The role of fibroblast growth factors (FGFs) in blood vessel formation has remained unclear. We used differentiating stem-cell cultures (embryoid bodies) and teratomas to show that FGF receptor-1 (FGFR-1) exerts a negative regulatory effect on endothelial cell function in these models. Embryoid bodies lacking expression of FGFR-1 as a result of gene targeting (Fgfr-1−/−) displayed increased vascularization and a distinct, elongated vessel morphology. Teratomas derived from FGFR-1–deficient stem cells were characterized by an increased growth rate and abundant, morphologically distinct vessels. Transmission electron microscopy of the Fgfr-1−/− teratomas showed a compact and voluminous but functional endothelium, which anastomosed with the host circulation. The increased vascularization and altered endothelial cell morphology was dependent on secreted factor(s), based on the transfer of the Fgfr-1−/− vascular phenotype by conditioned medium to Fgfr-1+/− embryoid bodies. Antibody and transcript arrays showed down-regulation of interleukin-4 (IL-4) and up-regulation of pleiotrophin in Fgfr-1−/− embryoid bodies, compared with the heterozygous cultures. We used neutralizing antibodies to show that IL-4 and pleiotrophin act as negative and positive angiogenic regulators, respectively. We conclude that FGFR-1 negatively regulates endothelial cell function by altering the balance of modulatory cytokines.
Introduction
Formation of new vessels from the preexisting vasculature, angiogenesis, involves migration, proliferation, and spatial organization of endothelial cells to shape the vascular tube. These coordinated events are regulated by angiogenic growth factors such as vascular endothelial growth factors (VEGFs) and fibroblast growth factors (FGFs). VEGF is regarded as the most specific angiogenic growth factor for endothelial cells.1 The first identified endothelial growth factor, FGF-2, is a potent stimulator of endothelial cell growth in vitro, but its role in endothelial cell function in vivo remains to be clarified.2 FGF-2 binds several members of the FGF receptor (FGFR-1, -2, -3, and -4) family, of which FGFR-1 is broadly expressed in the mesoderm during development.3 In vessels in teratomas and in different human tumor types, approximately 20% of the endothelial cells express FGFR-1.4
Inactivation of the mouse Fgfr-1 gene leads to embryonic death in conjunction with gastrulation between embryonic days (E) 7.5 to 9.5,3,5 primarily due to defects in patterning of the primitive streak. Using embryonic stem (ES) cells from these mice, the effects of FGFR-1 deficiency on the development of hematopoietic and endothelial cell lineages have been studied. Such cultures of differentiating embryonic stem cells, embryoid bodies, faithfully reproduce vasculogenesis and angiogenesis and allow the study of endothelial cells in a proper 3-dimensional context.6 Faloon et al showed that Fgfr-1−/− stem cells are defective in hematopoietic development.7 We extended these data to show that FGFR-1 expression indeed is required for development of hematopoietic cells but that it is dispensable for vasculogenesis and that loss of FGFR-1 expression is accompanied by increased vessel formation in Fgfr-1−/− embryoid bodies.4,8 Moreover, Fgfr-1−/− stem cells display characteristic changes in expression of different endothelial and hematopoietic markers, such as VEGFR-2 and CD41.4 Differentiating Fgfr-1−/− stem cells also lack expression of FGFR-4 and show delayed expression of FGFR-2 and -3.9 However, reintroduction of FGFR-1 into the Fgfr-1−/− ES cells leads to a complete rescue of the vascular phenotype.4
In this study, we describe a significantly increased growth rate and vessel formation in Fgfr-1−/− ES cell–derived teratomas compared with Fgfr-1+/− teratomas. Transmission electron microscopy (TEM) showed functional vessels with distinct morphologic features. In embryoid body cultures, the distinct Fgfr-1−/− vascular phenotype was dependent on secreted factor(s) as shown by transfer of the FGFR-1–deficient phenotype, such as vessel morphology and transcript regulation, by conditioned medium. Cytokine antibody arrays and microarray analyses of stem-cell genes showed changes in expression of several potential angiogenesis regulators in the Fgfr-1−/− cultures, such as reduced expression of interleukin-4 (IL-4) and increased expression of pleiotrophin (PTN). IL-4 has been shown to inhibit both VEGF- and FGF-induced angiogenesis in vitro10 and in vivo, leading to reduced growth of gliomas.11 PTN, also referred to heparin affin regulatory peptide (HARP) or heparin-binding growth-associated molecule (HB-GAM) is an 18-kDa secreted cytokine with high affinity for heparin, which is a potent regulator of tumor growth and vascularization.12,13 In agreement, neutralization of IL-4 led to enhanced angiogenesis, whereas neutralization of PTN suppressed angiogenesis, in the stem-cell cultures. We conclude that FGFR-1 regulates endothelial and hematopoietic development by modulating levels of angiogenic cytokines such as IL-4 and PTN.
Materials and methods
Embryonic stem-cell culture
J1 Fgfr-1+/− and Fgfr-1−/− embryonic stem (ES) cells were a kind gift from Dr Chuxia Deng, Mammalian Genetics Section, Genetics of Development and Disease Branch, National Institute of Diabetes and Digestive and Kidney Disease, National Institutes of Health (Bethesda, MD).3 The ES cells were cultured as described on growth-arrested murine embryonic fibroblast feeder cells in ES medium composed of Dulbecco modified Eagle medium/glutamax (Invitrogen, Carlsbad, CA) supplemented with 15% FBS, 25 mM HEPES, 1.2 mM Na-pyruvate, 19 μM monothioglycerol, and 1000 U/mL recombinant leukemia inhibitory factor (LIF; Chemicon International, Temecula, CA).8 Before differentiation, the ES cells were cultured for 1 to 2 passages on gelatin-coated tissue culture plastic to remove feeder cells. Differentiation of embryoid bodies started on day 0 when LIF was withdrawn from the medium. Formation of embryoid bodies was induced in hanging droplets. On day 4, the embryoid bodies were flushed down and plated on 8-well glass culture slides (Becton Dickinson Biosciences/Falcon [BD], Franklin Lakes, NJ) or on tissue culture plastic dishes. Cultures continued until day 8 when different analyses were performed. All analyses were performed on 4 or more embryoid bodies at 3 or more individual occasions.
Immunohistochemical staining
Peroxidase staining of embryoid bodies was performed as described.8 Embryoid bodies were fixed in zinc fix (0.1 M Tris HCl, pH 7.5, 3 mM calcium acetate, 23 mM zinc acetate, and 37 mM zinc chloride) overnight at 4°C. After peroxidase treatment and blocking in 3% BSA in Tris-buffered saline (TBS), samples were incubated overnight at 4°C with rat anti–mouse CD31/platelet-endothelial cell adhesion molecule (PECAM) antibody (BD) diluted in 3% BSA/TBS. This was followed by washes in TBS and incubation for 1 hour at room temperature with secondary biotinylated goat antirat antibody (Vector Laboratories, Burlingame, CA) diluted in 3% BSA/TBS and, finally, a 30-minute incubation with streptavidin-HRP (Vector Laboratories). Immune reactivity was visualized by the use of the chromogen AEC kit (Vector Laboratories).
