Abstract
A role for genetic susceptibility in non-Hodgkin lymphoma (NHL) is supported by the accumulating evidence of common genetic variations altering NHL risk. However, the pattern of NHL heritability remains poorly understood. We conducted a pooled analysis of 10 211 NHL cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph) to evaluate NHL risk among those with hematopoietic malignancies in first-degree relatives. Odds ratios (ORs) and 95% confidence intervals (CIs) of NHL and its subtypes were estimated from unconditional logistic regression models with adjustment for confounders. NHL risk was elevated for individuals who reported first-degree relatives with NHL (OR = 1.5; 95% CI = 1.2-1.9), Hodgkin lymphoma (OR = 1.6; 95% CI = 1.1-2.3), and leukemia (OR = 1.4; 95% CI = 1.2-2.7). Risk was highest among individuals who reported a brother with NHL (OR = 2.8; 95% CI = 1.6-4.8) and was consistent for all NHL subtypes evaluated. If a first-degree relative had Hodgkin lymphoma, NHL risk was highest if the relative was a parent (OR = 1.7; 95% CI = 1.0-2.9). If a first-degree relative had leukemia, NHL risk was highest among women who reported a sister with leukemia (OR = 3.0; 95% CI = 1.6-5.6). The pattern of NHL heritability appeared to be uniform across NHL subtypes, but risk patterns differed by specific hematopoietic malignancies and the sex of the relative, revealing critical clues to disease etiology.
Introduction
Non-Hodgkin lymphoma (NHL) is a multifactorial and heterogeneous group of diseases whose etiology likely involves both genetic and environmental risk factors. The increasing incidence rates of NHL not attributed to infection with the human immunodeficiency virus (HIV) in the latter half of the 20th century remain largely unexplained, and in the absence of identifiable risk factors and precursor lesions, opportunities for NHL prevention are limited. In 2002, there were 287 400 new NHL cases and 161 100 NHL deaths worldwide.1
Several lines of evidence support genetic contributions to NHL. Incidence rates among migrants resemble those in the country of origin rather than the adopted country.2-5 Population-based case-control studies and registry-based linkage studies have consistently reported a 2-fold excess NHL risk among individuals with a family history of a hematopoietic malignancy.6-16 There is also increasing evidence for a role of common genetic polymorphisms to alter NHL risk.17
A detailed pattern of NHL heritability has not been clarified, in part because very large study populations are needed to evaluate the risk of NHL subtypes and the role of family history of specific hematopoietic neoplasms. We present results from the largest pooled analysis of NHL to date. Our study includes data from 10 211 NHL cases and 11 905 controls from case-control studies participating in the International Lymphoma Epidemiology Consortium (InterLymph). We evaluated NHL risk among individuals who reported first-degree relatives with any of 4 hematopoietic malignancies (NHL, Hodgkin lymphoma [HL], leukemia, multiple myeloma [MM]) and further assessed familial aggregation by NHL subtype.
Because family history represents the interaction between shared environmental exposures, behaviors, and genetic susceptibility, a full understanding of NHL heritability can provide clues regarding underlying disease mechanisms, particularly as it relates to disease and subtype-specific heterogeneity.
