Abstract
Millions of women still use postmenopausal hormone therapy (HT). We genotyped 2090 women in Heart and Estrogen/progestin Replacement Study for functional polymorphisms in GP1BA and GP6 and assessed the coronary heart disease (CHD) event rate over 5.8 years of follow-up. In patients receiving placebo, there was an increased CHD death/myocardial infarction (MI)/unstable angina (UA) event rate in carriers of the GP1BA −5C allele (adjusted [adj] P = .006). HT increased the hazard ratio (HR) of CHD events in patients with the GP1BA −5TT genotype by 16% and reduced the HR in patients with the TC+CC genotypes by 46% (adj interaction P < .001). HT reduced the HR in patients with the GP6 13254TT genotype by 17% but increased the HR in patients with the TC+CC genotypes by 35% (adj interaction P < .001). Furthermore, HT increased the HR of CHD events in patients with the GP1BA −5TT plus GP6 13254TC+CC genotypes by 57% and reduced the HR in patients with the GP1BA −5TC+CC plus GP6 13254TT genotypes by 55% (adj interaction P < .001). In postmenopausal women with established CHD, these polymorphisms of platelet genes were predictors of CHD events and significantly modified the effects of HT on CHD risk. It will be important to replicate these findings in other studies.
Introduction
Coronary heart disease (CHD) is the most common cause of morbidity and mortality among American men and women. Based on laboratory investigations and observational studies, it was previously believed that postmenopausal hormone therapy (HT) was cardioprotective.1 However, randomized clinical trials with HT have either failed to support this benefit or have demonstrated adverse coronary outcomes in women receiving HT.2–5 As a result, HT use in the United States has dramatically decreased, although millions of women still use these agents.6 Because of the continued use of HT and because the absolute risk of adverse cardiovascular events is rather small, it would be highly desirable to have markers of HT risk that would permit a more rational approach to patient management.
Myocardial infarction (MI) and unstable angina (UA) develop when a platelet thrombus forms at the site of a ruptured or eroded unstable coronary atherosclerotic plaque.7 The essential pathophysiologic components of platelet thrombus formation are adhesion, activation, and aggregation. Two major platelet receptors involved in adhesion and activation are the von Willebrand factor receptor, glycoprotein (GP) Ibα, and a major signaling collagen receptor, GPVI.8 Upon arterial plaque rupture, the initial platelet contact to exposed subendothelial collagen requires the binding of the platelet membrane GPIbα to immobilized von Willebrand factor (VWF).8 The VWF-GPIbα interaction results in platelets “rolling” along the exposed subendothelium, and this slower velocity permits platelet GPVI to bind to collagen.9 Signaling between and through these crucial adhesive receptors causes platelet activation and subsequent aggregation.
Genetic contributions to CHD and arterial thrombosis are well established.10 The “Kozak” polymorphism of the gene encoding GPIbα (GP1BA) is a T>C substitution 5 base pairs upstream of the initiation codon that has been associated with increased platelet expression of GPIbα and with acute coronary syndrome (ACS) risk in some studies,11–13 although other studies have found no association.14–16 The gene encoding GPVI (GP6) harbors a 13254T>C polymorphism that induces a Ser219Pro substitution; the 13254CC genotype has been associated with an increased risk of MI17 and coronary thrombi,18 despite being associated with less in vitro thrombogenicity on collagen.19 Most of the genetic epidemiology studies on GP1BA and GP6 polymorphisms have been exclusively or predominantly in male patients, despite the fact that some studies suggest that genetic effects on arterial thrombosis may be greater for women than men.20–22
The Heart and Estrogen/progestin Replacement Study (HERS) was a large randomized clinical trial of HT in women with established coronary disease. Because HT use has been associated with increased platelet reactivity,23–26 we reasoned that the HERS trial provided a unique opportunity to test for (1) associations between inherited variations in platelet genes and CHD events, and (2) interactions between these platelet polymorphisms and HT in a population of women with established CHD.
