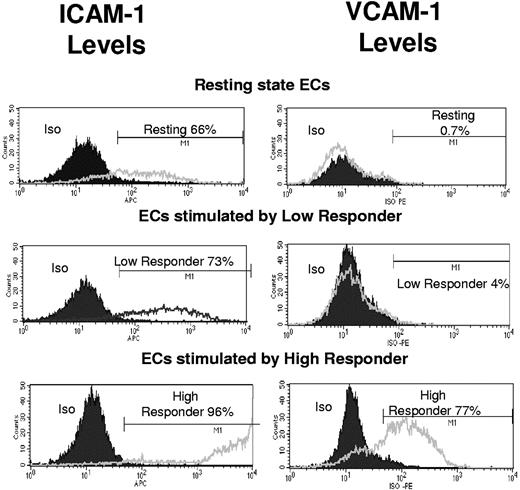
To determine whether coculture supernatants generated from donors with high CMV-specific T-cell frequency were associated with the ability to induce higher levels of adhesion-marker molecules on endothelial cells, cell-surface expression levels of adhesion molecules (intercellular adhesion molecule-1 [ICAM-1] and vascular cell adhesion molecule-1 [VCAM-1]) were compared in endothelial cells that were activated by either high- or low-responder coculture supernatants. Endothelial monolayers were treated for 24 hours with one of the following: conditioned coculture medium from high-responder donors, low-responder donors, or RPMI 1640/10% FCS as negative control medium. Cells were then dissociated and collected in a single-cell suspension using a nonprotease-based approach, and flow-cytometry analysis was used to monitor the surface expression levels of adhesion markers VCAM-1 and ICAM-1. In the resting state, 0.7% of the cells were positive for VCAM-1. This increased to 4% VCAM-1 expression in endothelial cells that were maintained in low-responder conditioned medium compared with 77% VCAM-1–positive in high-responder conditioned medium (Figure 4). At resting state, 66% of endothelial cells were positive for ICAM-1, 73% of cells treated with low-responder conditioned medium were positive, and 96% were positive with high-responder conditioned medium (Figure 4). Results are representative of 3 assays with 3 different pairs of high- and low-responder donors.
Higher levels of adhesion markers ICAM-1 and VCAM-1 are induced on endothelial cells (ECs) activated by high-responder coculture supernatants vs. low-responder supernatants. Endothelial monolayers were treated for 24 hours with one of the following: conditioned coculture medium from a representative high-responder donor, a representative low-responder donor, or RPMI 1640/10% FCS as negative control medium. Cells were then dissociated and collected in a single-cell suspension using a nonprotease-based approach, and flow-cytometry analysis was used to monitor the surface expression levels of adhesion markers VCAM-1 and ICAM-1. Results for VCAM-1 levels are shown in the right panels, where in the resting state 0.7% of the cells were positive for VCAM-1 (black line), and the isotype (Iso) control is indicated by a filled gray curve area that very closely follows the resting-state cell levels In endothelial cells maintained in low-responder conditioned medium, 4% were positive for VCAM-1 (line), and in those maintained in high-responder conditioned medium, 77% were positive (line). Results for ICAM-1 levels in the same sample are shown in the left panels, where 66% of resting-state endothelial cells were positive for ICAM-1 at a relatively low level (line), and 73% of the cells treated with low-responder conditioned medium were ICAM-1 positive (line). These ICAM-1 levels were slightly higher than those in the resting state. A total of 96% of the cells were ICAM-1 positive in cells treated with the high-responder conditioned medium (line), and many of these cells were expressing high levels of ICAM-1. Relative fluorescent intensity is represented on the x-axis, and the cell numbers as counts are shown on the y-axis.
Higher levels of adhesion markers ICAM-1 and VCAM-1 are induced on endothelial cells (ECs) activated by high-responder coculture supernatants vs. low-responder supernatants. Endothelial monolayers were treated for 24 hours with one of the following: conditioned coculture medium from a representative high-responder donor, a representative low-responder donor, or RPMI 1640/10% FCS as negative control medium. Cells were then dissociated and collected in a single-cell suspension using a nonprotease-based approach, and flow-cytometry analysis was used to monitor the surface expression levels of adhesion markers VCAM-1 and ICAM-1. Results for VCAM-1 levels are shown in the right panels, where in the resting state 0.7% of the cells were positive for VCAM-1 (black line), and the isotype (Iso) control is indicated by a filled gray curve area that very closely follows the resting-state cell levels In endothelial cells maintained in low-responder conditioned medium, 4% were positive for VCAM-1 (line), and in those maintained in high-responder conditioned medium, 77% were positive (line). Results for ICAM-1 levels in the same sample are shown in the left panels, where 66% of resting-state endothelial cells were positive for ICAM-1 at a relatively low level (line), and 73% of the cells treated with low-responder conditioned medium were ICAM-1 positive (line). These ICAM-1 levels were slightly higher than those in the resting state. A total of 96% of the cells were ICAM-1 positive in cells treated with the high-responder conditioned medium (line), and many of these cells were expressing high levels of ICAM-1. Relative fluorescent intensity is represented on the x-axis, and the cell numbers as counts are shown on the y-axis.