Patients, materials, and methods
Study population
Seventeen case-control studies (11 population-based, 6 hospital-based) with questionnaire-based data on family history of hematopoietic malignancies contributed data to this pooled analysis as part of the InterLymph Consortium. For consistency, we hereafter will refer to each study with their previously published names. For example, EpiLymph includes 6 studies and is shown as a single entity as it was published in this form (Table 1). InterLymph was established in 2000 as a voluntary consortium that facilitates collaboration among epidemiologic studies of lymphoma (http://epi.grants.cancer.gov/InterLymph).28 Study-specific information regarding participant recruitment for the 17 case-control member studies that contributed data are provided in Table 1.13,15,18,19,21-23,25,26,29-33 Sixteen studies enrolled both men and women, whereas the Connecticut study was restricted to women. HIV-positive NHL cases were excluded for this report. All studies except 1 hospital-based study in northern and southern Italy matched their cases and controls on age, sex, and geography. All studies were approved by participating institutional review boards, and written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Classification of NHL subtypes
NHL subtypes were classified within each study by independent expert pathology review. Twelve studies (Mayo Clinic, Nebraska, United Kingdom [UK], British Columbia, National Cancer Institute–Surveillance, Epidemiology, and End Results multicentre study [NCI-SEER], and EpiLymph studies: Italy, Spain, Germany, France, Finland, Ireland, Czech Republic) used the World Health Organization (WHO) classification system for defining lymphoid neoplasms.34 The studies conducted in northern and southern Italy and Connecticut defined NHL subtypes using the Revised European American Lymphoma (REAL) classification system.35 The University of California at San Francisco (UCSF) study used both the REAL and the Working Formulation.36 The University of Southern California (USC) and Italy multileft studies defined NHL subtypes using the Working Formulation.36
Classification systems from all studies were combined based on the International Classification of Diseases for Oncology,37,38 and as proposed by the InterLymph Pathology Working Group, with representative pathologists for each major study. Six subtypes were defined for further subtype-specific analyses: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, small-lymphocytic lymphoma and chronic-lymphocytic leukemia (SLL/CLL), marginal-zone lymphoma, mantle-cell lymphoma, and T-cell lymphoma. NHL subtypes evaluated in studies using the Working Formulation classification included DLBCL, follicular lymphoma, and SLL/CLL. Studies with NHL subtype classification by the REAL or WHO system allowed evaluation of the 6 subtypes. In all, we evaluated 10 211 NHL cases, including 3233 DLBCLs, 1979 follicular, 1336 SLL/CLL, 380 marginal-zone, 183 mantle-cell, and 447 T-cell lymphomas (Table 2). Further delineation between SLL and CLL were made when possible (Italy multileft, NCI-SEER, UCSF, Nebraska, Mayo Clinic, and Canada).
Definition of family history
Family history of hematopoietic malignancies was ascertained from questionnaire-based interviews either in-person or by telephone. We created uniform variables across all studies, defining “no family history” as reporting of no first-degree relative (parent, sibling, or child) with NHL, lymphoma not otherwise specified (NOS), HL, leukemia, or MM. Family history for specific hematopoietic malignancies was defined by ICD-9 or ICD-10 classification: NHL (202), HL (201), leukemia (204-208), MM (203). We categorized the affected relatives as parent-only, sibling-only, offspring-only, or parent/offspring, and also as father, mother, brother, sister, daughter, or son (brothers, sisters, daughters, and sons could not be distinguished in the Italian multileft study).
Statistical methods
The distributions of sex and age were similar between cases and controls. However, we adjusted for these variables because they were matching variables in many studies. We further adjusted for race and education, as these are known to be related to self-reported family history.6 Age was categorized in 10-year intervals and race was categorized as white, black, and other. Education was grouped as less than 12 years (did not graduate from high school), 12 to 15 years (graduated from high school), or 16+ years (college or greater; the UK study estimated socioeconomic status with a continuous deprivation indicator that was categorized into comparable tertiles). Final models included adjustment for sex, age, race, education, and study left, as adjustment for these variables altered our results by greater than 10%. The 6 study lefts within EpiLymph were considered as a single study and we note that the resulting risk estimates and confidence intervals were not altered when adjustment was made for all 6 studies separately. Risks for NHL and NHL subtypes were estimated by computing odds ratios (ORs) and their 95% confidence intervals (CIs) in dichotomous and polytomous unconditional logistic regression models, respectively. In analyses that compared study-specific estimates to pooled risk estimates (Figure 1), we adjusted for age using the year 2000 world standard population39 so that the referent age standard was comparable between each of the study estimates and the pooled estimate and was therefore further generalizable. Notably, adjustment using the year 2000 world standard and using the age distribution of the InterLymph Consortium resulted in equivalent risk estimates. In all other pooled estimates of risk, we standardized to the age distribution of the pooled study population (Table 1). Individuals with missing data for specific outcomes (eg, NHL subtype) and family history variables were excluded from respective analyses. All statistical tests were 2-sided with an alpha probability level of 0.05 as the threshold to reject the null hypothesis. All analyses were conducted using SAS version 9.1.3 (Cary, NC).