Patients, materials, and methods
Study subjects
The design, methods, and main outcomes of HERS have been previously reported.2,27 Participants were postmenopausal women younger than 80 years with established coronary artery disease and no prior hysterectomy who were randomly assigned to oral conjugated equine estrogen (0.625 mg per day) plus medroxyprogesterone acetate (2.5 mg per day) or placebo. The study was approved by an institutional review committee at each of the 18 US centers2 and the subjects gave informed consent. Established coronary disease was defined as 1 or more of the following: MI, coronary artery bypass graft surgery, percutaneous coronary revascularization, or angiographic evidence of at least a 50% occlusion of 1 or more major coronary arteries. The clinical trial ended after 4.1 years of follow-up, at which time the decision to use HT was left to the participant and her physician (details described in Grady et al27 ). Outcome follow-up continued for an additional 2.7 years, for a mean total follow-up of 6.8 ± 2.0 years.
Outcomes
The outcome of interest for the current analysis is the composite of all CHD events including CHD death, nonfatal MI, and UA requiring hospitalization. These outcomes are all felt to be dependent, in some part, upon platelet thrombus formation. CHD death included a documented fatal MI, sudden death within 1 hour of onset of symptoms, unobserved death that occurred out of the hospital in the absence of other known causes, and death due to coronary revascularization or congestive heart failure. The diagnosis of nonfatal MI was based on an algorithm that included ischemic symptoms, electrocardiogram (ECG) abnormalities, and elevated cardiac enzyme levels.28 UA was diagnosed in participants who were admitted to the hospital for management of anginal chest pain or an anginal equivalent but found not to have an MI. Details concerning the documentation of clinical events are described elsewhere.28
DNA isolation and genotyping
At the end of the trial, consent was obtained from participants to use their annually collected pap smears as a source of DNA for future studies of the cardiovascular and other effects of estrogen. Consent was also requested from family members of deceased participants. After consent was obtained, coverslips were removed with a razor blade after placing the slides on a metal stage in a liquid nitrogen bath. Slides were then treated in consecutive washes of Citrisolv (Fisher Scientific, Sommeville, NY) (first for 10 minutes, then for 5 minutes) to remove the coverslip adhesive followed by 2 washes in 100% ethanol (first for 10 minutes, then for 5 minutes). Slides were then allowed to air-dry. After the addition of 20 μL water, cellular material was scraped from the slides using a razor blade, transferred to a 1.5-mL microfuge tube, and resuspended in 600 μL Cell Lysis Solution (Gentra Systems, Minneapolis, MN). The cell lysis was treated with 4 μL proteinase K (20 mg/mL), followed by incubation at 55°C for at least 3 hours. Four microliters of RNase A (Gentra Systems) was added to each tube, mixed by inverting, and incubated at 37°C for 15 minutes. Next, 200 μL Protein Precipitation Solution (Gentra Systems) was added and mixed by vortexing for 20 seconds. The proteins were pelleted by centrifugation (13 000g to 16 000g) for 3 minutes, and the supernatant was transferred to a 1.5-mL microfuge tube. After the addition of 2.0 μL Gentra Glycogen solution (20 μg/mL), the DNA was precipitated with isopropanol and washed with 70% ethanol. Each sample was resuspended in 20 μL DNA Hydration solution (Gentra Systems), rehydrated at 60°C for 1 hour, quantified, and stored at −20°C.
Genotyping of the GP1BA −5T>C and GP6 13254T>C single-nucleotide polymorphisms (SNPs) was performed using the MassARRAY genotyping system (Sequenom, San Diego, CA). For GP1BA −5T>C, the polymerase chain reaction (PCR) primers 5′-ACGTTGGATGTTGGCAGCAGGAGCAGCAAG-3′ and 5′-ACGTTGGATGATCCACTCAAGGCTCCCTTGC-3′ were used in conjunction with the extension primer 5′-GAGGAGGAGAGGCATGAGG-3′. For GP6 13254T>C, the PCR primers 5′-ACGTTGGATGATGGACCCTGCAGAACCTAC-3′ and 5′-ACGTTGGATGTCTGATTTCCCAGGAACCTC-3′ were used with the extension primer 5′-AGAACCTACCTGCTACCG-3′. All reactions were performed according to the manufacturer's instructions, and scoring was performed using SpectroTyper software (Sequenom). Consent and usable DNA were obtained from 2229 of the original 2763 HERS participants (81%). From these subjects, 2090 (94%) were successfully genotyped for both the GP1BA variant and the GP6 variant. Analyses for the current report are based on data from this subset of the original HERS cohort. Participants were also genotyped for 5 other genetic variants in platelet genes: the PlA (HPA-1, Leu33Pro) polymorphism of ITGB3, a second variant in GP1BA (HPA-2, GPIbα Thr145Met), the 807T>C polymorphism of ITGA2, the1838G>A substitution of ADRA2A (α2-adrenergic receptor), and the 852C>T substitution of GNB3 (beta subunit of G proteins). None of these other 5 SNPs consistently interacted with HT at year 1 and follow-up (Tables S1–S3 available on the Blood website; see the Supplemental Tables link at the top of the online article).