CMV-specific T-cell frequency correlates with levels of endothelial cell damage
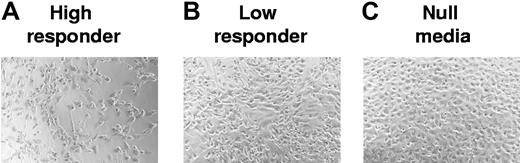
To determine whether the higher levels of PBMC adherence mediated by high-responder donors could also affect levels of endothelial damage, endothelial cells were activated for 24 hours by either high- or low-responder supernatants that were prepared previously from CMV-seropositive donors with high or low T-cell frequencies to CMV antigen. PBMCs were then added to these differentially activated endothelial cultures and allowed to adhere overnight. Nonadherent cells were then removed, and the resulting cocultures were monitored for endothelial damage over the course of 6 days. The most extensive damage was observed in high responder–treated endothelial cells, with only minor damage seen with low responder–treated endothelial cells (Figure 5). By using direct counts of cells per field of view under the microscope, the mean cell number per field of view (×20) in the negative control sample was 107 (± 8.6), 83 (± 5.7) for the low-responder cocultures, and 42 (± 15) for the high-responder cocultures. These results were used to determine the percentage of endothelial loss relative to the negative control cultures as 0%, where the low-responder donor had a 22.5% (± 5%) loss, and the high-responder donor had a 61% (± 13%) loss. This pathogenic effect was associated with the higher T-cell response to CMV and was never observed with CMV-seronegative donors.
High CMV-specific T-cell frequency is associated with more endothelial damage. Endothelial cells were activated by either high- or low-responder supernatants from CMV-seropositive donors with high or low T-cell frequencies to CMV antigen. PBMCs were then added to these differentially activated endothelial cultures and allowed to adhere overnight. Nonadherent cells were removed the next day. The resulting cocultures were monitored for endothelial damage over the course of 6 days. (A) Damage results to endothelial monolayer activated using coculture supernatants from a high-responder donor. (B) Results from endothelial monolayers activated by a low-responder donor. (C) Results from resting-state, nonactivated endothelial cells. For image information, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article.
High CMV-specific T-cell frequency is associated with more endothelial damage. Endothelial cells were activated by either high- or low-responder supernatants from CMV-seropositive donors with high or low T-cell frequencies to CMV antigen. PBMCs were then added to these differentially activated endothelial cultures and allowed to adhere overnight. Nonadherent cells were removed the next day. The resulting cocultures were monitored for endothelial damage over the course of 6 days. (A) Damage results to endothelial monolayer activated using coculture supernatants from a high-responder donor. (B) Results from endothelial monolayers activated by a low-responder donor. (C) Results from resting-state, nonactivated endothelial cells. For image information, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article.
Discussion
We previously demonstrated that the CMV-seropositive host CD4+ T-cell response to CMV antigen can produce IFN-γ and TNF-α at levels that are sufficient to drive induction of fractalkine in adjacent endothelial cells, which is a key marker of inflammation, whereas CMV-seronegative donors fail to show this effect.20 In this report, repeated analyses of a CMV-seropositive donor group demonstrated that the CD4+ populations from some donors induced approximately 10-fold higher fractalkine levels than others in response to CMV-antigen stimulation. This effect was directly related to the donor frequency of CMV-antigen–specific T cells (CD4+), in which high fractalkine responders have approximately 10- to 25-fold higher levels of CMV-specific T cells than low fractalkine responders. Because the driving force for fractalkine and other chemokine-induction levels was the antigen-specific T-cell–activation process, this range among the donors provided an opportunity to test whether the CMV-specific T-cell frequency also affected the levels of chemokine-mediated functions of chemoattraction and adherence and their impact on endothelial cells. We observed that cells mobilized by factors from high-responder donor-activated endothelial cells included CD14+, CD16+/56+, and CD8+ T cells. These cell types express fractalkine and/or RANTES receptors and comprise an important component of inflammatory cell populations. We consistently observed that PBMC populations from donors with higher frequencies of antigen-specific T cells to CMV induced higher levels of fractalkine and other chemokines, expressed higher levels of cell-adhesion markers, and led to more extensive endothelial damage.
