Abstract
The naturally occurring population of dedicated regulatory T cells that coexpress CD4 and CD25 is known to play a key role in the maintenance of peripheral T-cell tolerance; however, their mechanism of action has remained obscure. Here we report that a member of the family of β-galactoside–binding proteins, galectin-1, is overexpressed in regulatory T cells, and that expression is increased after activation. Most importantly, blockade of galectin-1 binding significantly reduced the inhibitory effects of human and mouse CD4+CD25+ T cells. Reduced regulatory activity was observed in CD4+CD25+ T cells obtained from galectin-1–homozygous null mutant mice. These results suggest that galectin-1 is a key effector of the regulation mediated by these cells.
Introduction
More than 10 years ago, T lymphocytes, which constitutively coexpress CD4 and CD25 and represent approximately 5% of murine peripheral T cells, were shown to play a key role in the prevention of autoimmunity.1,2 Similar cells have since been characterized in human peripheral blood.3,4 These cells differ from recently activated CD4+ T cells expressing CD25 because of their low-level expression of surface markers of activation such as CD69. In contrast to CD4+CD25− T cells, CD4+CD25+ T cells are hyporesponsive in vitro to polyclonal T-cell–receptor (TCR) stimuli. After activation they do not produce interleukin-2 (IL-2), IL-4, interferon (IFN)–γ, or IL-10, and their anergic state can be partially restored by IL-2. The mechanism of regulation appears to involve the inhibition of transcription of cytokine genes, most notably IL-2 and IFN-γ, leading to reduced proliferation.5,6
In mouse models, Papiernik et al have demonstrated that CD4+CD25+ T cells originate in the thymus, where they are induced to express CD25 at the CD4 single-positive stage, before migration to the periphery.7 Although the generation of CD4+CD25+ T cells during T-cell differentiation has not yet been fully characterized, it appears that the relatively high affinity of these cells for self-antigens falls between that required for positive and negative selection.8
The importance of naturally occurring CD4+CD25+ regulatory T (Treg) cells for maintenance of peripheral T-cell tolerance is well established, but their mechanisms of action remain elusive. Treg cells express high levels of cytotoxic T-lymphocyte–associated protein-4 (CTLA-4), which, it has been suggested, may act by sequestering B7 family molecules and inhibiting the costimulation of neighboring T cells.9 However, since CD4+CD25+ Treg cells effect suppression in the absence of B7-expressing antigen-presenting cells, this cannot be a universal explanation.3 Cell-surface–transforming growth factor β-1 (TGF-β1) has also been implicated, although this has not been reproduced.10,11 It has recently been reported that Lag-3 contributes to the function of naturally occurring regulatory cells, but the reversal of suppression achieved by Lag-3 blockade was only modest.5,6,12
Although cytokines such as IL-10 and TGF-β1 appear to play a key role in Treg function in vivo, regulatory effects in vitro are contact dependent and are not reversed by the neutralization of these cytokines. The antigen specificity of the suppressive function of Treg cells is also controversial, because evidence for it exists in some in vitro and in vivo systems, but it has been difficult to demonstrate in other contexts, particularly those involving adoptive transfer.13
In order to identify Treg-cell–specific molecules, we performed a transcriptomic and proteomic analysis of activated CD4+CD25+ T cells. This analysis revealed that galectin-1, a molecule that can function as a homeostatic agent, was selectively up-regulated in Treg cells. Here, we show that galectin-1 expression by the Treg cells is up-regulated upon TCR activation, and that specific blockade of galectin-1 by a monoclonal antibody (mAb) reversed the inhibitory effects of CD4+CD25+ T cells in humans.
Materials and methods
Culture media, reagents, and antibodies for flow cytometry
Unless otherwise stated, RPMI 1640 medium supplemented with penicillin/streptomycin (100 IU/mL and 100 μg/mL, respectively), l-glutamine (2 mM; Sigma, Gillingham, United Kingdom), and gentamicin (2 μg/mL; Sigma), referred to as supplemented RPMI, with 10% heat-inactivated human AB serum (Sigma), was used for all in vitro assays. Phycoerythrin (PE)–conjugated anti–human CD25 (2A3; BD Biosciences, Palo Alto, CA); Tri-color (TC)–conjugated anti–human CD4 (S3.5; Caltag Laboratories, Burlingame, CA) and allophycocyanin (APC)–conjugated rat anti–mouse CD4 (L3T4; BD Biosciences Pharmingen, Palo Alto, CA) monoclonal antibodies were used in flow cytometry. Mouse immunoglobulin G1 (IgG1) and IgG2a labeled with PE, TC, and APC, all from Caltag, were used as isotopic controls.
Staining for intracellular FoxP3 protein was performed using the cell-fixation/cell-permeabilization kit included in the FoxP3 protein staining kit (eBioscience, San Diego, CA) according to the manufacturer's instruction. For flow cytometric analysis, 1 × 105 cells were labeled in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]/2% fetal calf serum [FCS]/0.1% sodium azide), fixed in 1% paraformaldehyde (Sigma) and analyzed on a FACSCalibur using the CellQuest software (Becton Dickinson, Mountain View, CA).
Selection of primary human CD4+CD25+ and CD4+CD25− T cells
Peripheral blood mononuclear cells (PBMNCs) were derived from peripheral blood samples according to local regulations (buffy coats, Colindale, North London Transfusion Center, United Kingdom). All samples used in the study were obtained after informed consent and approval by the King's College London ethics committee according to the Declaration of Helsinki. For negative selection of CD4+ T cells, PBMNCs were depleted by incubating with a cocktail of antibodies composed of anti-CD8 (B-H7), anti-CD14 (B-A8), anti-CD33 (WM53), anti-CD19 (B-C8), anti-CD16 (B-E16), and anti-CD56 (B-A19) (all from Diaclone, Manchester, United Kingdom) and anti-γδ TCR (Becton Dickinson, Oxford, United Kingdom), followed by negative selection on goat antimouse magnetic beads (Pan Mouse IgGs; Dynal, Bromborough, United Kingdom) according to the manufacturer's instructions. The purified CD4+ T cells were then incubated with magnetic beads coated with anti–human CD25 (Dynal) for 30 minutes at 4°C. CD4+ T cells depleted of CD25 high-expressing cells (referred as to CD4+CD25−) were obtained from the supernatant. CD4+CD25+ T cells were recovered from t he beads after 1 hour of incubation at room temperature (RT) with CD4/CD8 Detachbeads (Dynal). The purity of the cell populations was in the range of 90% to 95% for CD4+CD25− T cells and between 85% to 95% for CD4+CD25+ T cells.