Western blotting and immunofluorescence staining
Embryoid bodies were plated out on day 4 in plastic tissue culture dishes to allow attachment. On day 8 of culture in basal medium, cultures were lysed in 20 mM Tris HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 1% NP-40, 2 mM EDTA, 500 μM Na3VO4, 1% aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethyl sulfonylfluoride. For analysis of phosphorylated signal transduction molecules, cultures were kept for 16 hours in 0.2% fatty acid–free bovine serum albumin (BSA; Sigma, Chicago, IL) in ES medium without LIF. When indicated, embryoid bodies were treated with the phosphatidyl inositide 3 kinase (PI3K) inhibitor LY294002 (10 μM; Sigma) for 30 minutes. Cell lysates were centrifuged for 15 minutes at 4°C, and the protein concentration of the supernatant was measured using the BCA protein detection kit (Pierce, Rockford, IL). Total cell lysates was separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) using 7% or 10% gels and transferred to Hybond C-Extra nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). The membranes were blocked in TBS/0.1% Tween 20 containing 3% BSA for 3 hours and then incubated with rabbit anti–mouse pAkt and Akt (Cell Signaling Technology, Danvers, MA), or rabbit anti-pErk1/2 or Erk1/2 (Cell Signaling Technology), overnight at 4°C. After vigorous washing with TBS/0.1% Tween 20, the membrane was incubated with peroxidase-conjugated anti-rabbit antibodies (Amersham Biosciences). Immune reactive bands were visualized using the enhanced chemiluminescence (ECL) Western blotting detection reagent (Amersham Biosciences). Quantification of blots was done using Image Gauge version 3.3 (FUJI Film Photo, Allendale, NJ). Embryoid bodies immunostained for pAkt expression were fixed on ice in acetone for 5 minutes. After blocking in 2% goat serum/PBS with 0.1 mM Na3VO4 on ice for 2 hours, incubation with rabbit anti–mouse pAkt (no. 9277; Cell Signaling Technology) and rat anti–mouse CD31 (BD) antibodies was done at 4°C overnight. Samples were incubated with secondary antibodies goat anti–rabbit 488 Alexa and goat anti–rat 568 Alexa (Molecular Probes, Eugene, OR) diluted in 2% goat serum/PBS for 1 hour at room temperature.
Preparation, sectioning, and staining of teratomas
J1 Fgfr-1+/− and Fgfr-1−/− ES cells (8 × 106/cell type and animal) were injected subcutaneously on the flank of female NMRI-nu mice (n = 5/group; M&B Taconic, Copenhagen, Denmark) and teratomas were grown for 27 to 45 days. Animal handling was performed with ethical permission approved by the Uppsala University ethics committee and according to the United Kingdom Coordinating Committee on Cancer Research guidelines for the welfare of animals in experimental neoplasia.14 Acetone-fixed, frozen, 6-μm sections were first blocked in 3% BSA in phosphate-buffered saline (PBS) and then immunostained with rat anti–mouse CD31 (BD) for 1 hour at room temperature followed by goat anti–rat Alexa 568 (Molecular Probes) in 3% BSA/PBS. Staining of nuclei was done by incubation in Hoechst 33342 (1.0 μg/mL) in PBS. Quantification of the area of CD31 staining was done on 4 individual sections per teratoma, using the Easy Image Analysis software (Rainfall, Stockholm, Sweden), and compensation for background was performed to avoid quantification of unspecific staining. Statistical analysis (t test) was done using the Stat View program (Eurodex, Stockholm, Sweden).
Microscopy
Immunohistochemical and immunofluorescence microscopy.
Immunohistochemical samples were mounted with Ultramount aqueous mounting medium (Dako, Glostrup, Denmark), and immunofluorescence samples were mounted with Fluoromount-G mounting medium (Southern Biotechnology, Birmingham, AL). Results were analyzed using a Nikon Eclipse E1000 microscope (Nikon, Tokyo, Japan; Figures 1,2,4,Figure 5,6) or an LSM 510 META confocal laser-scanning microscope with an inverted microscope (Carl Zeiss International, Oberkochen, Germany; Figures 1,3,5). The following objectives were used: Nikon Plan Apochromat 2×/0.1, 4×/0.2, 10×/0.45, 20×/0.75, 40×/0.95, and 60×/1.4 oil immersion; Zeiss confocal Plan Neofluar 40×/1.3 oil immersion 2 and Plan Apochromat 63×/1.4 oil immersion. Microphotographs were captured using a Nikon Eclipse DXM 1200 camera. The following software programs were used: Laser Scanning Microscope LSM 510 version 3.2 (Carl Zeiss International) and Easy Image 2000 Analysis version 2.7.4.03 (TeknoOptik, Stockholm, Sweden). Processing of microphotographs was done using Adobe Photoshop version CS2 (Adobe Systems, San Jose, CA).
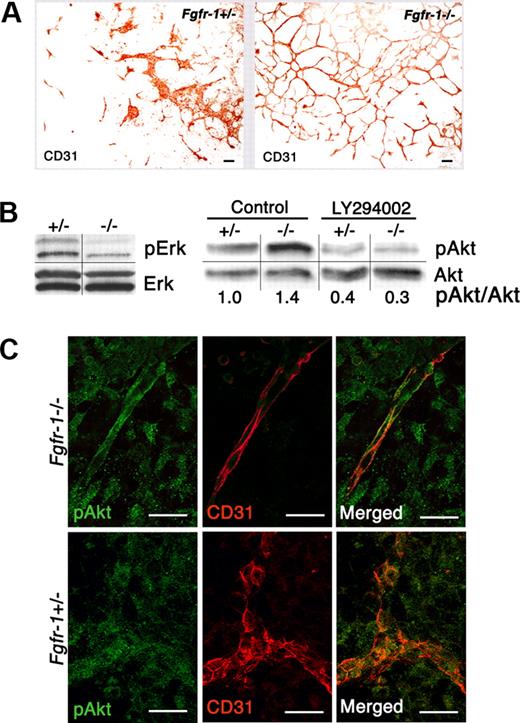
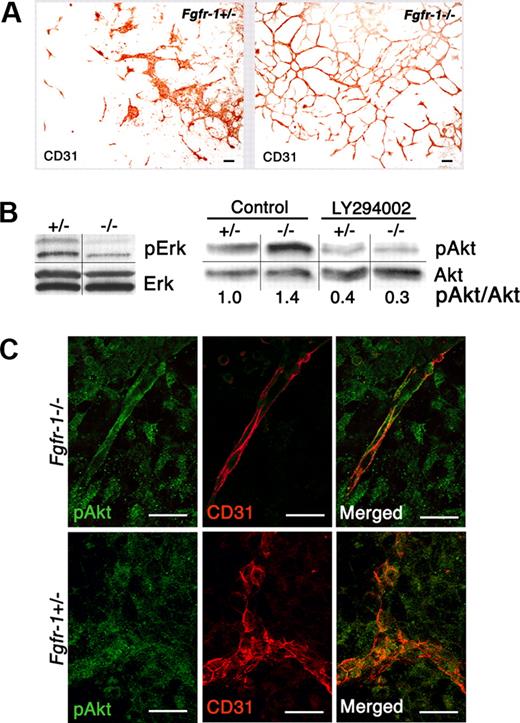
Changes in vascularization and signal transduction in Fgfr-1−/− embryoid bodies. (A) Increased vessel formation in the absence of exogenous growth factors in Fgfr-1−/− embryoid bodies compared with Fgfr-1+/−. Bars represent 100 μm. (B) Analysis of serine/threonine kinases Erk and Akt and their phosphorylated counterparts by immunoblotting. Phosphorylation of Akt was evaluated in the absence (control) and presence (LY294002) of the PI3K inhibitor (10 μM) in Fgfr-1+/− and Fgfr-1−/− embryoid bodies on day 8 of differentiation. Quantification shows fold induction of phosphorylated Akt/Akt protein (pAkt/Akt) in relation to pAkt/Akt in the +/− control (set to 1). The figure shows results representative of several individual experiments. Vertical lines indicate gel lanes repositioned from the same blot, whereas horizontal lines mark the boundary between consecutive blots for pErk/Erk and pAkt/Akt, respectively. (C) Immunofluorescence staining of pAkt (green) and CD31 (red) in Fgfr-1−/− and Fgfr-1+/− embryoid bodies at day 8. Bars represent 20 μm.