These results provide support for a model of the role of fractalkine and other chemokines induced by the host's CMV-antigen–specific CD4+ T-cell response and how this may contribute to endothelial damage.9,21 In the first step, CMV infection of endothelial cells leads to the release of infectious virus, noninfectious virion particles, and viral proteins. The latter 2 have the clear potential to be processed and presented as antigen by other adjacent endothelial cells to CMV-antigen–specific CD4+ T cells circulating in the peripheral blood and patrolling the vascular tissue system. In the second step, IFN-γ and TNF-α are released by the antigen-stimulated CD4+ cell. These cytokine factors interact with and activate the endothelium, which results in the induction of several chemokine factors, including fractalkine induction and secretion.22 Several adhesion molecules and receptors are also up-regulated on the activated endothelial surface, including ICAM-1 and VCAM, as examples.9,21,22 In the third step, the localized concentration of fractalkine on the apical surface of endothelial cells, together with the release of soluble fractalkine, results in a fractalkine gradient. This fractalkine gradient, together with other chemokines, can attract additional inflammatory cells to the site and can include platelets, neutrophils, natural killer cells, monocytes, and certain types of CD8+ T cells. These incoming inflammatory infiltrate cells can bind to membrane-bound fractalkine on the endothelial-cell surface via their fractalkine receptor, CX3CR1.23 Natural killer cells are known to cause endothelial damage in this setting after activation by fractalkine.24 Monocytes also can respond to and are recruited by chemokines and cytokines produced by activated endothelium, including fractalkine and macrophage colony-stimulating factor.4,21 Macrophage colony-stimulating factor induces differentiation of monocytes to macrophages and expresses scavenger receptors and toll-like receptors.15,25,26 Binding of these receptors activates the macrophage into release of inflammatory cytokines, chemokines, proteases, oxygen and nitrogen radicals, and other inflammatory molecules, which leads to inflammation and tissue damage.27
Compared with other pathogens, a relatively high percentage of the host T-cell response is invested in recognition of CMV antigens.28–30 Repeated antigen exposure over time may result in long-term changes of the immune response, including high levels of T-cell response.6,31 These changes can appear as high levels of CMV-specific T cells that control the virus and defend against CMV spread in the host.32–34 A potential outcome would be a shift toward a proinflammatory/T-helper 1–polarized type response of the host and may be the basis for many chronic inflammatory-type diseases.6,32–34 High T-cell response to CMV, together with increased levels of C-reactive protein, has been associated with increased carotid intima-media thickness, which is a marker for coronary artery disease in patients on highly active antiretroviral therapy.35 A similar problem may be occurring in immune recovery uveitis, a sight-threatening inflammatory condition that is seen in patients with AIDS and CMV retinitis who have an initial response to highly active antiretroviral therapy. During this process, reconstitution of immune function is associated with recovery of CMV-directed responses. In a recent study, active CMV infection such as CMV retinitis was rare, but immune recovery uveitis was common among patients with CMV retinitis regardless of whether they maintained or discontinued the use of anti-CMV therapy.36
Overall, our results emphasize the potential of the host antigen-specific T-cell population to respond aggressively to CMV where the magnitude of the cytokine response may initiate a chemokine cascade and direct an inflammatory infiltrate that has a self-destructive effect on the endothelium. The risk for this to occur may be greater with higher host CMV-specific T-cell frequencies (or with increased CMV levels) present in the host. Endothelial-cell damage may be the end effect of a repeated cycle in which the cell and tissue damage results from innate immune defense mechanisms (monocyte-macrophage cells) activated by a strong T-cell response to a chronic pathogen that is present as either a persistent infection or a latent infection undergoing periodic reactivation episodes. Although protective against the specific pathogen, a very high T-cell response may not be sufficiently down-regulated, and the consequences are chronic endothelial inflammation and damage. This may be a problem associated with many inflammatory disorders if they involve a chronic pathogen such as CMV, with which the antigen-specific T-cell response can result in chemokine induction and lead to chronic inflammation. Breaking the cycle of antigen presentation to the T cells by restricting virus replication may help to limit or even reverse the development of this aggressive T-cell response to CMV.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institute of Allergy and Infectious Diseases grants AI-41 089 and AI-36 214 (Virology Core, University of California, San Diego, Center for AIDS Research).
National Institutes of Health
Authorship
Contribution: C.A.B.-F. designed, performed, and analyzed experiments, collected and reviewed data, and prepared the manuscript; R.N.T. participated in performing experiments and collecting data; and S.A.S. designed the project, reviewed and analyzed data and experiments, and prepared and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen A. Spector, Department of Pediatrics, University of California San Diego, 9500 Gilman Dr, La Jolla, CA 92093-0672; e-mail: saspector@ucsd.edu.