Statistical analyses
Chi-square tests were used to test for Hardy-Weinberg equilibrium for each SNP after stratification by race/ethnicity. Cox proportional hazard models were used to estimate genotypic relative hazards in the placebo group after adjustment for age and potentially confounding baseline covariates. These baseline covariates were identified using a forward stepwise selection process from among all the variables listed in Table 1 that were associated (P < .1) with case status in univariate analyses. The variables that survived the forward stepwise selection process at a level of P < .05 included hypertension based on physician diagnosis or blood pressure higher than 140/90 mm Hg, diabetes requiring medication, creatinine clearance, waist-to-hip ratio, and aspirin and statin use. The addition of other variables reasonably predicted to influence the outcomes of interest based on a priori knowledge, but not included in the stepwise selection process, including race and smoking, did not change the qualitative result (data not shown). In separate analyses, the addition of each of the baseline covariates, including all of those retained in the stepwise procedure, was evaluated for its potential effect on the genotypic hazard ratios; however, none of them materially changed the magnitude or the significance of the univariate association between the genotypes of interest and risk for CHD events. Data on nonsteroidal anti-inflammatory and COX-2 inhibitor use were not available. The genetic associations were examined using both additive and dominant genetic models. There were too few variant allele homozygotes to support recessive models. Haplotype analyses were performed using Haplo.GLM (SAS, Cary, NC). To assess the possibility of an interaction between the platelet receptor protein polymorphisms and HT, additional Cox models were constructed using the entire study population. These models included treatment assignment, gene polymorphism, and treatment assignment*gene polymorphism interaction terms. The likelihood ratio test was used to assess the significance of the interactions. The interaction analyses assumed a dominant genetic model for each allelic variant and were performed according to intention to treat. Relative hazards for the composite outcome were determined based on CHD events that occurred after 1 year of follow-up as well as all CHD events that occurred prior to closeout. The year-1 results were evaluated because of a previously documented pattern of excess risk during the first year in the HT group. Additional analyses were performed after stratification of cases as MI/CHD deaths or UA. In general, the results produced similar patterns to that observed with all cases, although with less power and statistical significance than with the combined cases (data not shown). All analyses were performed using SAS version 9.1.
Results
Patient characteristics
Two thousand ninety (76%) of the original 2763 HERS subjects were successfully genotyped for both the GP1BA −5T>C and the GP6 13254T>C variants. These subjects were followed for a mean ± SD of 5.8 ± 2.0 years. During that time, 526 women (25%) suffered a CHD event (non-fatal MIs, 248; CHD deaths, 77; hospitalizations for UA, 201). The baseline characteristics of the study population, stratified by the presence or absence of a CHD event at any time during follow-up, are shown in Table 1. There were no significant differences between the subjects included in this report and the remainder of the cohort with respect to the characteristics included in Table 1 (data not shown). As expected, women who suffered a CHD event had more traditional cardiovascular risk factors than those who did not have events. However, there were no significant differences between the cases and noncases for baseline low-density lipoprotein (LDL) or high-density lipoprotein (HDL) cholesterol levels. After stratification for race, the platelet gene variants were in Hardy-Weinberg equilibrium in all 3 racial categories except for the GP6 variant in African Americans (P = .01; Table 2;Table S1).
Platelet receptor genotypes and risk for CHD
In analyses limited to the placebo group, carriers of 1 or 2 copies of the GP1BA −5C allele had higher rates of CHD events than T-allele homozygotes (Table 3)In adjusted models, assuming an additive genetic effect, the data provided modest evidence of an association at 1 year and strong evidence of an association after 5.8 years of follow-up. Dominant models produced comparable evidence of association (year 1: hazard ratio [HR] 1.68, 95% confidence interval [CI] 0.93-3.01, P = .090; closeout: HR 1.35, 95% CI 1.04-1.76, P = .026). The probability of a false-positive report for the dominant-model association between the GP1BA −5C allele and CHD events is less than 25%, assuming a true odds ratio (OR) of at least 1.5 and a prior probability of at least 10%.29 Models limited to white women produced a similar pattern of results (additive models, year 1: P = .09; closeout: P = .001). Haplotype analyses for the 3 common (> 5%) haplotypes defined by the −5C>T and the Met145thr SNPs showed that the only common haplotype containing the −5C allele was also associated with CHD events in the entire cohort after adjustment for race and covariates (year-1 OR = 1.6, P = .07; closeout OR = 1.4, P = .01) and in similar analyses restricted to white individuals only (year-1 OR = 2.0, P = .01; closeout OR = 1.3, P = .07). There were too few events in African Americans and other ethnicities to evaluate separately.