Changes in vascularization and signal transduction in Fgfr-1−/− embryoid bodies. (A) Increased vessel formation in the absence of exogenous growth factors in Fgfr-1−/− embryoid bodies compared with Fgfr-1+/−. Bars represent 100 μm. (B) Analysis of serine/threonine kinases Erk and Akt and their phosphorylated counterparts by immunoblotting. Phosphorylation of Akt was evaluated in the absence (control) and presence (LY294002) of the PI3K inhibitor (10 μM) in Fgfr-1+/− and Fgfr-1−/− embryoid bodies on day 8 of differentiation. Quantification shows fold induction of phosphorylated Akt/Akt protein (pAkt/Akt) in relation to pAkt/Akt in the +/− control (set to 1). The figure shows results representative of several individual experiments. Vertical lines indicate gel lanes repositioned from the same blot, whereas horizontal lines mark the boundary between consecutive blots for pErk/Erk and pAkt/Akt, respectively. (C) Immunofluorescence staining of pAkt (green) and CD31 (red) in Fgfr-1−/− and Fgfr-1+/− embryoid bodies at day 8. Bars represent 20 μm.
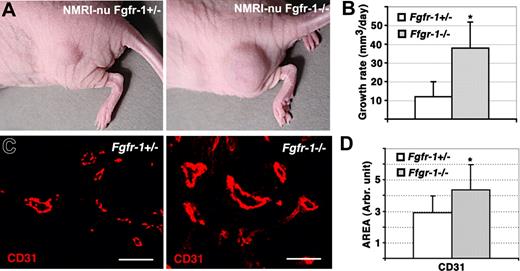
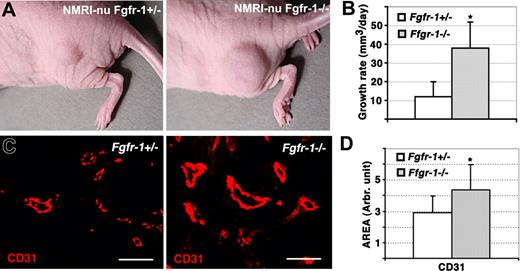
Increased tumor growth and vascularization of Fgfr-1−/− teratomas. (A) Growth of Fgfr-1+/− and Fgfr-1−/− teratomas in NMRI-nu mice. (B) The volume of the teratomas was determined regularly by measuring with a caliper, allowing estimation of the teratoma growth rate (mm3/day). The difference in growth rate between Fgfr-1+/− and Fgfr-1−/− teratomas was statistically different; *P = .007. (C) Staining of teratoma sections for CD31 expression (red). Bars represent 50 μm. (D) Quantification of the CD31+ area in Fgfr-1+/− and Fgfr-1−/− teratomas. The CD31+ area was significantly increased (*P = .002) in the Fgfr-1−/− teratomas.
Increased tumor growth and vascularization of Fgfr-1−/− teratomas. (A) Growth of Fgfr-1+/− and Fgfr-1−/− teratomas in NMRI-nu mice. (B) The volume of the teratomas was determined regularly by measuring with a caliper, allowing estimation of the teratoma growth rate (mm3/day). The difference in growth rate between Fgfr-1+/− and Fgfr-1−/− teratomas was statistically different; *P = .007. (C) Staining of teratoma sections for CD31 expression (red). Bars represent 50 μm. (D) Quantification of the CD31+ area in Fgfr-1+/− and Fgfr-1−/− teratomas. The CD31+ area was significantly increased (*P = .002) in the Fgfr-1−/− teratomas.
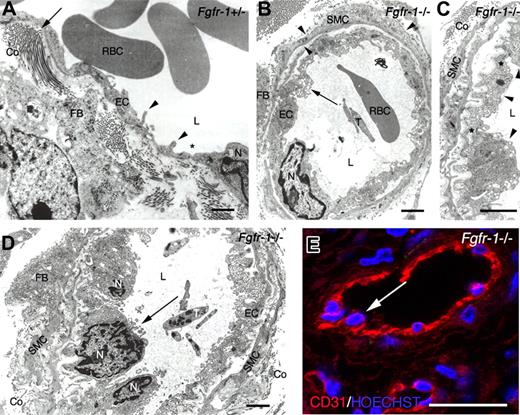
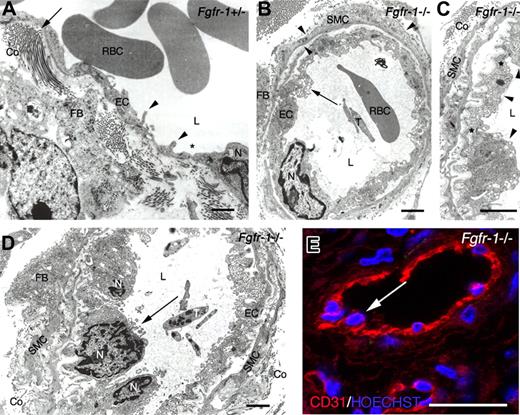
Ultrastructural analysis of teratoma tissue. (A) TEM on a representative Fgfr-1+/− teratoma micrograph shows elongated, flattened endothelial cells with cytoplasmic infoldings (◀) and occasional fenestrations (∗). Endothelial basement membrane and perivascular smooth muscle cell coat was scant, but fibroblasts producing collagen (←) were identified in the perivascular area. (B) A representative Fgfr-1−/− micrograph shows a vessel with endothelium resting on an endothelial basement membrane and surrounded by smooth muscle cells invested by basement membrane (◀). ← indicates abundant transport vesicles in protruding endothelial cell cytoplasm. (C) Higher magnification of vessel in panel B, showing increase in endothelial cell surface by basal invaginations covered by basement membrane and luminal protrusions. Tight junctions (∗) indicate the presence of 3 endothelial cells (◀) in this micrograph. Perpendicularly cut collagen is seen outside the smooth muscle cell layer. (D) Fgfr-1−/− micrograph shows several adjacent, protruding endothelial cells (←). (E) Confocal analysis of immunofluorescent CD31 staining (red) shows similar image as in panel D with protruding endothelial cell nuclei (←) in Fgfr-1−/− teratoma. Nuclei were visualized by Hoechst 33342 staining (blue). RBCs indicates red blood cells; L, vascular lumen; EC, endothelial cell; N, endothelial cell nucleus; FB, fibroblast; T, thrombocyte; Co, collagen; and SMC, smooth muscle cell. Original magnifications ×12 500 (A,B,D) and ×20 000 (C). Bars represent 500 nm (A-D) and 30 μm (E).
Ultrastructural analysis of teratoma tissue. (A) TEM on a representative Fgfr-1+/− teratoma micrograph shows elongated, flattened endothelial cells with cytoplasmic infoldings (◀) and occasional fenestrations (∗). Endothelial basement membrane and perivascular smooth muscle cell coat was scant, but fibroblasts producing collagen (←) were identified in the perivascular area. (B) A representative Fgfr-1−/− micrograph shows a vessel with endothelium resting on an endothelial basement membrane and surrounded by smooth muscle cells invested by basement membrane (◀). ← indicates abundant transport vesicles in protruding endothelial cell cytoplasm. (C) Higher magnification of vessel in panel B, showing increase in endothelial cell surface by basal invaginations covered by basement membrane and luminal protrusions. Tight junctions (∗) indicate the presence of 3 endothelial cells (◀) in this micrograph. Perpendicularly cut collagen is seen outside the smooth muscle cell layer. (D) Fgfr-1−/− micrograph shows several adjacent, protruding endothelial cells (←). (E) Confocal analysis of immunofluorescent CD31 staining (red) shows similar image as in panel D with protruding endothelial cell nuclei (←) in Fgfr-1−/− teratoma. Nuclei were visualized by Hoechst 33342 staining (blue). RBCs indicates red blood cells; L, vascular lumen; EC, endothelial cell; N, endothelial cell nucleus; FB, fibroblast; T, thrombocyte; Co, collagen; and SMC, smooth muscle cell. Original magnifications ×12 500 (A,B,D) and ×20 000 (C). Bars represent 500 nm (A-D) and 30 μm (E).