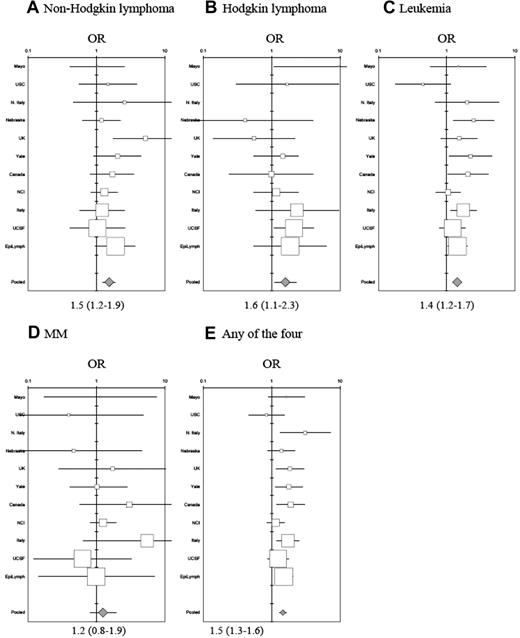
Study-specific and pooled risk estimates for NHL. Estimates are ordered by study size with family history of (A) non-Hodgkin lymphoma, (B) Hodgkin lymphoma, (C) leukemia, (D) multiple myeloma, and (E) any hematopoietic malignancy, adjusted for education, race, sex, age (2000 world standard), and study left for InterLymph.
Study-specific and pooled risk estimates for NHL. Estimates are ordered by study size with family history of (A) non-Hodgkin lymphoma, (B) Hodgkin lymphoma, (C) leukemia, (D) multiple myeloma, and (E) any hematopoietic malignancy, adjusted for education, race, sex, age (2000 world standard), and study left for InterLymph.
Results
A description of the participating case-control studies is shown in Table 1. The pooled study population of 10 211 cases and 11 905 controls included 11 562 men and 10 554 women, 93% of whom were white (Table 2). The median age of cases and controls was 58 years (range, 18-84 years). More controls (25%) were in the highest category of education than cases (22%), although the difference was not statistically significant. Four percent of controls (n = 500) and 6% of cases (n = 648) reported having had a first-degree relative affected with any of the 4 hematopoietic malignancies (NHL, HL, leukemia, or MM). Among controls, family history of leukemia was the most prevalent (n = 278; 2%) followed by NHL (n = 140; 1%), HL (n = 55; 0.5%), and MM (n = 38; 0.3%).
NHL overall
In the combined study population, the risk for NHL in individuals who reported a first-degree relative with NHL was elevated with a pooled estimate of 1.5 (95% CI = 1.2-1.9; Figure 1A; Table 3). In sensitivity analyses alternately excluding each study 1 at a time, risk estimates remained elevated and statistically significant. NHL risk among individuals who reported a first-degree relative with HL was similarly elevated (OR = 1.6, 95% CI = 1.1-2.3; Figure 1B) as was NHL risk among individuals who reported a first-degree relative with leukemia (OR = 1.4, 95% CI = 1.2-2.7; Figure 1C). We observed a slight increase in NHL risk for individuals who reported a first-degree relative with MM (OR = 1.2, 95% CI = 0.8-1.9; Figure 1D), although confidence limits overlapped unity. Risk of NHL increased with the number of affected family members. NHL risk among individuals who reported only 1 first-degree relative with any of the 4 hematopoietic malignancies had a 1.4-fold (95% CI = 1.2-1.6) risk increase compared with a 2.7-fold (95% CI = 1.4-4.9) risk increase for those who reported 2 or more first-degree relatives with any of the 4 hematopoietic malignancies.
Risks varied according to the relationship of the study participants with affected family members. For instance, NHL risk among those who reported a sibling with NHL (OR = 2.0, 95% CI = 1.4-2.8) was higher than those who reported a parent with NHL (OR = 1.4, 95% CI = 1.0-1.8; Table 3). Among both men and women, the risk was higher if the relative with NHL was male (OR = 2.1, 95% CI = 1.5-3.0) than if the relative was female (OR = 1.3, 95% CI = 1.0-1.8). NHL risk was highest for both men and women who reported a brother with NHL (OR = 2.8, 95% CI = 1.6-4.8), followed by having a father with NHL (OR = 1.8, 95% CI = 1.1-2.9).