In vitro activation of human CD4+CD25− and CD4+CD25+ T cells and proliferation assays
The selected cells were activated by the addition of anti-CD3/anti-CD28–coated beads (T-Cell Expander; Dynal). The cells were diluted to 1 × 106 cells/mL with supplemented RPMI 1640 plus 10% human AB serum and 0.4 μL of anti-CD3/anti-CD28–coated beads per 1 × 106 cells. The cultures were incubated for the indicated days at 37°C and 5% CO2. For protein and gene expression studies, the cells were counted, and the dry pellets stored at −80°C until use.
To assess proliferation in allogeneic mixed lymphocyte responses (MLRs), 5 × 104 CD4+CD25+, and CD4+CD25− T cells from the same donor and cocultures of both cell populations at different ratios were set up in triplicate as responders. As stimulators, 1 × 105 allogeneic 40 Gy–irradiated CD2-depleted PBMNCs were added to each well. The cells were then incubated for 5 days at 37°C and 5% CO2. On day 4, the cultures were pulsed with 0.037 MBq (1 μCi) 3H-thymidine ([H3]TdR, 925 GBq/mmol [25 Ci/mmol]; Amersham Biosciences, Freiburg, Germany); 16 hours later, the cells were harvested using a Wallac harvester (Wallac, Turku, Finland). The radioactivity measured in a scintillation counter (Wallac).
IL-2 bioassay
IL-2 production was measured using the murine cell line CTLL-2 (ATCC, Rockville, MD), which responds to human and mouse IL-2. Supernatants harvested from the T-cell cultures were added to the CTLL-2 cells (5000 cells/well) in 150 μL of medium. The cultures were labeled with [3H]TdR (0.0185 MBq/well [0.5 μCi/well]) after 8 hours of incubation and harvested 18 hours later. [3H]TdR incorporation was then measured in a Betaplate liquid scintillation counter (Wallac). Mean counts per minute (cpm) of triplicate cultures and standard deviation of the mean were calculated. In each experiment scalating concentrations of standard recombinant human (rh) IL-2 were used as positive controls.
IFN-γ determination by ELISA
A 96-well plate was coated with 10 μg/mL anti–IFN-γ antibody (Immunokontact, Frankfurt, Germany) in PBS and incubated overnight at 4°C. From a stock solution of IFN-γ at 250 ng/mL (Immunokontact), standards were set up. Sample supernatants (20 μL) plus 80 μL of the culture medium were also added to the relevant. The biotin-conjugated antibody (50 μL; Immunokontact) diluted to 1:1000 in Tween 20–bovine serum albumin (BSA)–PBS was added to each well and incubated for 2 hours at RT. Streptavidin–horseradish peroxidase (HRP) (100 μL; Biosource International, Nivelles, Belgium) diluted 1:2500 in Tween 20–BSA-PBS was added to each well and incubated for 45 minutes at RT. The plate was then washed at least 8 times with Tween 20–PBS and once with PBS. TMB substrate (100 μL; Zymed, San Francisco, CA) was added to each well and incubated for 20 minutes at RT. After incubation, 50 μL of the stop solution consisting of 10% of 97% H2SO4 (Sigma) was added to each well, and the plate was read at 450 nm on a Titertek Multiskan Plus spectrophotometer (Labsystem, Helsinki, Finland).
2-DE gel electrophoresis
Total-cell lysates from 10 × 106 of day 3–activated CD4+CD25− and activated CD4+CD25+ T cells were prepared according to the protocol used by Nyman et al14 with some modifications. The lysed samples were loaded onto an 18-cm pH 3-10 NL IPG strip (Amersham Biosciences) and incubated for 18 hours at RT. The isoelectrofocusing was carried out at 15°C to 70 kV. After focusing, the strips were equilibrated. For the second dimension, 12% SDS-PAGE, 1-mm-thick gels were used and the samples were run at 250 V. The gels were silver-stained using a mass spectrometry–compatible protocol.15 The gel images were analyzed with ImageMaster 2D Elite software (Amersham Biosciences, Becks, United Kingdom).
Tryptic digestion, peptide mass fingerprint by matrix-assisted laser desorption/ionization mass spectrometry, and database searching
In-gel trypsinolysis was performed using an Investigator Progest (Genomic Solutions, Huntingdon, United Kingdom) robotic digestion system, as previously described.16 Proteins were identified by correlation of uninterpreted tandem mass spectra to entries in SwissProt/TREMBL, using ProteinLynx Global Server (versions 1 and 1.1; Micromass, Elstree, United Kingdom). One missed cleavage per peptide was allowed, and the fragment ion tolerance was set to 100 parts per million (ppm). Carbamidomethylation of cysteine was assumed, but other potential modifications were not considered in the first pass search. All matching spectra were reviewed manually, and in cases where the score reported by the ProteinLynx global server (Waters, Milford, MA) was less than 100, additional searches were performed against the National Center for Biotechnology Information (NCBI) nonredundant database using MASCOT (Matrix Science, London, United Kingdom), which uses a robust probalistic scoring algorithm.17 Identifications were verified by manual sequencing using the MassLynx program Pepseq (Micromass).
Transcript omic analysis using high-density Affymetrix U95Av2 arrays
Two independent experiments were performed to compare the gene expression profiling of activated CD4+CD25+ and CD4+CD25− T cells. Protocols for sample preparation and array hybridization were performed according Affymetrix protocols (Santa Clara, CA).
Initial data analysis was performed with Affymetrix Microarray Suite 5 software and Microsoft Excel (Redmond, WA). First, the median intensity of each chip (after antibody amplification) was calculated from average difference values generated by the Affymetrix software (“raw data”). Then, the median intensities of all the chips were scaled to be the same, by arbitrarily selecting 1 chip and scaling the median intensities of the other chips to this value. This transformation generated the datasets that are referred to as the “normalized signal intensity values.”
Western blot analysis
Fresh and activated CD4+CD25− and CD4+CD25+ T-cell lysates (5 × 105 cells) were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA) in a semidry transfer system (Amersham Pharmacia Biotech, Uppsala, Sweden) and reacted with a mouse anti–human galectin-1 mAb (clone 25C1; Vector Laboratories, Burlingame, CA) in 5% milk/PBS plus 0.2% Tween 20 (Sigma). Blots were then incubated with an HRP-conjugated goat anti–mouse IgG1 (Amersham Pharmacia Biotech). rhGalectin-1 (PeproTechEC, Oxford, United Kingdom) was used as positive control. Loading controls were checked by incubation of the blots with mouse anti–β-actin mAb (Sigma).