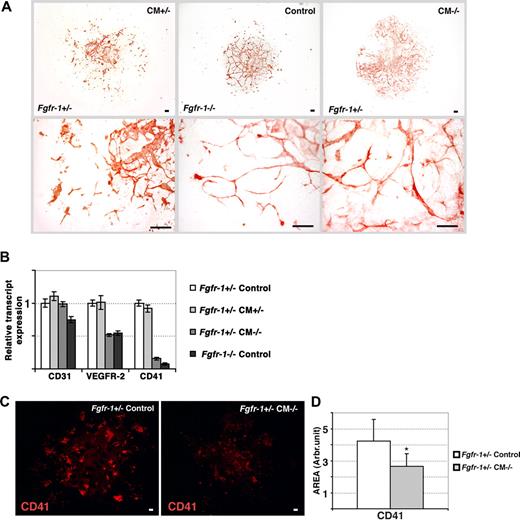
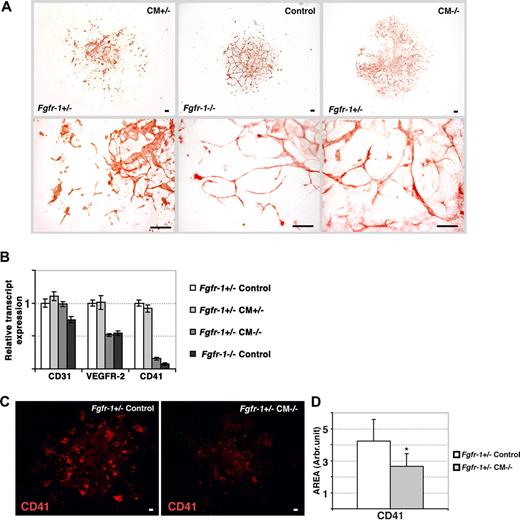
Transfer of the Fgfr-1−/− phenotype to Fgfr+/− embryoid bodies by conditioned medium. (A) Immunohistochemical staining for CD31 expression in Fgfr-1+/− embryoid bodies kept in Fgfr-1+/−–conditioned medium (CM+/−), Fgfr-1−/− embryoid bodies in basal medium (control), and Fgfr-1+/− embryoid bodies in Fgfr-1−/−–conditioned medium (CM−/−). Note the change in vessel morphology in Fgfr-1+/− embryoid bodies in CM−/−, mimicking that in Fgfr-1−/− embryoid bodies. Bottom row of panels shows representative magnifications of the top panels. Bars represent 100 μm. (B) Real-time PCR analyses of Cd31, Vegfr2, and Cd41 transcript levels in embryoid bodies cultured as indicated. (C) Immunostaining for CD41 in Fgfr-1+/− embryoid bodies kept in basal medium (control) or in CM−/−. Bars represent 100 μm. (D) Quantification of CD41+ area showed significantly lower expression of CD41 in Fgfr-1+/− embryoid bodies cultured in CM−/− compared with the regular culture medium (control). *P = .005.
Transfer of the Fgfr-1−/− phenotype to Fgfr+/− embryoid bodies by conditioned medium. (A) Immunohistochemical staining for CD31 expression in Fgfr-1+/− embryoid bodies kept in Fgfr-1+/−–conditioned medium (CM+/−), Fgfr-1−/− embryoid bodies in basal medium (control), and Fgfr-1+/− embryoid bodies in Fgfr-1−/−–conditioned medium (CM−/−). Note the change in vessel morphology in Fgfr-1+/− embryoid bodies in CM−/−, mimicking that in Fgfr-1−/− embryoid bodies. Bottom row of panels shows representative magnifications of the top panels. Bars represent 100 μm. (B) Real-time PCR analyses of Cd31, Vegfr2, and Cd41 transcript levels in embryoid bodies cultured as indicated. (C) Immunostaining for CD41 in Fgfr-1+/− embryoid bodies kept in basal medium (control) or in CM−/−. Bars represent 100 μm. (D) Quantification of CD41+ area showed significantly lower expression of CD41 in Fgfr-1+/− embryoid bodies cultured in CM−/− compared with the regular culture medium (control). *P = .005.
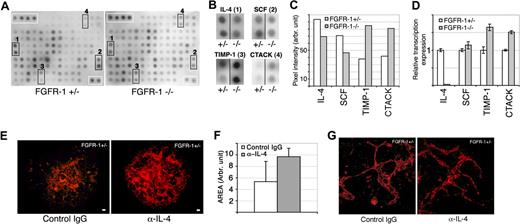
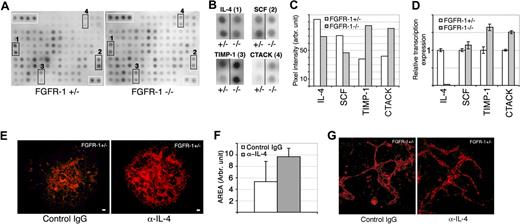
Cytokine antibody arrays show decreased IL-4 expression in Fgfr-1−/− embryoid bodies. (A) Filters spotted in duplicate with antibodies against 62 different cytokines were incubated with conditioned medium from Fgfr-1+/− or Fgfr-1−/− embryoid bodies. Immunoreactivity was visualized by ECL. Positive control protein spots are indicated by white boxes in the upper left corner of each filter. Specific changes in antibody reactivity are boxed (marked 1-4). (B) Enlargement of marked filter areas (1-4) in panel A, showing spots with reduced or increased cytokine levels in conditioned media (CM−/− compared with CM+/−). (C) Quantification of average pixel intensity of duplicate spots for each cytokine, IL-4, SCF, TIMP-1, and CTACK, in CM−/− and CM+/−. (D) Real-time PCR analysis of candidate cytokines in Fgfr-1+/− and Fgfr-1−/− embryoid bodies at 4 days of differentiation. (E) Induced vessel formation shown by VEGFR-2 immunostaining (red) in Fgfr-1−/− embryoid bodies as a result of treatment with neutralizing anti–IL-4 antibodies (5 μg/mL) from day 0 to day 4 compared with control isotype-matched IgG treatment. Bars represent 100 μm. (F) Quantification of Fgfr-1+/− embryoid body vascularization in response to treatment with control IgG or anti–IL-4 antibody. (G) Higher magnification of vessels treated with IgG or anti–IL-4 antibody. Bars represent 50 μm.
Cytokine antibody arrays show decreased IL-4 expression in Fgfr-1−/− embryoid bodies. (A) Filters spotted in duplicate with antibodies against 62 different cytokines were incubated with conditioned medium from Fgfr-1+/− or Fgfr-1−/− embryoid bodies. Immunoreactivity was visualized by ECL. Positive control protein spots are indicated by white boxes in the upper left corner of each filter. Specific changes in antibody reactivity are boxed (marked 1-4). (B) Enlargement of marked filter areas (1-4) in panel A, showing spots with reduced or increased cytokine levels in conditioned media (CM−/− compared with CM+/−). (C) Quantification of average pixel intensity of duplicate spots for each cytokine, IL-4, SCF, TIMP-1, and CTACK, in CM−/− and CM+/−. (D) Real-time PCR analysis of candidate cytokines in Fgfr-1+/− and Fgfr-1−/− embryoid bodies at 4 days of differentiation. (E) Induced vessel formation shown by VEGFR-2 immunostaining (red) in Fgfr-1−/− embryoid bodies as a result of treatment with neutralizing anti–IL-4 antibodies (5 μg/mL) from day 0 to day 4 compared with control isotype-matched IgG treatment. Bars represent 100 μm. (F) Quantification of Fgfr-1+/− embryoid body vascularization in response to treatment with control IgG or anti–IL-4 antibody. (G) Higher magnification of vessels treated with IgG or anti–IL-4 antibody. Bars represent 50 μm.