By contrast, family history of HL in either parent (OR = 1.7, 95% CI = 1.0-2.9) was more strongly associated with NHL risk than HL history in a sibling (OR = 1.3, 95% CI = 0.7-2.4; Table 3). The elevated NHL risk among those with a parent with HL was largely observed among men (OR = 2.1, 95% CI = 1.1-4.1) and highest if the relative was their father (OR = 2.4, 95% CI = 1.0-5.9). No statistically significant risk for NHL was found in those who reported a sibling with HL.
Among individuals who reported a first-degree relative with leukemia, NHL risk was slightly higher if the relative was a sibling (OR = 1.7, 95% CI = 1.3-2.2) rather than a parent (OR = 1.4, 95% CI = 1.1-1.7; Table 3). However, the NHL risk observed among those with siblings with leukemia was higher in women (OR = 2.2, 95% CI = 1.5-3.2) and highest among women who reported a sister with leukemia (OR = 3.0, 95% CI = 1.6-5.6).
The confidence limits for the overall NHL risk for individuals who reported a first-degree relative with MM could not rule out chance. However, an increased risk was observed if the relative was a male (OR = 2.4, 95% CI = 1.1-5.1) and in particular, the father (OR = 2.9, 95% CI = 1.2-6.9). No excess NHL risk was observed among those who reported a family history of MM in female relatives or in siblings.
NHL subtypes
For each of the 5 major B-cell NHL subtypes, risks were higher if the relative with NHL was either a male or a sibling (Table 4). Cases were more likely to report a brother with NHL no matter what subtype they developed (DLBCL OR = 2.7, 95% CI = 1.4-5.4; follicular lymphoma OR = 2.6, 95% CI = 1.2-5.7; marginal-zone lymphoma OR = 6.1, 95% CI = 2.3-16.1; mantle-cell lymphoma OR = 4.9, 95% CI = 1.4-17.6). Elevated but not statistically significant risks were seen for SLLs/CLLs (OR = 2.5, 95% CI = 0.9-7.0) and T-cell lymphomas (OR = 2.1, 95% CI = 0.5-9.1).
Individuals who reported a first-degree relative with HL had higher risks of DLBCL (OR = 1.7, 95% CI = 1.1-2.7; Table 4). Although risks for all B-cell lymphoma subtypes were elevated for those reporting a parent with HL, the estimates were not statistically significant.
If the participant reported that a first-degree relative had leukemia, risks were elevated for DLBCL (OR = 1.3, 95% CI = 1.0-1.6), marginal-zone lymphoma (OR = 2.2, 95% CI = 1.4-3.6), mantle-cell lymphoma (OR = 2.3, 95% CI = 1.2-4.4), and SLL/CLL (OR = 2.2, 95% CI = 1.7-2.9) but not for follicular or T-cell lymphomas. Similar female and sibling specificity was observed for DLBCL where risk was elevated largely among individuals who reported a female relative with leukemia (OR = 1.5, 95% CI = 1.1-2.2), a sibling with leukemia (OR = 1.9, 95% CI = 1.3-2.8), and highest if family history included a sister with leukemia (OR = 2.6, 95% CI = 1.5-4.5). Risks were similarly elevated for both marginal-zone and mantle-cell lymphomas if the first-degree relative was a female with leukemia and highest if the relative was a sister.
To assess whether our results were due to recall bias, we evaluated risks separately for SLL and CLL, since the primary difference is in first presentation and hence “leukemia” in the CLL diagnosis. We found equivalent risks for SLL and CLL for those who reported a first-degree relative with leukemia (SLL OR = 2.3, 95% CI = 1.4-3.7; CLL OR = 2.0, 95% CI = 1.1-3.5). We further found SLL/CLL risk among those with a parent with leukemia (OR = 2.5, 95% CI = 1.7-3.6) to be slightly higher than those with a sibling with leukemia (OR = 2.0, 95% CI = 1.2-3.1). No risk elevation was observed for follicular lymphoma among individuals who reported any relative with leukemia.