Immunoprecipitation of galectin-1 from culture supernatants
The culture supernatants were incubated with mouse anti–human galectin-1 mAb (clone 25C1; Vector Laboratories) at 4°C for 18 hours. Galectin-1 was precipitated with immunopure immobilized Protein G (Pierce Biotechnology, Rockford, IL). Precipitates were separated in Novex 4% to 20% Tris-glycine gels (Invitrogen, Carlsbad, CA) under reducing conditions. After transfer to PVDF membranes, blots were probed with anti–human galectin-1 mAb and HRP-conjugated goat anti–mouse IgG1 and developed with SuperSignal West Femto (Pierce Biotechnology). rhGalectin-1 was used as positive control (Peprotech, London, United Kingdom).
Confocal microscopy
Cells (1 × 105) were washed in PBS, plated on l-poly-lysine (Sigma)–coated coverslips, and allowed to bind for 15 minutes. Cells were fixed with 4% PFA and permeabilized with 0.2% Triton X-100 (Sigma). For localization of galectin-1, cells were incubated with anti–human galectin-1 (Clone 25C1; Novo Castra, Newcastle, United Kingdom)mAb for 60 minutes at 20°C. Bound antibody was detected with FITC–goat antimouse mAb (Jackson ImmunoResearch, West Grove, PA) for 30 minutes at 37°C. Counterstaining was conducted using phalloidin TRITC-labeled (mixed isomers from Amanita phalloides; Sigma; Aldrich, Poole, United Kingdom). Coverslips were mounted on slides with a drop of mounting medium from DAKO Cytomation (Cambs, United Kingdom). Confocal laser-scanning microscopy was carried out with an LSM 510 microscope (Zeiss, Welwyn Garden City, United Kingdom) mounted over an affinity-corrected Axioplan microscope (Zeiss, Göttingen, Germany) using an EC Plan-Neofluar 40×/1.30 numerical aperture (NA) oil DIC or a Plan-Apochromat 100×/1.40 NA oil DIC objective. Dual-emission fluorescent images were collected in separate channels. Images were recorded using a Zeiss Axiocam HR camera and were acquired using Zeiss LSM5 software. Images were analyzed using Zeiss LSM5 Image Browser version 3.2.
Cell division analysis by CFSE staining
To analyze the number of cell divisions, 1 × 107 CD4+CD25− T cells were washed twice in PBS incubated in 1 μM CFSE (carboxyfluorescein diacetate succinimidyl diester; Molecular Probes, Eugene, OR) in PBS at 37°C for 3 minutes. Cells were then washed twice in cold PBS and once more in 5% FCS-PBS. CFSE-labeled cells were assessed by flow cytometry, and the purity of CFSE staining of cells was greater than 99%. Cells were stained with Annexin V–PE (Bender MedSystems, Vienna, Austria) and 7-amino-actinomycin D (7-AAD; BD Biosciences Pharmingen) according to manufacturer instructions to determine the number viable cells in the cultures (Annexin V−/7-AAD−). For accurate cell counting in each cell division, equal amounts of reference beads (CALIBRITE microbeads; Becton Dickinson) were added to all the tubes immediately prior to data acquisition following the recommendations of the manufacturer. Acquisition was terminated when 2000 microbeads were acquired.
Selection of primary mouse CD4+CD25+ and CD4+CD25− T cells and cocultures
Male and female 129P3/J (galectin-1+/+; Jackson Laboratories, Bar Harbor, ME) mice and 129P3/J galectin-1–null mice kindly provided by Prof D. Watt (University of Sussex, Brighton, United Kingdom) at the age of 6 to 10 weeks were used for these experiments. The generation of galectin-1–null mice has been described.18 Mice were maintained and handled in accordance with the Home Office regulations for Animal Care.
Spleens and lymph nodes were macerated through a 70-μm cell strainer and then treated with red-cell lysis buffer consisting of 155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA. Negative magnetic selection of CD4+ T cells was performed with sheep anti–rat IgG DynaBeads (Dynal, Oslo, Norway) to capture cells labeled with Antibody Mix (Mouse CD4 Negative Selection Kit; Dynal). CD4+CD25+ T cells were positively selected on MiniMACS columns (Miltenyi, Bergish Gladbach, Germany), using Streptavidin MicroBeads (Miltenyi) to capture CD4+ cells labeled with biotinylated anti-CD25 mAb (clone 7D4; BD Biosciences, Heidelberg, Germany). Purity of both CD4+CD25+ and CD4+CD25− T cells was greater than 93%.
Purified CD4+CD25+ T cells (1 × 105/well) were cultured in 96-well round-bottomed plates with CD4+CD25− T cells. T cells were activated with 1 μg/mL anti-CD3ϵ (145-2C11; BD Biosciences) in the presence of 1 × 105 antigen-presenting cells obtained by depleting mouse T cells from the wild-type 29P3/J mice with anti–mouse CD8 (53.6.72) and CD4 (YTS 151) mAbs. After 3 days of culture, the incorporation of [3H]TdR (Amersham Biosciences, Little Chalfont, United Kingdom) over 16 hours was measured.
Statistical analysis
Comparisons were done by multiple regression analysis. P values below .05 were considered significant.
Results
Isolation and characterization of CD4+CD25− and CD4+CD25+ T-cell populations
It is now widely accepted that it is the CD4+ T cells with the highest levels of CD25 expression (shown boxed in Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article) that corresponds to the naturally occurring Treg cells. In humans, these cells represent 2% (range, 0.5%-3.5%) of total CD4+ T-cell population; of those, approximately 75% (range, 70%-90%, from 5 donors) expressed FoxP3 by FACS (Figure S1B).
Selected T-cell populations were stimulated with anti-CD3/anti-CD28–coated beads for 5 days. The CD4+CD25− T cells responded to TCR cross-linking with a strong proliferative response, whereas the CD4+CD25+ T cells showed a very poor response (Figure S1C). Moreover, CD4+CD25+ T cells were able to strongly inhibit the proliferation of CD4+CD25− T cells in the cocultures (1:1 ratio).
Supernatants harvested from the cell cultures were used to analyze IL-2 and IFN-γ secretion by CTLL-2 bioassay and enzyme-linked immunosorbent assay (ELISA), respectively. CD4+CD25− T cells produced high levels of IL-2 and IFN-γ, whereas in the CD4+CD25+ T cells, low levels of both cytokines were detected. When CD4+CD25− T cells were cocultured with CD4+CD25+ T cells the cytokine production was dramatically reduced (Figure S1C). These data confirmed that culture conditions reproducibly reflected the suppressive phenotype of CD4+CD25+.