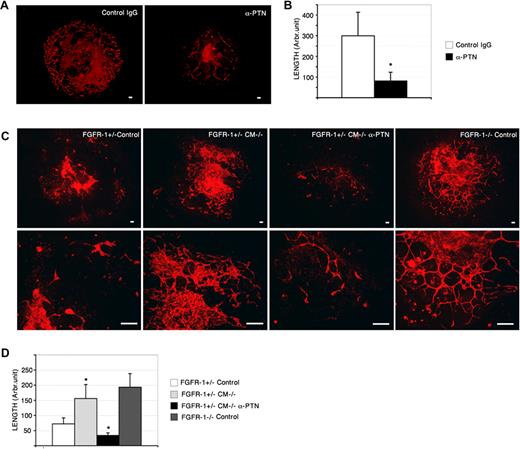
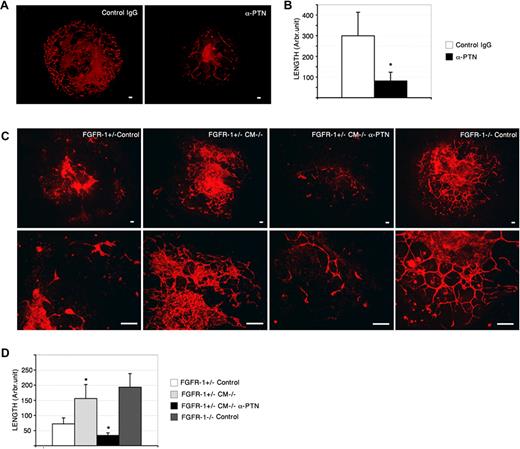
PTN neutralization attenuates Fgfr-1−/−–mediated vascularization. (A) Fgfr-1−/− embryoid bodies treated with PTN neutralizing antibodies (anti-PTN, 3 μg/mL) from day 4 to day 8 showed reduced CD31 immunostaining (red) compared with control IgG treatment (control IgG). Bars represent 100 μm. (B) Quantification of CD31+ vessel length in Fgfr-1−/− embryoid bodies treated with anti-PTN or control IgG. *P < .001. (C) CD31 immunoreactivity (red) in Fgfr-1+/− or Fgfr-1−/− embryoid bodies kept in basal medium (control) or treated with CM−/− with and without anti-PTN antibodies (3 μg/mL) as indicated from day 4 to day 8. Bars represent 100 μm. Lower panels show higher magnification. Bars represent 100 μm. (D) Quantification of CD31+ length in the embryoid body cultures (n = 6-8 for each condition) kept in the different conditions in panel C. Statistical analysis of the different conditions (FGFR-1+/− CM−/− compared with FGFR-1+/− Control, and FGFR-1+/− CM−/− α-PTN compared with FGFR-1+/− CM−/−) showed significant differences (*P < .028).
PTN neutralization attenuates Fgfr-1−/−–mediated vascularization. (A) Fgfr-1−/− embryoid bodies treated with PTN neutralizing antibodies (anti-PTN, 3 μg/mL) from day 4 to day 8 showed reduced CD31 immunostaining (red) compared with control IgG treatment (control IgG). Bars represent 100 μm. (B) Quantification of CD31+ vessel length in Fgfr-1−/− embryoid bodies treated with anti-PTN or control IgG. *P < .001. (C) CD31 immunoreactivity (red) in Fgfr-1+/− or Fgfr-1−/− embryoid bodies kept in basal medium (control) or treated with CM−/− with and without anti-PTN antibodies (3 μg/mL) as indicated from day 4 to day 8. Bars represent 100 μm. Lower panels show higher magnification. Bars represent 100 μm. (D) Quantification of CD31+ length in the embryoid body cultures (n = 6-8 for each condition) kept in the different conditions in panel C. Statistical analysis of the different conditions (FGFR-1+/− CM−/− compared with FGFR-1+/− Control, and FGFR-1+/− CM−/− α-PTN compared with FGFR-1+/− CM−/−) showed significant differences (*P < .028).
Transmission electron microscopy (TEM).
At dissection of teratomas, small (1 × 1 × 1 mm) samples were immediately transferred into 2% glutaraldehyde (Agar Scientific, Stansted, United Kingdom) in 0.1 M sodium cacodylate buffer, pH 7.2, supplemented with 0.1 M sucrose, and incubated for 6 hours. The primary fixation was followed by 1.5 hours of postfixation in 1% osmium tetroxide (Agar Scientific) in the cacodylate buffer, dehydration in graded series of ethanol, and embedding in epoxy plastic Agar 100 (Agar Scientific). Ultrathin sections were cut and placed on polyvinyl formal plastic (Formvar; Agar Scientific)–coated copper grids and contrasted with uranyl acetate and lead citrate before analyzing in a Hitachi H-1700 electron microscope (Hitachi, Tokyo, Japan).
Culture and analysis of embryoid bodies in conditioned medium
Differentiation of Fgfr-1+/− ES cells in hanging drops was initiated in medium conditioned by parallel Fgfr-1+/− or Fgfr-1−/− embryoid body cultures, collected between day 0 (withdrawal of LIF) and day 2 (ie, 48 hours). Conditioned medium from Fgfr-1+/− embryoid bodies will henceforth be indicated as CM+/−, whereas conditioned medium from Fgfr-1−/− embryoid bodies will be denoted CM−/−. The medium was carefully centrifuged to ensure that no contaminating cells were transferred to the conditioned cultures. On day 4, the embryoid bodies were flushed down and plated on glass culture slides or plastic Petri dishes with fresh conditioned medium from 4-day-old, parallel Fgfr-1+/− or Fgfr-1−/− embryoid body cultures. Fresh conditioned medium was added to the test cultures on days 5 and 7. The embryoid bodies were examined by immunostaining or by real-time polymerase chain reaction (PCR) on day 8. Fluorescence staining was performed on zinc-fixed embryoid bodies, blocked for 1 hour in 3% BSA/TBS. Rat anti–mouse CD41 (BD) antibody in 3% BSA/TBS was incubated overnight at 4°C. After washes in TBS, secondary goat anti–rat Alexa 568 antibody in 3% BSA/TBS was incubated for 1 hour at room temperature. Quantification of the area of CD41 staining was performed with Easy Image Analysis software (Rainfall), and compensation for background was performed to avoid quantification of unspecific staining. Statistical analysis (t test) was done using the Stat View program.
Cytokine antibody array
Conditioned medium from Fgfr-1+/− or Fgfr-1−/− embryoid bodies, cultured between days 4 to 8, was incubated overnight at 4°C on ChemiArray Mouse Antibody Array III Map (no. AA1003M; Chemicon International) containing antibodies against 62 different cytokines. Detection of protein signals was done according to the manufacturer's instructions, except that ECL Plus (Amersham Biosciences) was used in the final exposure step. Quantification of blots was done using Image Gauge version 3.3 (FUJI Film Photo). Every spot was marked separately and close to every single spot, the respective background was marked and quantified. The background was withdrawn from each spot before comparison between the 2 groups. Two different exposure times of the blots were quantified, one longer and one shorter. Spots were quantified using the appropriate exposure, that is, where the control spots (marked with white squares in Figure 4A) were of similar intensity between the Fgfr-1+/− and Fgfr-1−/− membranes. All antibodies were spotted in duplicate on each membrane. When duplicates differed, data were excluded from the evaluation.