Conversely, there was no convincing evidence of an association between the GP6 13254 variant and risk for CHD events at either time point using additive (Table 3) or dominant models or in analyses limited to the white-only sample (data not shown). At 1 year, the ADRA2A SNP was associated with CHD events, but this relationship did not persist upon follow-up (Table S2). None of the other 4 platelet gene SNPs was associated with CHD events (Table S2).
Effect of HT on CHD events according to platelet receptor genotypes
Carriers of the common GP1BA −5TT genotype assigned to receive HT had more events during the first year than GP1BA −5TT women assigned to receive placebo (HR = 1.62, 95% CI 1.03-2.55; Table 4)This increased risk was attenuated after 5.8 years of follow-up (HR = 1.16, 95% CI 0.95-1.41; Table 4). In contrast, women with the less common −5TC and −5CC GP1BA genotypes assigned HT had fewer CHD events than similar women that received placebo, an effect that was evident at both 1 and 5.8 years of follow-up. This pattern of excess or neutral CHD risk with HT in GP1BA −5TT women and reduced risk in GP1BA −5TC or CC women produced evidence of a drug*gene interaction at both time points (P < .001 for both). When analyses were limited to white individuals only, the evidence of interaction remained (P = .002 and .001, respectively).
Although the GP6 genotypes were not associated with CHD events in patients randomized to placebo (Table 3), there was modest evidence of interaction with treatment assignment. Women assigned to receive HT who were GP6 T-allele homozygotes had lower rates of CHD, whereas similarly treated women who were carriers of the C allele had higher rates of CHD when compared with women taking placebo (test for interaction, year 1: P < .001; closeout: P < .001; Table 4). The same pattern was also evident in the white-only sample (P for interaction, year 1: .046; closeout: .008). None of the other 5 platelet gene SNPs interacted with HT for CHD events (Table S3).
Gene-gene and gene–gene-HT interactions
In light of the results from the individual loci, we also considered whether combinations of genotypes would impart a greater or lesser risk for CHD events, with and without HT. Classifying women taking placebo according to carriership for the variant (C) alleles for the 2 platelet receptor polymorphisms defined 4 groups (Table 5)In women assigned to receive placebo, carriers of the GP1BA −5C allele who were also homozygous for the GP6 T allele (group 3, n = 168, 16% of the study population) had a higher rate of CHD events than the other 3 groups; however, there was only marginal evidence of heterogeneity across the 4 groups (year 1: P = .07; closeout: P = .04) and the formal test of a GP1BA * GP6 interaction was not significant (year 1: P = .38; closeout: P = .51).
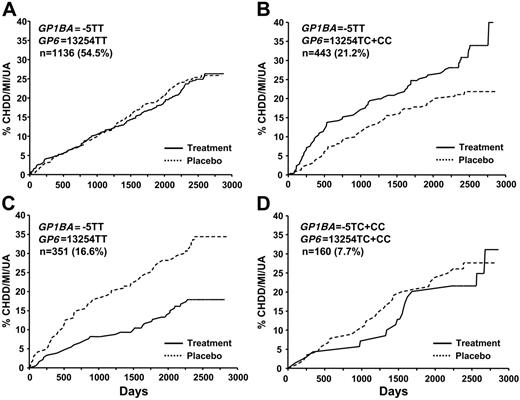
In contrast, the effect of HT varied considerably across the 4 groups (Table 6)Women with the −5TT GP1BA genotype who were also carriers of the GP6 C allele (group 2, n = 443, 21%) had significantly greater risk for CHD events with HT, whereas women who were carriers for the GP1BA −5C allele and homozygous for the GP6 T allele (group 3, n = 351, 17%) experienced dramatic reductions in risk (Table 6; Figure 1). These data provided evidence for a genotype group * HT interaction at both 1 year and closeout (P = .004 and P < .001, respectively).