Constitutive galectin-1 expression in freshly isolated and TCR-activated Treg cells
In pursuit of the molecular basis of action of naturally occurring Treg cells, we examined the patterns of protein and gene expression in human activated CD4+CD25− and CD4+CD25+ T-cell populations using 2D electrophoresis (2-DE), mass spectrometry, and microarray methods. Although the 2D electrophoretograms obtained from whole-cell lysates of TCR-activated CD4+CD25+ and CD4+CD25− cells were similar overall, a few proteins were clearly up-regulated in the Treg cells. One spot, with an apparent mass of about 14.1 kDa, and a pI of 5.4, was consistently up-regulated 3-fold in the CD4+CD25+ population (Figure 1A), and was identified as galectin-1 by tandem mass spectrometry.
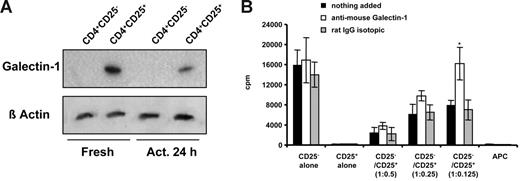
Expression and secretion of galectin-1 in human CD4+CD25− and CD4+CD25+ T cells. (A) Galectin-1 up-regulation is observed by comparative analysis of 2-DE of total-cell lysates obtained from activated CD4+CD25− and CD4+CD25+ human peripheral blood CD4+ T cells. Human CD4+CD25− and CD4+CD25+ T cells were activated with anti-CD3/anti-CD28–coated beads and cell lysates were prepared for 2-DE analysis. Proteins were separated using 18-cm 3-10 nonlinear IPG strips in the first dimension and 12% SDS-PAGE in the second dimension. Shown are the silver-stained gel images. Protein spots were sequenced by electrospray tandem mass spectrometry on the Micromass Q-Tof (high-performance liquid chromatography/tandem mass spectrometry [HPLC-MS/MS]). These results are representative of 3 independent experiments. (B) Kinetic analysis of the expression of galectin-1 in freshly isolated and in vitro–activated human CD4+, CD4+CD25−, and CD4+CD25+ T-cell populations by Western blot analysis. Human samples were prepared from total-cell lysates obtained from fresh and anti-CD3/anti-CD28–activated human T cells. The membrane was blotted with anti–human galectin-1 mAb (Clone 25C1; Novo Castra). Data are representative of 5 samples analyzed. (C) Detection of galectin-1 in the supernatants of anti-CD3/anti-CD28–activated human CD4+CD25− and CD4+CD25+ T cells and in cocultures of both populations (1:1 and 1:4 ratios) by immunoprecipitation. Cell-free supernatants were harvested from 48-hour cultures and galectin-1 was detected by immunoprecipitation with anti–human galectin-1 mAb. Data are representative of 2 experiments.
Expression and secretion of galectin-1 in human CD4+CD25− and CD4+CD25+ T cells. (A) Galectin-1 up-regulation is observed by comparative analysis of 2-DE of total-cell lysates obtained from activated CD4+CD25− and CD4+CD25+ human peripheral blood CD4+ T cells. Human CD4+CD25− and CD4+CD25+ T cells were activated with anti-CD3/anti-CD28–coated beads and cell lysates were prepared for 2-DE analysis. Proteins were separated using 18-cm 3-10 nonlinear IPG strips in the first dimension and 12% SDS-PAGE in the second dimension. Shown are the silver-stained gel images. Protein spots were sequenced by electrospray tandem mass spectrometry on the Micromass Q-Tof (high-performance liquid chromatography/tandem mass spectrometry [HPLC-MS/MS]). These results are representative of 3 independent experiments. (B) Kinetic analysis of the expression of galectin-1 in freshly isolated and in vitro–activated human CD4+, CD4+CD25−, and CD4+CD25+ T-cell populations by Western blot analysis. Human samples were prepared from total-cell lysates obtained from fresh and anti-CD3/anti-CD28–activated human T cells. The membrane was blotted with anti–human galectin-1 mAb (Clone 25C1; Novo Castra). Data are representative of 5 samples analyzed. (C) Detection of galectin-1 in the supernatants of anti-CD3/anti-CD28–activated human CD4+CD25− and CD4+CD25+ T cells and in cocultures of both populations (1:1 and 1:4 ratios) by immunoprecipitation. Cell-free supernatants were harvested from 48-hour cultures and galectin-1 was detected by immunoprecipitation with anti–human galectin-1 mAb. Data are representative of 2 experiments.
Transcriptomic analysis using Affymetrix U95Av2 arrays identified 435 genes that were overexpressed by a factor of 2 or more in the CD4+CD25+ cells, including 2 different galectin-1 gene transcripts (Table S1).
The up regulation of galectin-1 in human CD4+CD25+ Treg cells was confirmed by Western blotting of lysates of human CD4+CD25+ and CD4+CD25− T cells with anti–galectin-1 mAb (Figure 1B). Expression of galectin-1 was increased 24 hours after activation with anti-CD3/anti-CD28–coated beads.
Detection of galectin-1 in culture supernatants by immunoprecipitation
As shown in Figure 1C, high levels of galectin-1 were detected by immunoprecipitation with anti–human galectin-1 mAb only in those supernatants in which the CD4+CD25+ cells were present (either alone or in coculture at 1:1 ratio). Lower amounts of galectin-1 were detected in those supernatants in which the number of CD4+CD25+ cells seeded was reduced (cocultures at 1:0.25 ratios). Galectin-1 was barely detectable in supernatants derived from CD4+CD25− cultures. rhGalectin-1 was used as positive control.
Galectin-1 is predominantly expressed on human CD4+CD25high cells
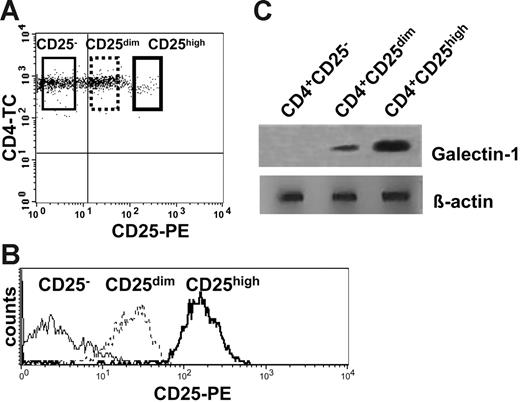
Coexpression of CD4 and CD25 has been used to identify Treg cells in mice and humans. Unlike the mouse, CD4+CD25+ Treg cells in the human are not a clearly distinct population. Rather, CD25 expression is a continuum from no expression to high levels of expression. Consequently, it is likely that some of the CD4+CD25dim T cells, which predominantly represent recently activated cells, may fall into the CD25high gate. Since the goal of this study was the identification of specific molecules in the Treg cells, we aimed at isolating them at the highest purity. CD4+ T cells with the highest CD25 expression level were FACS sorted to more than 98% purity (Figure 2). These CD4+CD25high T cells represented, on average, 1.8% of CD4+ T cells (range, 0.5%-2.2%, from 3 donors) and showed a slightly, but reproducibly, lower CD4 expression than the CD4+CD25dim and CD4+CD25− cells. CD4+CD25high, CD4+CD25dim, and CD4+CD25− T cells were sorted using the gates shown in Figure 2A and 2B. To address which of the 3 subpopulations of CD4+ cells expressed galectin-1, freshly sorted cells were lysed and assessed by Western blot using a human anti–galectin-1 mAb. Interestingly, the expression of galectin-1 was mainly confined to the CD4+CD25high population (Figure 2C). CD4+CD25dim T cells also expressed galectin-1, although the expression level was lower than in the CD4+CD25high T cells.