Real-time PCR analysis
Total RNA was extracted from day-8 Fgfr-1+/− and Fgfr-1−/− embryoid bodies. Contaminating genomic DNA was digested with Dnase I (Amersham Biosciences), and 1 μg total RNA was used for first-strand cDNA synthesis using oligo dT primer and the Advantage RT-for-PCR-Kit (Clontech, Mountain View, CA). β-Actin was used as an endogenous reference and non–reverse-transcribed RNA was used as a negative control. The PCR samples, containing cDNA, primers (0.25 μM final concentration), and 2 × SYBR Green PCR master mix (Applied Biosystems, Foster City, CA), were run in triplicate on an ABI Prism 7700 Sequence Detection System instrument (Applied Biosystems) with an initial 10-minute activation at 95°C, followed by 45 cycles at 95°C for 15 seconds and 60°C for 1 minute. Primer sequences were as follows: Il4 (accession number: NM 021283) CTCATGGAGCTGCAGAGACTCTT (5′-3′ sense), CATTCATGGTGCAGCTTATCGA (5′-3′ antisense); Scf (accession number: M59915) TGTGATGAAGGACGAAAAGGAA (5′-3′ sense), GGCCTGAACAAACAATCTCTC (5′-3′ antisense); Timp1 (accession number: NM 011593) CCTCGTGGGCTCTGAGGAC (5′-3′ sense), AGCCTGGATTCCGTGGC (5′-3′ antisense); Ctack (accession number: NM 011336) TCGGCGCAGTGTCTGTGT (5′-3′ sense), TCCCTTGGCGTTCTAACCAC (5′-3′ antisense); and βactin (accession number XD3765) CACTATTGGCAACGAGCG (5′-3′ sense), TCCATACCCAAGAAGGAAGG (5′-3′ antisense). The threshold cycle (CT) value was calculated for each sample by the ABI Prism 7700 instrument. Transcript levels were then normalized against βactin transcript levels and changes were expressed as relative values.
IL-4 neutralizing antibodies
Fgfr-1−/− embryoid bodies were cultured in the presence of either of 2 neutralizing rabbit anti–mouse IL-4 antibodies (5 μg/mL, no. AB1451 [Chemicon International] and no. 500-M04 [Peprotech, Rocky Hill, NJ]) or control IgG antibodies from day 0 to day 4 of differentiation. The concentration of the anti–IL-4 antibodies was chosen by titration using 1, 3, or 5 μg/mL where 3 μg/mL showed partial and 5 μg/mL, full effect. On day 8, the embryoid bodies were washed once in PBS, fixed in ice-cold acetone for 5 minutes, and blocked in 3% BSA in PBS for 1 hour at room temperature. The primary goat anti–mouse VEGFR-2 (R&D, Minneapolis, MN) antibody was diluted in 3% BSA/PBS and incubated on the membrane overnight at 4°C. Incubation with the secondary antibody donkey anti–goat Alexa 594 (Molecular Probes) diluted in 3% BSA/PBS was for 1 hour at room temperature.
Stem-cell microarray analysis
Total RNA was extracted from day-8 Fgfr-1+/− and Fgfr-1−/− embryoid bodies, and quantity and quality were determined using a Bioanalyser equipped with a RNA 600 Nano Chip (Agilent Technologies, Life Sciences and Chemical Analysis, Foster City, CA). The Promega Pronto! Hybridization kit (Promega/Corning, South San Francisco, CA) was used according to the manufacturer's instructions, and cDNA was fluorescently labeled by Cy3 dCTP or Cy5 dCTP (Amersham Biosciences). A stem-cell cDNA gene array STEM 4.1 (Kungliga Tekniska Högskolan, Royal School of Technology, Stockholm, Sweden; http://www.ktharray.se) was used and each slide (n = 5) was incubated with Cy3- or Cy5-labeled Fgfr-1+/− and Fgfr-1−/− cDNA (30 pmol/each slide). The arrays contain 20 000 probes, representing approximately 16 000 unique genes. Analysis of hybridized cDNA was performed with the use of Axon 4000B scanner and Gene Pix Pro 4.0 software (Molecular Devices, Sunnyvale, CA) with a ratio of 1 for photomultiplier tube setting.
Bioinformatic analysis
Array data were analyzed using software within the Linneus Center of Bioinformatics Data warehouse (Uppsala University, Uppsala, Sweden; http://www.lcb.uu.se/lcbdw.php),15 a system built from the BASE platform for microarray data management.16 Following background correction, spot signals were extracted from the median pixel intensities and data were normalized using the print-tip loess algorithm.17 Spots flagged as “bad” or “not found” by the Gene Pix Pro 4.0 software (flag value −50 or −100) were filtered from the data, and intensities were merged for reporters printed multiple times on each array. Genes with signal to noise ratio higher than 2 were kept, and the geometric mean was calculated from 5 arrays (including dye-swap experiments). The mean log2 ratios were calculated from replicate arrays (dye-swap experiments) to obtain one single expression level for each reporter. Gene filtering was done to keep reporters in at least 3 of 5 arrays. B-statistics was performed according to parametric empiric Bayes approach to distinguish differentially expressed genes (cutoff higher than 3).18
Neutralizing PTN antibodies
Fgfr-1−/− embryoid bodies were cultured in the presence of neutralizing rabbit anti–human PTN antibody (3 μg/mL, no. PC187; Merck Biosciences, Darmstadt, Germany) from day 4 to day 8 of differentiation. Treatment of Fgfr-1+/− with CM−/− was performed as described above and with addition of anti-PTN antibodies (3 μg/mL; Merck Biosciences) from day 4 to day 8. On day 8, embryoid bodies were prepared for immunofluorescence staining of vessels by rat anti–mouse CD31 (BD) followed by goat anti–rat Alexa 568 (Molecular Probes). Analysis was performed on 6 to 8 embryoid bodies at 3 or more individual occasions. Quantification of the length of CD31 staining was performed with Easy Image Analysis software (Rainfall), and compensation for background was performed to avoid quantification of unspecific staining. Statistical analysis (t test) was done using the Stat View program.
Results
Altered endothelial cell signal transduction in embryoid bodies lacking expression of FGFR-1
We have previously shown that Fgfr-1−/− embryoid bodies develop a more abundant vascular plexus, compared with embryoid bodies derived from FGFR-1 heterozygous (Fgfr-1+/− stem cells (Figure 1A) or wild-type stem cells.4,8 Activation of serine/threonine kinases Akt and Erk1/2 is known to lead to transduction of signals for survival and proliferation, respectively. Since expression of FGFR-1 in endothelial cells has been associated with increased mitogenicity (Magnusson et al4 and references therein), we analyzed the state of activation of these signaling molecules. Western blotting showed lower levels of pErk1/2 in Fgfr-1−/− compared with Fgfr-1+/− embryoid bodies (Figure 1B). Immunoblotting for pAkt showed slightly elevated levels in the Fgfr-1−/− cells, which were attenuated by treatment with the PI3K inhibitor LY294002, indicating induction of a PI3K signaling pathway. Immunofluorescent analysis showed pAkt immunostaining in CD31+ vessel structures in the Fgfr-1−/− embryoid bodies as well as Fgfr-1+/− embryoid bodies (Figure 1C), but with a clearly distinct morphology of endothelial cells in the FGFR-1–deficient cells. Therefore, survival rather than proliferation of FGFR-1–deficient endothelial cells may contribute to the vascular abundance in the Fgfr-1−/− stem-cell cultures.