Kaplan-Meier estimates of the cumulative incidence of primary end points by time to occurrence. The combined primary end point of cardiovascular death, nonfatal MI, or unstable angina is shown on the y-axis. Patients were grouped by treatment assignment and genotypes. The number of subjects (and percentage of total) with each genotype is shown.
Kaplan-Meier estimates of the cumulative incidence of primary end points by time to occurrence. The combined primary end point of cardiovascular death, nonfatal MI, or unstable angina is shown on the y-axis. Patients were grouped by treatment assignment and genotypes. The number of subjects (and percentage of total) with each genotype is shown.
Discussion
Data from the Women's Health Initiative indicate that estrogen plus progesterone HT increases MI and stroke despite its beneficial effects on cholesterol levels. Since blood platelets play a central role in the pathophysiology of MI and stroke, we hypothesized that certain inherited variations in platelet genes could be associated with CHD outcomes and that the prothrombotic effects of HT are modified by such genetic variants. This latter possibility could lead to HT being a risk factor for CHD events in some, but not all, postmenopausal women. The data from the current study indicate that the −5T>C polymorphism in GP1BA is associated with increased risk for CHD events in women with established coronary disease and that both the −5T>C polymorphism in GP1BA and the 13254T>C polymorphism in GP6 modify the effect of HT on risk for CHD events. Specifically, in the 21% of women with the detrimental genotypes for both genes (Table 6 group 2), HT was associated with a 6% absolute increase in risk for CHD events compared with placebo. Conversely, women with the complementary genotypes for both loci (Table 6 group 3) experienced roughly a 5% absolute risk reduction with HT. If our findings can be replicated in other studies, these HT pharmacogenetic findings may permit more individualized HT treatment decisions for women suffering from significant menopausal symptoms.
Only one of the platelet polymorphisms we examined was a risk for CHD events in the placebo-treated group. Patients expressing the −5C “Kozak” polymorphism of GP1BA were at increased risk for recurrent CHD events (Table 3). Previous studies have been inconsistent in their findings regarding an association between this polymorphism and a heterogeneous group of CHD phenotypes, although the −5C allele has consistently been found to be associated with stroke (reviewed in Yee and Bray30 ). Previous studies on the association of the −5C SNP of GP1BA with ACSs differed with respect to design, number of cases, sex composition of the subjects, age of the subjects, and even the measured end point. The 3 studies that found no significant association between −5C and ACSs were small and cross-sectional.14–16 Perhaps more weight regarding the importance of the −5T>C alleles should be given to the 2 studies that found an association because they were large and prospective,12,13 similar to HERS.
We did not observe a significant relationship between the GP6 13254T>C polymorphism and CHD events, consistent with a report in the Japanese population31 and with findings showing that homozygosity for the 13254C (uncommon) allele was associated with reduced GPVI receptors and a reduced thrombogenicity on collagen.19,32 Although the GP6 13254T>C polymorphism changes an amino acid (Ser219Pro), there is no evidence that this substitution causes any reported functional alteration. Of note, Croft et al17 found that the GP6 13254CC genotype was associated with MI in women but not men. However, hormone use was not considered as a confounder, and perhaps the apparent sex difference was due to female hormone use.
In the HERS population, each platelet polymorphism interacted individually (Table 4) and together (Table 6) with HT to modify the risk for CHD events. HT has been shown to interact with polymorphisms in other genes to modify risk for a variety of clinical events. For example, HT is associated with an increased risk of venous thromboembolic events in women with the factor V Leiden polymorphism33 and an increased risk of MI among hypertensive postmenopausal women with the prothrombin 20210G>A polymorphism.34 HT has also been shown to interact with polymorphisms of estrogen receptor (ER)α for HDL levels35 and for osteoporosis.36 In the Framingham Heart Study, HT was associated with increased platelet reactivity.26 However, our study is the first to examine the relationship between inherited platelet variations and HT for a CHD clinical end point.