Galectin-1 expression correlates with the expression levels of the CD25 marker. (A) Freshly isolated human CD4+ T cells were labeled with anti-CD4 and anti-CD25 mAbs and sorted according to the gates illustrated to isolate CD4+CD25−, CD4+CD25dim, and CD4+CD25+ populations. (B) Histogram plot showing the expression of CD25 in the sorted populations. (C) Lysates of freshly isolated CD4+CD25−, CD4+CD25dim, and CD4+CD25high T cells were examined by Western blot analysis for galectin-1 expression.
Galectin-1 expression correlates with the expression levels of the CD25 marker. (A) Freshly isolated human CD4+ T cells were labeled with anti-CD4 and anti-CD25 mAbs and sorted according to the gates illustrated to isolate CD4+CD25−, CD4+CD25dim, and CD4+CD25+ populations. (B) Histogram plot showing the expression of CD25 in the sorted populations. (C) Lysates of freshly isolated CD4+CD25−, CD4+CD25dim, and CD4+CD25high T cells were examined by Western blot analysis for galectin-1 expression.
Galectin-1 is found in the nucleus and the cytoplasm of T cells
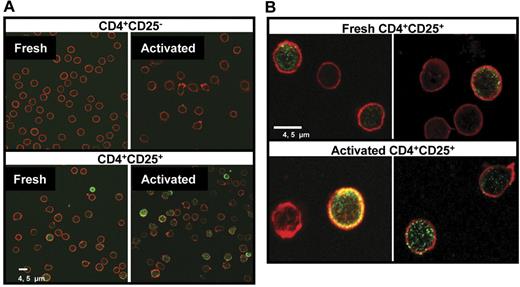
Cellular localization of galectin-1 on freshly isolated and TCR-activated Treg cells was determined by confocal microscopy. We found that approximately 25% of the cells in the freshly isolated CD4+CD25+ fraction (range, 7%-34%, from 10 donors) contained high levels of galectin-1, with staining localized mainly in the nucleus and the cytoplasm (Figure 3), whereas less than 8% of the CD4+CD25− population (range, 1.5%-12%, from 10 donors) showed positive staining. After activation with anti-CD3/anti-CD28 Ab–coated beads, approximately 35% of the CD4+CD25+ Treg cells (range, 20%-40%, from 10 donors) were positive for galectin-1, with brighter fluorescence intensity than the corresponding non-activated cells.
Detection of galectin-1 by confocal microscopy. (A) Freshly isolated and anti-CD3/anti-CD28–activated CD4+CD25− and CD4+CD25+ T cells were fixed with 4% PFA and permeablized by 0.2% Triton X-100. Samples were stained with mouse anti–human galectin-1 mAb followed by FITC conjugated goat antimouse antibody (green). Cytoskeleton was stained by phalloidin-TRITC (red). (B) Images of fresh and activated human CD4+CD25+ T cells at high magnification showing the localization of galectin-1 inside the cells.
Detection of galectin-1 by confocal microscopy. (A) Freshly isolated and anti-CD3/anti-CD28–activated CD4+CD25− and CD4+CD25+ T cells were fixed with 4% PFA and permeablized by 0.2% Triton X-100. Samples were stained with mouse anti–human galectin-1 mAb followed by FITC conjugated goat antimouse antibody (green). Cytoskeleton was stained by phalloidin-TRITC (red). (B) Images of fresh and activated human CD4+CD25+ T cells at high magnification showing the localization of galectin-1 inside the cells.
As shown in Figure 3B, galectin-1 was found in the nuclei of the cells as well as in the inner side of the plasma membrane. In a few cases galectin-1 was detected as single or double clusters in the plasma membrane of the cells.
Anti–galectin-1 mAb can reverse the in vitro suppressive activity of naturally occurring CD4+CD25+ Treg cells
To determine whether galectin-1 played a role in the mechanism of suppression, we investigated the effect of anti–galectin-1 Ab on the ability of human CD4+CD25+ Treg cells to suppress the in vitro proliferative responses of CD4+CD25− T cells. As shown in Figure 4A and 4B, CFSE dye dilution analysis by flow cytometry of CFSE-labeled responder CD4+CD25− T cells demonstrated that the anti–galectin-1 mAb dramatically reversed the Treg-cell–mediated suppression as judged by the number of cells at the end of the culture, whereas the corresponding isotype-matched control IgG1 did not diminish the suppressive effects of the Treg cells. Several groups have suggested that human CD4+CD25+ Treg cells induce the production of IL-10 in CD4+CD25− cells when cocultured with CD4+CD25+ Treg cells in vitro. To test the contribution of IL-10 to the observed suppression anti–IL-10 mAb was added to the cocultures. As indicated in Figure 4B, anti–IL-10 did not alter the suppressive function of CD4+CD25+ Treg cells. This observation further stresses the key role of galectin-1 in the suppressive function of the CD4+CD25+ Treg cells.
Anti–galectin-1 mAb can block the in vitro suppressive activity of naturally occurring human CD4+CD25+ Treg cells. Freshly isolated and CFSE labeled CD4+CD25− and CD4+CD25+ T cells or a combination of both (1:1 ratio) were stimulated with anti-CD3/anti-CD28–coated beads for 5 days. (A) Number of viable (Annexin V−/7-AAD−) CFSE-labeled CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and the number of viable CFSE-CD4+CD25− T cells in the cocultures. Data are mean ± SD, representative of 1 representative experiment of 10 experiments (*P < .015). (B) The number of viable CFSE-labeled CD4+CD25− T cells was determined in each cell division. The CFSE-labeled CD4+CD25− T cells were cultured alone or in combination with CD4+CD25+ T cells (1:1 ratio) with or without 0.1 μg/mL of anti–galectin-1 mAb. Data represent mean + SD of 4 experiments. Proliferation of anti-CD3/anti-CD28–activated (C) and allogeneic PBMNC-stimulated (D) CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and at decreasing ratios from 1:1 to 1:0.125, respectively. Cells were cultured for 5 days, 16 hours before harvesting cultures were pulsed with 3[H]TdR. Data are means ± SD from 1 experiment representative of 3 experiments that were performed (*P < .015).