Enhanced growth and vascularization of teratomas from embryonic stem cells lacking FGFR-1 expression
To determine whether FGFR-1 deficiency would affect vascularization of tissues in vivo, embryonic stem cells heterozygous or homozygous null for FGFR-1 were inoculated to grow as teratomas in nude mice. It has been shown previously that Fgfr-1−/− teratomas develop normally with regard to representation of cells from the 3 different germ layers.3 The growth rate of the teratomas over the period of one month was increased 4-fold in the absence of FGFR-1 compared with Fgfr-1+/− teratomas (Figure 2A,B). We have previously shown that the in vitro growth rates of Fgfr-1+/− and Fgfr-1−/− ES cells do not differ,4 indicating that the difference in teratoma growth rates may be due to changes in vascularization. Sectioning of the tumors and analysis by staining for expression of CD31 indeed revealed significantly increased vascularization of the Fgfr-1−/− teratomas (Figure 2C; see Figure 2D for quantification). Notably, vessels in the Fgfr-1−/− teratomas became more intensely labeled compared with vessels in the Fgfr-1+/− teratomas.
TEM analysis of teratoma vessel morphology
Ultrastructural analysis (TEM) of Fgfr-1+/− teratomas showed vessels coated by flattened and elongated endothelial cells with occasional fenestration. These vessels often lacked both endothelial basement membrane and pericyte/smooth muscle cell coating (Figure 3A). The perivascular area appeared translucent with collagen in close approximation, or even anchored to, the endothelial cells. In the Fgfr-1−/− teratomas, endothelial cells frequently displayed a plasma membrane profile with multiple invaginations, protrusions, and abundant cytoplasmic transport vesicles (Figure 3B). This increase in cytoplasmic volume corresponds to the “thick” endothelium in the immunofluorescent staining shown in Figure 2C. The Fgfr-1−/− teratoma vessels were covered with an intact basement membrane and 1 or 2 layers of smooth muscle cells. Inspection at higher magnification (Figure 3C) showed a compact organization of endothelial cells that occasionally appeared to overlap (Figure 3C arrowheads), rather than forming a single layer of endothelial cells around the vessel circumference. Moreover, nuclei of such crowded endothelial cells protruded into the vascular lumen as detected by both TEM (Figure 3D) and confocal analysis (Figure 3E) of the Fgfr-1−/− teratomas. Clearly, vessels in both types of teratomas anastomosed with the host circulation as judged from the presence of erythrocytes and thrombocytes in the vessels. These data show that FGFR-1 deficiency led to abundant vascularization in teratomas and to atypical vessel features, such as thickening of the endothelium due to an increased number of endothelial cells. However, the vessels were functional and may have contributed to the increased growth rate of the Fgfr-1−/− teratomas.
Transfer of angiogenic phenotype by conditioned medium
We sought to identify the molecular mechanisms underlying the enhanced vascularization and distinct vessel morphology of Fgfr-1−/− embryoid bodies and teratomas. For this purpose, we examined medium conditioned by the Fgfr-1−/− cultures, for transfer of the endothelial phenotype to the Fgfr-1+/− embryoid bodies. Medium conditioned for 24 to 48 hours by the Fgfr-1−/− embryoid bodies (denoted CM−/−) was harvested on days 2, 4, 5, and 7 of culture and transferred to Fgfr-1+/− embryoid bodies cultured in parallel. As shown in Figure 4A, addition of CM−/− induced a change in vessel morphology in the Fgfr-1+/− embryoid bodies, mimicking that of the Fgfr-1−/− embryoid bodies. As a control, medium conditioned by Fgfr-1+/− embryoid bodies (denoted CM+/−) was transferred to parallel Fgfr-1+/− cultures, which, however, did not affect the morphology of the vasculature (Figure 4A).
FGFR-1 deficiency is associated with altered levels of expression of VEGFR-2 and CD41, markers of endothelial and hematopoietic development, respectively.4 We analyzed whether the switch in vessel morphology in CM−/−-treated Fgfr-1+/− embryoid bodies was accompanied by regulation of VEGFR-2 and CD41. As shown in Figure 4B, incubation of Fgfr-1+/− embryoid bodies with CM−/− induced a 50% reduction in Vefgr2 transcript levels and a close to complete disappearance of Cd41. In contrast, expression of Cd31 was not affected. The Vegfr2 and Cd41 transcript levels in CM−/−-treated Fgfr-1+/− embryoid bodies mirrored those in Fgfr-1−/− embryoid bodies (Figure 4B). In contrast, transfer of CM+/− to parallel Fgfr-1+/− cultures did not affect the expression levels of VEGFR-2 or CD41, arguing for a specific effect of the CM−/−.
The reduction in Cd41 transcript levels by incubation of Fgfr-1+/− embryoid bodies in CM−/− was accompanied by a corresponding decrease in CD41 protein levels (Figure 4C; for quantification see Figure 4D). This is in agreement with our previous data showing that FGFR-1–deficient embryonic stem cells fail to undergo hematopoietic development.4 Our present data indicate this block in hematopoietic development is dependent on a soluble factor transferred by conditioned medium.
IL-4 is absent in Fgfr-1−/−–conditioned medium
To identify factors transferred by conditioned medium, we used a cytokine antibody array containing antibodies against 62 different cytokines and growth factors spotted in duplicates. Filters were incubated with conditioned medium from the different cultures (CM+/− or CM−/−; Figure 5A). The spots were carefully quantified and uneven background was equalized with the aid of control spots that were compared between filters. Most spots showed similar intensity between the 2 conditions after compensation for differences in background, but significant changes were seen for 4 different antibodies (Figure 5B). There was increased expression of CTACK/CCL27, a regulator of lymphocyte migration, and of TIMP-1, a matrix metalloproteinase inhibitor, in the FGFR-1–deficient condition. On the other hand, IL-4 and stem-cell factor (SCF) expression decreased in the absence of FGFR-1 (see Figure 5C for quantification). Changes in expression were further examined using real-time PCR, which allowed validation of the decreased expression of both TIMP-1 and CTACK in the Fgfr-1−/− embryoid bodies (Figure 5D). In contrast, transcript levels of SCF were similar between Fgfr-1+/− and Fgfr-1−/− embryoid bodies, which do not exclude posttranslational changes that may affect protein levels and angiogenesis.
Since IL-4 has been implicated in regulation of angiogenesis previously, we evaluated the effect of IL-4 neutralizing antibodies on embryoid body vascularization. As shown in Figure 5E, neutralization of IL-4 during days 0 to 4 of differentiation of Fgfr-1+/− embryoid bodies resulted in marked induction of vessel formation (see Figure 5F for quantification). Two different neutralizing anti–IL-4 antibodies were tested with the same result (data not shown). In parallel cultures, embryoid bodies were treated with purified IL-4, which tended to result in reduced vascularization but also in general effects on stem-cell differentiation that hampered conclusions as to specific changes on endothelial cell development (data not shown). As shown in Figure 5G, the morphology of vessels in the anti–IL-4–treated cultures was similar to that in control, indicating that additional factor(s) contributed to the particular features of the Fgfr-1−/− vessels.
Microarray analysis of stem-cell gene expression shows increased expression of PTN in Fgfr-1−/− embryoid bodies
To further pinpoint changes in gene regulation in differentiating embryonic stem cells as a consequence of FGFR-1 deficiency, we performed comparative microarray analyses using a stem-cell cDNA array. One of the most markedly induced transcripts in the Fgfr-1−/− embryoid bodies was PTN (3-fold induction; Table 1), a finding that was confirmed by real-time PCR analysis (data not shown).
To determine the contribution of PTN to the Fgfr-1−/− vascular phenotype, neutralizing anti-PTN antibodies were added to the embryoid body cultures from day 4 to day 8. As shown in Figure 6A, a low concentration of PTN neutralizing antibodies strongly reduced vascularization in Fgfr-1−/− embryoid bodies. Quantification showed a 3-fold reduction in vessel length (Figure 6B).