When we examined the interaction of the 2 modifying alleles with HT, we found that HT showed no increased risk for CHD/MI/UA for approximately 62% of the women in this study (Table 6; Figure 1A,D). However, those women with the GP1BA TT and GP6 TC or GP6 CC genotypes randomized to HT had a significantly increased risk of events (Table 6; Figure 1B). Conversely, we found 1 genotype combination (GP6 TT and GP1BA TC or CC) that was associated with lower rates of CHD with HT use. Considering the myriad of genetic and environmental factors that coalesce into the overall CHD risk for any 1 subject, it is not surprising that some genetic variants interact with HT in a beneficial manner and others in an adverse manner. As has been reported for the main findings of the entire HERS trial, the sum total of all factors interacting with HT indicates that HT is no benefit in this population. It should be noted that many of the effects of these platelet genes seen at 1 year were lessened by the end of the follow-up period (Tables 3,Table 4,Table 5–6). The effects of HT on platelet function have been reported to be maximal at 1 and 3 months and reduced or lost at 6 months,37 which would be consistent with a more prominent role for platelets in the early adverse CHD effects of HT in HERS.2 However, for those genotype combinations that had significant interactions, the curves separated in the first year, and a clear difference persisted throughout the follow-up years.
There are several potential mechanisms for the observed associations of the platelet polymorphisms with CHD events. The −5C allele of GP1BA has been associated with increased surface expression of GPIbα on platelets and increased adhesion to collagen under medium flow rates.11,38 In contrast, the 13254C allele of GP6 has been associated with decreased GPVI receptors and a reduced thrombogenicity on collagen,19,32 which would be consistent with our findings in Table 3.
The relationship between HT and these genetic variations is less clear. We have shown that human megakaryocytes and platelets express ERα and ERβ,39,40 raising the possibility that there is a direct hormonal effect on this cell lineage. We observed no change in GPIbα expression in ovariectomized mice treated with oral conjugated equine estrogen (CEE).41 However, oral CEE does increase platelet GPVI expression41 and the GP6 genotype affects receptor levels,19 although no study has directly assessed whether the genotype effect is modified by hormones. Because the 13256T>C SNP of GP6 defines 2 haplotypes that have altered amino acids, it is possible that HT alters expression of another gene that interacts with platelets in a manner affected by the GPVI isoform that is expressed. In addition, because GPIbα and GPVI can physically associate in the platelet membrane,42 perhaps this association is favored with 1 GPVI isoform.
Genetic association studies may suffer from bias due to population variations in allele frequency and linkage disequilibrium (LD).43 In the current study we found similar patterns of association and interaction after excluding African Americans; however, we are unable to rule out more subtle forms of population stratification within the white subset. Bias related to population variations in LD is less likely to be an issue for functional variants altering regulatory regions or coding sequences such as the ones included in this study. Genetic subgroup analyses are also susceptible to type I error. It is important to remember that exceptionally small P values, such as the one found in Table 4, may still represent false-positive results, especially if the prior probability of the finding is low. The fact that there are biologically attractive mechanisms that are consistent with our data does not greatly diminish the need to interpret these findings with caution. It is also important to recognize that the women in this study all had established coronary disease. Whether or not similar findings might occur in younger healthier women, the magnitude of the effect, if present, remains to be established. The distribution of GP6 genotypes was out of Hardy-Weinberg in African Americans but not in white individuals. While we cannot rule out a genotyping error in the African Americans, the departure from Hardy-Weinberg is modest, the percentage of the cohort that is African American is small, and the results for the total cohort are similar to results from analyses restricted to white individuals only. There were too few African Americans with the GP6 variant allele to explore other possible explanations or to rule out the play of chance with a high degree of confidence. Despite these limitations, the dramatic nature of some of the interactions presented here, coupled with frequency of the allelic variants in question and the number of women contemplating HT use, suggest that these associations and interactions warrant further study at both the clinical and molecular level.
Authorship
Contribution: P.F.B. and D.M.H. conceived, designed, and analyzed the study and wrote the manuscript. D.M.H., E.V., and D.C.S. contributed to the design and execution of the original HERS study. T.D.H. designed genotyping primers and performed the genotyping.
Conflict-of-interest disclosure: P.F.B., T.D.H., E.V., and D.C.S. declare no competing financial interests. In the past 5 years D.M.H. has received research support and consulting fees from companies that manufacture postmenopausal hormone therapy.
Correspondence: Paul F. Bray, Jefferson Medical College, 1015 Walnut St, Curtis 705, Philadelphia, PA 19107; e-mail: paul.bray@jefferson.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section 1734.
This work was supported by grants HL68829 and HL74729 from the National Institutes of Health (NIH).
We would like to thank Georgia Saylor for data management and programming.