Evaluation of risk by NHL subtype for individuals who reported a family history of MM was limited by small numbers. Nevertheless, among those who reported a male relative with MM, significantly elevated risks were observed for follicular (OR = 3.1, 95% CI = 1.2-8.3), marginal-zone (OR = 6.7, 95% CI = 2.0-22.4), mantle-cell (OR = 5.3, 95% CI = 1.1-25.7), and T-cell lymphomas (OR = 5.8, 95% CI = 1.5-21.4; Table 4). No risk was observed for the 2 common NHL subtypes, DLBCL or SLL/CLL, in individuals who reported a family history of MM.
NHL subtypes and sex
We found no significant differences between men and women for NHL subtype risk among those who reported a family history of NHL. The magnitude of risk was somewhat elevated among women with a family history of HL for marginal-zone (OR = 3.4, 95% CI = 1.0-11.9) and T-cell lymphoma (OR = 2.2, 95% CI = 0.5-9.5). Family history of leukemia elevated risks particularly among women for marginal-zone lymphoma (OR = 2.9, 95% CI = 1.6-5.1), T-cell lymphoma (OR = 1.6, 95% CI = 0.8-3.1), and SLL/CLL (OR = 2.8, 95% CI = 1.9-4.1), whereas among men, risk was increased for mantle-cell lymphomas (OR = 3.1, 95% CI = 1.6-6.2). The excess NHL risk from family history of MM among men was most evident for marginal-zone (OR = 10.4, 95% CI = 2.9-37.3) and T-cell lymphomas (OR = 9.2, 95% CI = 2.4-36.0) and, although elevated risks were observed, chance could not be ruled out for mantle-cell (OR = 5.3, 95% CI = 0.8-35.1) and follicular lymphoma (OR = 2.0, 95% CI = 0.6-6.3).
Evidence for early onset of disease
We evaluated evidence for early disease onset in stratified analyses of NHL and NHL subtype risk among individuals 50 years or younger and those older than 50 years at diagnosis of NHL. We found no evidence of early onset for NHL or NHL subtypes in any report of a first-degree relative with NHL. However, among individuals who reported a parent or offspring with HL, NHL risks were pronounced among men and women 50 years old or younger (OR = 3.1, 95% CI = 1.2-7.8) compared with those who were older than 50 years (OR = 1.3, 95% CI = 0.8-2.2). And among individuals who were 50 years old or younger who reported a family history of MM, pronounced but not statistically significantly elevated NHL risks were observed among those with affected parents/offspring (OR = 2.2, 95% CI = 0.6-7.8) or affected siblings (OR = 3.1, 95% CI = 0.3-35.0), whereas risks were null among individuals who were older than 50 years.
Although there was no evidence of early onset for overall NHL risk among those who reported a family history of leukemia, SLL/CLL risks appeared higher among individuals who were 50 years old or younger and who reported a family history of leukemia (OR = 3.2, 95% CI = 1.5-6.9) compared with individuals who were older than 50 years (OR = 2.1, 95% CI = 1.6-2.9). Similarly, among those who reported a family history of leukemia, T-cell lymphoma risk was elevated among individuals who were 50 years old or younger who reported a family history of leukemia (OR = 2.3, 95% CI = 1.0-5.6) compared with individuals who were older than 50 years (OR = 1.0, 95% CI = 0.5-2.2).
Discussion
Family members of lymphoma and leukemia patients face a well-established increase in risk for developing NHL. Genetic and other risk factors have been difficult to determine because even large individual studies have lacked a sufficient number of participants to detail risk estimates according to type of malignancy of affected family members, family relationships, or NHL subtype. In this large pooled analysis, several features of NHL risk emerge in relation to familial patterns of hematopoietic malignancies in first-degree relatives of study participants. In general, it appears that NHL confers a consistently stronger familial association among men than among women. Siblings are also a more powerful marker of personal risk of NHL than is history of NHL in a parent. Overall, NHL presented an unremarkable pattern of age-specific risk and no suggestion of early onset. The overall risk estimate from this pooled analysis reflected a 50% increased risk that is equivalent to estimates from registry-based data where family history data are verified,6-8,11 lending general support to the credibility of these findings despite the inevitable inaccuracies and the possible bias in recollection of cancer diagnoses in family members.