To confirm that the effect of CM−/− on vascularization of Fgfr-1+/− embryoid bodies was dependent on transfer of PTN, neutralizing anti-PTN antibodies were added to CM−/− prior to incubation on the cultures. Figure 6C shows that inclusion of PTN neutralizing antibodies efficiently attenuated the ability of CM−/− to enhance vascularization in the Fgfr-1+/− cultures (see Figure 6D for quantification of vessel length). Lower panels in Figure 6C show details of vessels in all conditions. Combined, these data demonstrate that FGFR-1 negatively regulates PTN expression and that PTN exerts a potent angiogenic effect in the differentiating stem-cell cultures.
Discussion
In this study, we confirm and extend our previous data on the negative regulatory role of FGFR-1 in angiogenesis. We have shown that FGFR-1–deficient mouse embryonic stem cells fail to develop into hematopoietic cells; in contrast, endothelial cell development is exaggerated. Using several FGFR-1–specific antibodies for immunostaining as well as detection of Fgfr-1 promoter-driven LacZ activity, we previously showed expression of FGFR-1 in approximately 20% of the endothelial cell population.4 Interestingly, in the absence of exogenous stimuli, these FGFR-1–expressing cells were mitogenically active and progressed through the cell cycle. In contrast, the FGFR-1–nonexpressing endothelial cell pool, derived from embryoid body cultures, was resting. The reduced Erk1/2 activity in the Fgfr-1−/− embryoid bodies (Figure 1) may thus be a direct consequence of loss of mitogenic signaling through the receptor. It is possible that the increased Akt activity we observed in the Fgfr-1−/− condition was an indirect compensatory consequence of the reduced mitogenicity.
We now show that developmental tumors, teratomas, devoid of FGFR-1 expression, grow faster and are more densely vascularized than FGFR-1–expressing teratomas. In vitro cultures of Fgfr-1−/− ES cells show the same growth rate as wild-type ES cells,4 suggesting that increased vascularization of the FGFR-1–deficient teratomas may indeed have promoted an increased tumor growth. Vessels in the teratomas may be derived both from the ES cells and from the host. Fluorescent in situ hybridization showed Y chromosome–positive cells in vessel structures in the male-derived Fgfr-1−/− teratomas growing in female nude mice (data not shown); however, with available reagents we were unable to draw firm conclusions as to the contribution of host-derived endothelial cells. Nevertheless, since we show that elimination of FGFR-1 expression is accompanied by altered balance of secreted angiogenic modulators, it is highly likely that both stem cell– and host-derived endothelial cells would be similarly affected by loss of FGFR-1 expression in the teratoma.
Inhibition of FGF/FGFR function in tumors has been shown to lead to decreased tumor growth.19,20 Our data on increased vascularization and growth of Fgfr-1−/− teratomas appear to oppose the concept that FGF/FGFRs are suitable drug targets in tumor therapy. These situations are, however, not quite comparable, as teratoma is a developmental tumor model. It is still important to understand the potential molecular consequences of loss of FGFR-1 function, particularly in a perspective of long-term treatment of patients with FGF/FGFR-targeted therapies. As shown in Figure 3, the morphology of the vessels in the Fgfr-1−/− teratomas was altered, and frequently the vessels showed tightly arranged endothelial cells protruding into the vessel lumen. A prominent feature of the Fgfr-1−/− teratoma vessels was the abundance of transport vesicles, indicative of active transport over the plasma membrane. Formation of pinocytotic vesicles in endothelial cells may increase in various pathological conditions,21 such as in rat models of brain edema,22 or in pulmonary inflammatory processes.23 The abundant transport vesicles and the compact protruding endothelial cells of the Fgfr-1−/− teratoma vessels are indicative of changes in metabolic activity and uncoordinated organization of the endothelial cells, which may be a consequence of the patterning defects observed in Fgfr-1−/− embryos.3 Nevertheless, the vessels were functional and may have contributed to the observed increased growth rate.
By transfer of conditioned medium between cultures, we demonstrated that secreted factor(s) produced by Fgfr-1−/− embryoid bodies contributed to the specific vessel phenotype in the Fgfr-1−/− condition and to decreased expression of VEGFR-2 and CD41. A cytokine antibody array was used to show regulation of 2 interesting angiogenic modulators, TIMP-1 and IL-4. We chose to focus on IL-4, which has been implicated in regulation of angiogenesis previously. Thus, IL-4 inhibits both VEGF- and FGF-induced angiogenesis in vitro10 and in vivo, leading to reduced growth of gliomas.11 The decreased IL-4 production in the Fgfr-1−/− embryoid bodies may be due to the attenuated hematopoiesis and loss of IL-4–producing cells such as T-helper cells and mast cells.24 Our data using neutralizing anti–IL-4 antibodies concur with the classification of IL-4 as a negative regulator of angiogenesis.
A stem-cell array was used to further determine changes in angiogenic regulation as a consequence of loss of FGFR-1. One of the most prominently up-regulated transcripts in Fgfr-1−/− embryoid bodies was PTN. Whether expression of PTN is regulated directly by FGFR-1 or whether expression was increased indirectly as a downstream consequence of loss of FGFR-1 is not known. PTN is a ligand for the receptor-type protein tyrosine phosphatase RPTP β/ζ.25 Interestingly, binding of PTN seems to decrease the activity of RPTP β/ζ (see Deuel et al, 2002, for a review13 ). The receptor tyrosine kinase anaplastic lymphoma kinase (ALK) of the insulin receptor family has also been implicated as a receptor for PTN.26 PTN promotes differentiation and proliferation of a wide variety of normal and transformed fibroblasts, and epithelial and endothelial cells; whether these effects are mediated via RPTP β/ζ or ALK remains to be shown. It is noteworthy that RPTP β/ζ transcript levels were 3-fold increased in the Fgfr-1−/− embryoid bodies compared with Fgfr-1+/−, as determined by real-time PCR (data not shown), indicating that loss of FGFR-1 expression leads to up-regulation of both the PTN ligand and its receptor. PTN has been shown to regulate neuronal differentiation and neurite outgrowth,27 and PTN gene inactivation leads to enhanced hippocampal long-term potentiation28 in otherwise viable and fertile mice. Importantly, PTN has been shown to promote angiogenesis in vivo in ischemic myocardium,29 and PTN expression has been directly correlated to tumor growth and vascularization (Zhang et al and references therein30 ). Our data implicate FGFR-1 as a modulator of PTN expression and a role for PTN in the balanced regulation of vascular development.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Swedish Research Council (project no. K2005-32X-12552-08A), the Swedish Cancer foundation (project no. 3820-B05-10XBC), and the Association for International Cancer Research (AICR Grant 04-0069) to L.C.-W.
We gratefully acknowledge expert advice from Drs Gunnar Nilsson (Department of Medicine, Clinical Immunology and Allergy Unit, Karolinska Institutet, Stockholm, Sweden), Hanna Göransson (Department of Genetics and Pathology, Uppsala University, Uppsala, Sweden), and Adam Ameur and Jakub Orzechowski Westholm (Linneus Center for Bioinformatics, Uppsala University, Uppsala, Sweden).
Authorship
Contribution: P.U.M. designed and performed research, analyzed data, and wrote the paper; A.D. performed statistical analysis and wrote the paper; S.M. designed the microarray study and performed bioinformatic analyses; A.L. performed transmission electron microscopy and analyzed data; L.C.-W. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lena Claesson-Welsh, Department of Genetics and Pathology, Uppsala University, The Rudbeck Laboratory, Dag Hammarskjöldsv. 20, 751 85 Uppsala, Sweden; e-mail:lena.welsh@genpat.uu.se.