Anti–galectin-1 mAb can block the in vitro suppressive activity of naturally occurring human CD4+CD25+ Treg cells. Freshly isolated and CFSE labeled CD4+CD25− and CD4+CD25+ T cells or a combination of both (1:1 ratio) were stimulated with anti-CD3/anti-CD28–coated beads for 5 days. (A) Number of viable (Annexin V−/7-AAD−) CFSE-labeled CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and the number of viable CFSE-CD4+CD25− T cells in the cocultures. Data are mean ± SD, representative of 1 representative experiment of 10 experiments (*P < .015). (B) The number of viable CFSE-labeled CD4+CD25− T cells was determined in each cell division. The CFSE-labeled CD4+CD25− T cells were cultured alone or in combination with CD4+CD25+ T cells (1:1 ratio) with or without 0.1 μg/mL of anti–galectin-1 mAb. Data represent mean + SD of 4 experiments. Proliferation of anti-CD3/anti-CD28–activated (C) and allogeneic PBMNC-stimulated (D) CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and at decreasing ratios from 1:1 to 1:0.125, respectively. Cells were cultured for 5 days, 16 hours before harvesting cultures were pulsed with 3[H]TdR. Data are means ± SD from 1 experiment representative of 3 experiments that were performed (*P < .015).
The reversion of the suppressive activity of CD4+CD25+ Treg cells by anti–galectin-1 antibody was then assessed in proliferation assays stimulated by anti-CD3/anti-CD28–coated beads (polyclonal; Figure 4C), or by allogeneic CD2-depleted PBMNCs (MLR; Figure 4D). In anti-CD3/anti-CD28–activated cultures, significant reversion of suppression was detected in cocultures at 1:1 and 1:0.25 ratios of CD4+CD25− to CD4+CD25+, respectively (Figure 4C). When allogeneic cells were used as stimulus, the blockade of suppression by anti–galectin-1 antibody was only observed at 1:1 ratios (Figure 4D).
Galectin-1 is required for optimal CD4+CD25+ Treg function in mice
To further dissect the role of galectin-1 in Treg cells, we extended these studies to a murine system and confirmed by Western blot up-regulation of galectin-1 in both freshly isolated and activated CD4+CD25+ Treg cells (Figure 5A). In contrast to human cells, the constitutive expression of galectin-1 by mouse cells follows a different kinetics since the levels of galectin-1 detected after 24 hours of in vitro activation were slightly reduced with respect to freshly isolated cells. As in humans, the anti–galectin-1 mAb treatment fully restored the in vitro proliferative response of mouse CD4+CD25− T cells when cells were cocultured with CD4+CD25+ Treg cells (Figure 5B).
Expression of galectin-1 in mouse CD4+CD25− and CD4+CD25+ T cells and blockade of in vitro suppressive activity by anti–galectin-1. (A) Expression of galectin-1 in freshly isolated and in vitro–activated mouse CD4+CD25− and CD4+CD25+ T-cell populations by Western blot analysis. Samples were prepared from total-cell lysates obtained from fresh and activated T cells stimulated by anti-CD3ϵ antibody and T-cell–depleted antigen-presenting cells. The membrane was blotted with anti–mouse galectin-1 mAb (clone 201002; R&D Systems). (B) In vitro proliferation of mouse CD4+CD25− and CD4+CD25+ T cells cultured alone and in cocultures at the indicated ratios. Data are mean ± SD of 1 representative experiment of 3 experiments (*P < .011).
Expression of galectin-1 in mouse CD4+CD25− and CD4+CD25+ T cells and blockade of in vitro suppressive activity by anti–galectin-1. (A) Expression of galectin-1 in freshly isolated and in vitro–activated mouse CD4+CD25− and CD4+CD25+ T-cell populations by Western blot analysis. Samples were prepared from total-cell lysates obtained from fresh and activated T cells stimulated by anti-CD3ϵ antibody and T-cell–depleted antigen-presenting cells. The membrane was blotted with anti–mouse galectin-1 mAb (clone 201002; R&D Systems). (B) In vitro proliferation of mouse CD4+CD25− and CD4+CD25+ T cells cultured alone and in cocultures at the indicated ratios. Data are mean ± SD of 1 representative experiment of 3 experiments (*P < .011).
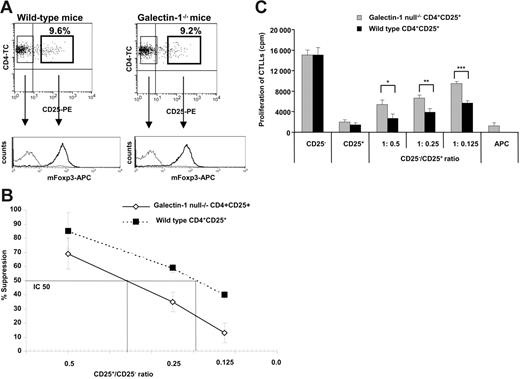
If galectin-1 played a key role in regulation, Treg cells from mice genetically deficient in galectin-1 would be less able to suppressed immune responses than Treg cells from the corresponding wild-type littermates. As shown in Figure 6A, similar percentages of CD4+ cells coexpressed CD25 in both strains of mice. By flow cytometry, the expression of FoxP3, a transcription factor that has been associated with the suppressive function of the Treg cells, was similar in the wild-type and in the galectin-1 homozygous null mutant (galectin-1−/−) mice, suggesting that the genetic alteration induced in the knockout mice did not affect the transcription and expression of FoxP3. Similarly, the expression of molecules such as CD69, CD45RB, CD152 (CTLA-4), CD62L, and GITR were indistinguishable in both strains of mice (data not shown). Selected CD4+CD25− and CD4+CD25+ T-cell populations were activated in vitro in the presence of T-cell–depleted antigen-presenting cells and anti-CD3ϵ antibody. Interestingly, increased cell proliferation was observed for both galectin-1–null CD4+CD25− and CD4+CD25+ T cells compared with the wild-type mice (Figure S2).
Galectin-1 is required for maximal regulatory T cell function. (A) Phenotype of CD4+CD25− and CD4+CD25+ T cells of wild-type and galectin-1−/− 129P3/J mice. Dotplots showing similar percentages of CD4+CD25+ T cells in both strains of mice and expression of Foxp3 in the selected CD4+CD25− and CD4+CD25+ T-cell populations by FACS analysis from both strain of mice. Plots are from 1 representative experiment of 3 experiments. (B) The suppressive capacity of the wild-type and galectin-1−/− CD4+CD25+ T cells was compared by mixing the cells at different ratios with galectin-1−/− CD4+CD25− T cells. The cells were stimulated with anti-CD3ϵ antibody and T-cell–depleted antigen-presenting cells for 72 hours. The IC50 line is represented to indicate the CD25+/CD25− ratio that gives 50% suppression (n = 3). (C) Secretion of IL-2 as measured by CTLL cell proliferation in the supernatants harvested after 48 hours of culture. *P ≤ .038; **P ≤ .049; ***P ≤ .026. The data are the mean ± SD values of 1 representative experiment of 3 independent experiments.
