To the editor:
In the July 1, 2006, issue of Blood, Meshinchi et al1 comment on the role of stem-cell transplantation (SCT) in FLT3/ITD-positive acute myeloid leukemia (AML). In their interpretation of data previously presented by Gale et al,2 they conclude that the occurrence of a FLT3/ITD may not be regarded as a negative prognostic factor in patients undergoing allogeneic SCT. The dispute between both authors concerns the fact that although an “intention-to-treat” analysis does not suggest a clinically relevant improvement in survival in patients for whom a donor could be identified, the “as-treated” comparison shows a lower probability of relapse and better overall survival in FLT3/ITD-positive patients undergoing allogeneic transplantation compared with chemotherapy.
We would like to add to this discussion by presenting the results of the AML 96 study of the DSIL (German study initiative leukemia), in which 999 patients 60 years or younger were prospectively included between 1996 and 2003 and stratified according to cytogenetic risk category.3 Of 555 intermediate-risk patients evaluable for FLT3 mutation status, 175 (31.5%) were FLT3/ITD-positive. The treatment protocol included 2 cycles of induction chemotherapy including high-dose Ara-C for all patients as previously described. Since the rate of remission was not different in patients with and without a FLT3/ITD (68% vs 63%),4 we decided to determine the impact of different consolidation therapies on overall survival (OS) and probability of relapse in patients with respect to FLT3/ITD mutation status. Allogeneic SCT using an HLA-matched sibling donor was performed in 103 patients after standard conditioning therapy. If no donor was available, the next treatment priority was to obtain G-CSF–mobilized autologous blood stem cells after induction and/or first postremission chemotherapy and to perform an autologous SCT (n = 141). If patients could not mobilize autologous cells, conventional consolidation chemotherapy consisted of 2 cycles of high-dose Ara-c (n = 132).
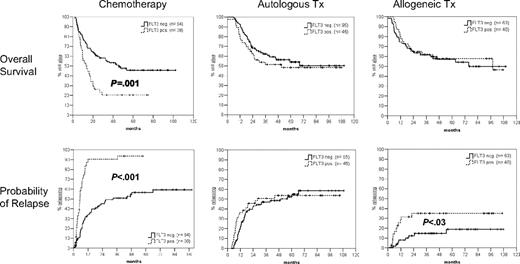
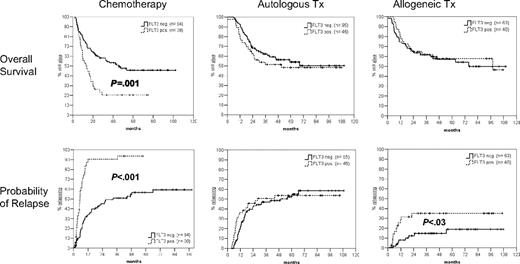
Figure 1 shows that after a median follow-up of 53 months for surviving patients, overall survival is not significantly different between FLT3/ITD-positive and -negative patients having undergone either autologous or allogeneic SCT. In contrast, FLT3/ITD-positive patients receiving chemotherapy as consolidation therapy had an inferior probability of survival (21% vs 46%; hazard ratio [HR] = 2.2; 95% confidence interval [CI], 1.4-3.5; P = .001). The probability of relapse in the group of patients receiving chemotherapy was significantly higher in FLT3/ITD-positive compared with FLT3/ITD-negative patients (94% vs 59%; HR = 4.0; 95% CI; 2.5-6.6; P < .001). Of interest, as reported by Gale et al,2 the probability of relapse tended to be higher in FLT3/ITD-positive than FLT3/ITD-negative cases (35% vs 19%; HR = 2.7; 95%, CI, 1.1-6.5; P = .03) after allografting but not after autologous transplantation.
Probability of overall survival and relapse according to postremission therapy. After a median follow-up of 53 months for surviving patients, the probability of overall survival and relapse (from remission) are shown for patients having received either chemotherapy (n = 132), autologous transplant (n = 141), or allogeneic transplant (n = 103) as postremission therapy. Tx indicates transplantation.
Probability of overall survival and relapse according to postremission therapy. After a median follow-up of 53 months for surviving patients, the probability of overall survival and relapse (from remission) are shown for patients having received either chemotherapy (n = 132), autologous transplant (n = 141), or allogeneic transplant (n = 103) as postremission therapy. Tx indicates transplantation.
Although we absolutely agree with Gale et al in that a donor–versus–no donor or a good mobilizer–versus–poor mobilizer comparison should be the cornerstone of valid prognostic analyses, we believe it is fair to say that patients with a FLT3/ITD-positive AML achieving remission should be scheduled for an autologous or allogeneic SCT within a controlled clinical study. Our data as well as reports from other study groups suggest that until alternative strategies including new targeted therapies will be introduced, allogeneic or autologous SCTs in first remission seem to be warranted to compensate for the negative prognostic impact of FLT3/ITD.5
Authorship
Correspondence: Martin Bornhäuser, Medizinische Klinik und Poliklinik I, Universitätsklinikum Carl Gustav Carus, Fetscherstrasse 74, 01307 Dresden, Germany; e-mail: martin.bornhaeuser@uniklinikum-dresden.de
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete membership list for the AML SHG 96 study group is provided in Document S1, available at the Blood website; click on the “Supplemental Document” link at the top of the online article.