Evidence for a stronger familial association of NHL in men than women has been reported in some but not all previous studies, including registry linkage reports, cohort studies, and case-control data not included in this report.11,15,16 Our pooled analyses further demonstrate that the male- and sibling-specific aggregation was consistent in the 5 B-cell lymphoma subtypes that we evaluated. Further pursuit of multiple-case families or twin studies may shed light on the familial association of NHL among men. Among our 10 multiple-case families where cases reported more than 2 relatives with NHL, all who reported a sibling with NHL reported it in a brother. If, as the overall body of data now suggests, familial NHL association is stronger in men, environmental and genetic factors that are shared, particularly among male relatives and brothers, should be considered.
Our results demonstrate the strength of using consortia data such as InterLymph to provide sufficient statistical power to estimate the effects of uncommon exposures such as family history on the risk of all NHL and its histologic subtypes. We combined various classification schemas as dictated by the InterLymph pathology working group, providing confidence in our evaluation of NHL subtypes. The large sample size afforded by the pooled analyses also allowed us to examine the age-, sex-, and relationship-specific risk of NHL according to family history of HL, MM, and leukemia. For DLBCL, we observed a female-specific aggregation where risk was highest for women who reported a sister with leukemia. We also found elevated DLBCL risk in individuals who reported a relative with HL. Follicular lymphoma risk was elevated in individuals who reported a male relative with MM, consistent with reports from a previous registry-based study.6 For SLL/CLL, the familial patterns are of interest because the patients were told that they had a different diagnosis (lymphoma or leukemia) for what is now known to be a single malignancy with variability in the first presentation of disease. These patients disproportionately reported leukemia in the family regardless of whether their first diagnosis was SLL or CLL, suggesting that their own diagnosis did not influence the condition that they reported for their relative, thus indicating that our results are less likely to be attributed to recall bias. For both HL and MM, we found that higher NHL risk was conferred upon individuals with a parent with either disease, particularly when the father had lymphoma and when the study participant was diagnosed at 50 years of age or older.
In our data, we observed a wide variation of risk estimates in individual studies that likely reflected the variety of study designs (hospital- versus population-based), sampling variability, response rates, and wording of the questionnaires. However, sensitivity analyses of these heritability patterns demonstrated robustness and results were not strongly influenced by any 1 study. The main limitation of our analyses is in the use of self-reported family history, which may lead to overestimates of the risk estimate, as described previously.6,29,40 Despite our efforts to reduce the effects of recall bias by adjustment for age, sex, education, and race, which are important factors related to accuracy of self-reported family history, any residual bias from differential case-control reporting would have remained in our analyses and should thus be considered in the interpretation of our results. We believe that misclassification would have been more likely to occur among NHL and leukemia that was reported among parents. Whereas we cannot exclude the possibility that NHL and leukemia misclassification among parents resulted in the lower risks conferred by parents compared with siblings, it would not have accounted for the sex specificity of our findings. Nevertheless, because of the potential misclassification and differential reporting of each of the hematopoietic malignancies, we therefore also summarized all of the results for NHL risk among individuals who reported any of the 4 hematopoietic malignancies.29,40
Because family size has been suggested as a possible risk factor for NHL,41 we also assessed its potential as a confounder to family history. In our data from 5 studies where family size could be evaluated, the number of individuals who reported first-degree relatives with NHL increased with family size, as expected. However, we did not find that family size differed by case or control status. Therefore, if the data were representative of our entire pooled population, family size would not explain the association between family history and NHL. Further, the association between first-degree relatives with NHL and family size was not specific to men and therefore could not explain the predominance of NHL risk observed among those reporting a brother with NHL.
Other limitations in our analyses included the lack of information on the age or NHL subtype in the relative where specificity may be important7 and our inability to evaluate HL by age. We also cannot rule out bias related to ascertainment practices in early onset disease. Because our population was largely white, our data did not permit evaluation by nonwhite race groups that may have yielded important clues regarding NHL heritability from other hematopoietic malignancies such as MM that have striking differences by race (eg, higher among blacks).42 Because 93% of our population was self-reported as white, analyses restricted to white individuals provided results that were consistent with our race-adjusted results.