Galectin-1 is required for maximal regulatory T cell function. (A) Phenotype of CD4+CD25− and CD4+CD25+ T cells of wild-type and galectin-1−/− 129P3/J mice. Dotplots showing similar percentages of CD4+CD25+ T cells in both strains of mice and expression of Foxp3 in the selected CD4+CD25− and CD4+CD25+ T-cell populations by FACS analysis from both strain of mice. Plots are from 1 representative experiment of 3 experiments. (B) The suppressive capacity of the wild-type and galectin-1−/− CD4+CD25+ T cells was compared by mixing the cells at different ratios with galectin-1−/− CD4+CD25− T cells. The cells were stimulated with anti-CD3ϵ antibody and T-cell–depleted antigen-presenting cells for 72 hours. The IC50 line is represented to indicate the CD25+/CD25− ratio that gives 50% suppression (n = 3). (C) Secretion of IL-2 as measured by CTLL cell proliferation in the supernatants harvested after 48 hours of culture. *P ≤ .038; **P ≤ .049; ***P ≤ .026. The data are the mean ± SD values of 1 representative experiment of 3 independent experiments.
To examine the contribution of galectin-1 to the suppressive effects of murine Treg cells, we compared the ability of CD4+CD25+ Treg cells purified from galectin-1–null and wild-type mice to inhibit the proliferation of galectin-1–null CD4+CD25− T cells. In these experiments, a titration of CD4+CD25+ Treg cells was assessed. As shown in Figure 6B, galectin-1–null CD4+CD25+ T cells were less capable (IC50 = 0.36) of inhibiting the proliferation of the responder cells than their wild-type counterparts (IC50 = 0.19; P = .007). The production of IL-2 was determined in the supernatants of the cocultures by CTLL proliferation assay (Figure 6C). The capacity of the CD4+CD25− and CD4+CD25+ T cells to produce IL-2 was similar in both strains of mice. Interestingly, when galectin-1–null CD4+CD25+ T cells were used in the cocultures, the levels of IL-2 detected were significantly higher than in those cocultures seeded with wild-type CD4+CD25+ T cells, suggesting that the galectin-1–null CD4+CD25+ T cells have a reduced capacity to inhibit the transcription of IL-2 by the responder cells. Altogether, these results suggest that galectin-1 plays a significant role in suppression mediated by murine as well as human CD4+CD25+ Treg cells.
Discussion
The data described here demonstrate that galectin-1 is markedly overexpressed in human and mouse CD4+CD25+ Treg cells, as assessed by gene expression analysis, 2-DE gels, and Western blotting. Confocal microscopy confirmed these findings, and revealed a marked increase in levels of galectin-1 expression following activation. Galectin-1 was also detected in the supernatants of those cultures where the CD4+CD25+ Treg cells were present. Most importantly, addition of an anti–galectin-1 specific antibody led to almost complete reversal of the suppression caused by CD4+CD25+ Treg cells. Galectin-1 expression was confined to CD4+CD25+ Treg cells. Furthermore, galectin-1–null murine Treg cells were less potent suppressors than their wild-type counterparts.
Galectin-1 is a member of a highly conserved family of β-galactoside–binding proteins that are widely distributed in lymphoid and nonlymphoid tissues and perform a variety of immunoregulatory functions. Functional galectin-1 is a homodimer and is secreted by a nonclassical mechanism that bypasses the endoplasmic reticulum and the Golgi apparatus.19–21
Recently, galectin-1 has attracted the attention of immunologists as a master regulator of cell homeostasis and inflammation.20,22 Galectin-1 expression has been reported in thymic epithelial cells,23 activated CD8+ T cells,24 macrophages,25 activated B cells,26 and mouse CD4+CD25+ T cells by DNA microarray.27 In addition, it is highly expressed by many tumors, in which it appears to protect from T-cell attack.28 In the regulation of T-cell activation the key target glycoproteins ligated by galectin-1 are CD45, CD43, and CD7. The consequences of galectin-1 binding by its ligands in T cells include cell-cycle arrest and/or apoptosis, and the inhibition of proinflammatory cytokine release, including IL-2 and IFN-γ.20,29
Interestingly, galectin-1 has been successfully used to treat several models of T helper 1 cell (Th1)–mediated autoimmune diseases, including experimental autoimmune encephalomyelitis in rats30 and collagen-induced arthritis.31 Amelioration of graft-versus-host disease following allogeneic bone marrow transplantation has also been demonstrated in a murine model.32
Galectin-1 is known to be an important contributor to T-cell homeostasis, although the mechanisms by which it mediates immunomodulation have not been elucidated. An important point to determine would be whether it acts as a soluble “cytokine” or exerts its effects via cell-cell contact. Conditioned media from melanomas containing soluble galectin-1 induced T-cell apoptosis,28 whereas supernatants from CD4+CD25+ T cells did not possess suppressive effects.3,4 Since 10-fold greater amounts of soluble than surface galectin-1 are required for an equivalent biological effect,33 the need for cell-cell contact in in vitro assays of Treg function may be due to insufficient secretion by CD4+CD25+ T cells. Our confocal microscopy data demonstrate that galectin-1 is present in both the cytoplasm and the nuclei of human CD4+CD25+ T cells (Figure 3). Upon activation, higher levels of galectin-1 were detected in the nuclei and particularly in the cytoplasm of the CD4+CD25+ T cells, from where it can be rapidly transported to the surface or secreted. This is consistent with our suggestion that surface-bound galectin-1 is a key mediator of CD4+CD25+ T-cell suppression.
Galectin-1 inhibits effector functions by promoting growth arrest and apoptosis of activated T cells,19,24 possibly via partial activation34 and increased susceptibility to activation-induced cell death,31 or by blocking proinflammatory cytokine secretion.29 These properties of galectin-1 are well correlated with the known mechanisms of Treg-cell–mediated suppression. The increase in galectin-1 expression following activation would account for the requirement for prior activation of the Treg cells before suppression can occur. The ability of galectin-1 to bind glycoproteins such as CD45, CD43, and CD7 on the T-cell surface35 is consistent with the non–antigen-specific nature of suppression. Cell-contact–dependent suppression by CD4+CD25+ T cells could be explained by their expression of surface-bound galectin-1 homodimers able to cross-link glycoprotein receptors on responding T cells.36 The observation that galectin-1-treatment of collagen-sensitized mice causes decreased IFN-γ and IL-2 production29 mirrors the in vitro effects of CD4+CD25+-mediated suppression.