In summary, our results describe variation in NHL heritability according to family history of the hematopoietic neoplasms and NHL subtypes. While we cannot exclude the possibility that our results and specific patterns of heritability are due to chance, the magnitude of our risk estimates, the specificity of our associations, and the consistency of our results that have remained statistically significant with substantial adjustment for confounders argue against this possibility. Our data support further examination of all sources of familial aggregation,43 including investigation of common gene variations that alter NHL risk44 in the search for etiologic mechanisms. The use of family history in the evaluation of gene variations may further improve the efficiency of such a search.45 The observed patterns of inheritance support the role for heritable factors that can affect all NHL subtypes uniformly (eg, family history of NHL and NHL risk) and genes that are NHL subtype specific (eg, family history of leukemia and SLL/CLL risk). Future efforts in sib-pair or twin studies46 may further our understanding of the sibling relationships, particularly for the male- and female-specific associations for family history of NHL and leukemia, respectively. Family studies in those with family history of HL or MM may also reveal factors influenced by the father that confer excess risk. Finally, family-based linkage studies in high-risk families may help to identify genes of higher penetrance for specific subtypes where there was early onset disease.
Authorship
Contribution: The project was conceived and led by cochairs of the InterLymph Family Working Group: S.S.W., P.H., S.L.S., and P. Brennan. Investigators who carried out and provided data from each study are as follows: NCI-SEER (P.H., S.S.W., J.R.C., W.C., S.D., R.K.S.); University of California San Francisco (E.A.H., P.M.B.); University of Southern California (L.B.); Mayo Clinic (J.R.C., S.L.S.); Connecticut (T.Z., Y.Z.); United Kingdom (E.R., F.M.); British Columbia (J.J.S.); Nebraska (B.C.H.C., D.W.); Multileft Italian (WILL) study (A.S.C., P.V.); northern and southern Italy (S.F., C.L.V., L.D.M.); EpiLymph-Germany (N.B., A.N.); EpiLymph-France (M.M.); EpiLymph-Spain (S.D.S.); EpiLymph-Italy (P.L.C.); EpiLymph-Ireland (A.S.); EpiLymph-Finland (M.V.); and EpiLymph–Czech Republic (L.F.). EpiLymph is coordinated by P. Brennan and P. Boffetta. The statistical analysis was carried out by S.S.W. and P.H. The manuscript was drafted and revised by S.S.W., P.H., S.L.S., and P. Brennan. All authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sophia S. Wang, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd, EPS MSC# 7234, Bethesda, MD 20892-7234; e-mail: wangso@mail.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This pooled analysis was supported by the Intramural Research Program at the US National Institutes of Health (NIH) National Cancer Institute (NCI). Individual studies that contributed data to this pooled analysis were supported by the following sources: grant CA62006 from NCI (Connecticut study); contracts PC65064, PC67008, PC67009, PC67010, and PC71105 from NCI (NCI-SEER study); grant 99B083 from the American Institute for Cancer Research (Nebraska study); grants CA45614, CA89745, CA87014, and CA104682 (UCSF study); grant CA50850 from NCI (USC study); grants CA92153 and CA94919 (Mayo Clinic study); the Italian Association for Cancer Research (northern and southern Italy study); the Leukaemia Research Fund (United Kingdom study); grant CA51086 from NCI, the European Community (Europe Against Cancer Programme), and the Lega Italiana per la Lotta Contro I Tumori (Multicentre Italian Study); a European Commission Grant no. QLK4-CT-2000-00422 (EpiLymph studies); Association pour la Recherche contre le Cancer (ARC no. 5111) and the Fondation de France (1999 008471; EpiLymph-France); Compagnia di San Paolo di Torino, Programma Oncologia 2001 (EpiLymph-Italy); Spanish Ministry of Health grant PI040091 and RCESP 03/09 (EpiLymph-Spain); the German Federal Office for Radiation Protection (StSch4261 and StSch4420; EpiLymph-Germany); and the National Cancer Institute of Canada, the Chan Sisters Foundation, and the Canadian Institutes for Health Research (British Columbia study).
We thank present and founding InterLymph executive committee members Bruce Armstrong, Martha Linet, and Carol Kasten; Lindsay Morton from the InterLymph pathology working group; Lynn Goldin from the NCI; Tom Mack from the University of Southern California for their review of the manuscript; and Peter Hui and Mary McAdams at Information Management Systems for programming support.