Taken together, our observations implicate galectin-1 as a very plausible candidate effector molecule for CD4+CD25+ Treg function. That this molecule has immunoregulatory effects in other contexts is a further example of the economy of the immune system. It remains to be determined whether our findings can be exploited to harness and/or inhibit immune regulation in the context of transplantation, autoimmune disease, and cancer therapy.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Lechler, Vice-Principal (Health), King's College London, Rm 1.4, Hodgkin Bldg, Guy's Campus, London SE1 1UL United Kingdom; e-mail: robert.lechler@kcl.ac.uk, jane.pearson@kcl.ac.uk
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Dr S. Gamble, Dr R. Edwards, and Mrs Shajna Begum for their advice and skilful technical assistance with the preparation of samples for the 2-DE gel analysis and mass spectrometry; Prof A. J. Ridley for facilitating the confocal studies at the Ludwig Institute for Cancer Research; Prof D. Watt for supplying the galectin-1–null mice used in these studies; Dr D. Marín for statistical analysis; and Dr M. P. Hernández-Fuentes for critical review of this manuscript. We are grateful to the Department of Immunology, Faculty of Medicine of Imperial College London, Hammersmith Campus, where most of the experimental work was conducted.
This work was supported by the Swiss National Science Foundation (grant number 3235 to D.G.), the Spanish Ministerio de Educación y Ciencia (Postdoctoral Fellowship MEC, to E.C.-M.), and grants from the Medical Research Council (MRC), the British Heart Foundation (BHF), and the Arthritis Research Campaign (ARC), United Kingdom, and Reprogramming the Immune System for the Establishment of Tolerance (RISET; from the European Union [EU]).

![Figure 1. Expression and secretion of galectin-1 in human CD4+CD25− and CD4+CD25+ T cells. (A) Galectin-1 up-regulation is observed by comparative analysis of 2-DE of total-cell lysates obtained from activated CD4+CD25− and CD4+CD25+ human peripheral blood CD4+ T cells. Human CD4+CD25− and CD4+CD25+ T cells were activated with anti-CD3/anti-CD28–coated beads and cell lysates were prepared for 2-DE analysis. Proteins were separated using 18-cm 3-10 nonlinear IPG strips in the first dimension and 12% SDS-PAGE in the second dimension. Shown are the silver-stained gel images. Protein spots were sequenced by electrospray tandem mass spectrometry on the Micromass Q-Tof (high-performance liquid chromatography/tandem mass spectrometry [HPLC-MS/MS]). These results are representative of 3 independent experiments. (B) Kinetic analysis of the expression of galectin-1 in freshly isolated and in vitro–activated human CD4+, CD4+CD25−, and CD4+CD25+ T-cell populations by Western blot analysis. Human samples were prepared from total-cell lysates obtained from fresh and anti-CD3/anti-CD28–activated human T cells. The membrane was blotted with anti–human galectin-1 mAb (Clone 25C1; Novo Castra). Data are representative of 5 samples analyzed. (C) Detection of galectin-1 in the supernatants of anti-CD3/anti-CD28–activated human CD4+CD25− and CD4+CD25+ T cells and in cocultures of both populations (1:1 and 1:4 ratios) by immunoprecipitation. Cell-free supernatants were harvested from 48-hour cultures and galectin-1 was detected by immunoprecipitation with anti–human galectin-1 mAb. Data are representative of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-04-016451/4/m_zh80050708800001.jpeg?Expires=1765912807&Signature=X6Bbq8Ix~kruWEKpMnGIG-eOkpJX7hbg7wxi6-DLhDwUnUPCEPshj4z86~extayYgGt00UWBsIblFLEa1bvrIXKiSpRNu2itQy-qoFNanmlBvoKmC6TgMZmJ2AkuQyy9eYzs7PQKGdPUlDQoCcSCVSm1EClK15SCiXX7c-pex4VG8U161ewLnXUM1Jl4nat0bydbNkXGaAgk3esScKrLS9fWh69T4WMVKNlOC2rApOlCXKbCmBUtPlvM5UEQHTbv4qmYxChcgOIn9RE0U6M7OjTjKrObmbiRQBZbZ3vUQhylqOP0uuogJEwmocDOw41QJzbqIhzE8dleicouiJEk2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Anti–galectin-1 mAb can block the in vitro suppressive activity of naturally occurring human CD4+CD25+ Treg cells. Freshly isolated and CFSE labeled CD4+CD25− and CD4+CD25+ T cells or a combination of both (1:1 ratio) were stimulated with anti-CD3/anti-CD28–coated beads for 5 days. (A) Number of viable (Annexin V−/7-AAD−) CFSE-labeled CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and the number of viable CFSE-CD4+CD25− T cells in the cocultures. Data are mean ± SD, representative of 1 representative experiment of 10 experiments (*P < .015). (B) The number of viable CFSE-labeled CD4+CD25− T cells was determined in each cell division. The CFSE-labeled CD4+CD25− T cells were cultured alone or in combination with CD4+CD25+ T cells (1:1 ratio) with or without 0.1 μg/mL of anti–galectin-1 mAb. Data represent mean + SD of 4 experiments. Proliferation of anti-CD3/anti-CD28–activated (C) and allogeneic PBMNC-stimulated (D) CD4+CD25− T cells and CD4+CD25+ T cells cultured alone and at decreasing ratios from 1:1 to 1:0.125, respectively. Cells were cultured for 5 days, 16 hours before harvesting cultures were pulsed with 3[H]TdR. Data are means ± SD from 1 experiment representative of 3 experiments that were performed (*P < .015).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-04-016451/4/m_zh80050708800004.jpeg?Expires=1765912807&Signature=nBHFhET7TDVBW6kfcNqrzrgCMdyow0qWjiQoza07hINlHrXVT1b-uWb5VS-sRjYA2OPRKKysVatqJkNNo6VqY6FQwLLrgdvx0Gfym7W2VReiiWt6lQbMsIvzbHLdrHH7Fyz0sR7OTTCxzOjDFQ8ab3CJINdAv~7sIoYELA~XygF4MS3u~4IzXzdv1kwvW6MK-Eh6J6HzEZ3GQ2Rp7FnLJ~h8WuAcaqYFZKfJE2~ymL8WZMcd0ec4hMazZatnr4ertzh2ipCdC1okd7UVtu4rFWXOxuf4Y~aJIkoOHvLyari7OUJM3bENWFDAjQ2fFtG5537IqV3ANkZuphb34q5Kbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)