Abstract
We studied the clinical outcomes of 171 adults with hematologic malignancies who received unrelated cord blood transplantation (CBT) as a primary unrelated stem-cell source (n = 100), or bone marrow transplant (BMT) or peripheral blood stem-cell transplant (PBSCT) from related donors (n = 71, 55 BMT and 16 PBSCT). All patients received myeloablative regimens including 12 Gy total body irradiation. We analyzed the hematologic recovery, and risks of graft-versus-host disease (GVHD), transplantation-related mortality (TRM) and relapse, and disease-free survival (DFS) using Cox proportional hazards models. Significant delays in engraftment occurred after cord blood transplantation; however, overall engraftment rates were almost the same for both grafts. The cumulative incidences of grades III to IV acute and extensive-type chronic GVHDs among CBT recipients were significantly lower than those among BMT/PBSCT recipients. Multivariate analysis demonstrated no apparent differences in TRM (9% in CBT and 13% in BMT/PBSCT recipients), relapse (17% in CBT and 26% in BMT/PBSCT recipients), and DFS (70% in CBT and 60% in BMT/PBSCT recipients) between both groups. These data suggest that unrelated cord blood could be as safe and effective a stem-cell source as related bone marrow or mobilized peripheral blood for adult patients when it is used as a primary unrelated stem-cell source.
Introduction
Recently, cord blood has been increasingly used in adults as a stem-cell source for allogeneic transplantation to treat hematologic malignancies.1-5 We previously reported on a comparative analysis of cord blood transplant (CBT) versus bone marrow transplant (BMT) from unrelated donors in our institute.6 The overall results for CBT recipients were better than for BMT recipients in terms of graft-versus-host disease (GVHD), transplant-related mortality (TRM), and disease-free survival (DFS). In our previous assessments, the availability of grafts containing sufficient cell numbers, the shorter time from donor search to transplantation, the low requirements of steroid therapy for GVHD, the conditioning regimen, the GVHD prophylaxis used in our institution, and Japanese genetic issues regarding low alloreactivity7-9 might have contributed to our favorable results of cord blood transplantation in adults.
Two other registration-based studies comparing both CBT and BMT from unrelated donors in adult patients with acute leukemia have recently been published; both studies showed almost the same results between cord blood transplantation and bone marrow transplantation.10,11 However, some results in those reports were conflicting, especially for TRM. The US study demonstrated a poor outcome for TRM in CBT recipients compared with HLA (human leukocyte antigen)–matched BMT recipients.10 The European study11 showed similar TRM in both groups.
We speculated that the key difficulty in interpreting retrospective comparative studies, including ours, may be related to patient selection. Most recipients of CBT did not have an HLA-matched unrelated donor, and their disease tended to progress to advanced or high-risk stage while searching, unsuccessfully, for marrow donors.12 However, when a patient was eligible for allogeneic transplantation but did not have a related donor, we performed cord blood transplantation at the same timing as for patients who had a related donor.
In the present study, we compared our results of CBT from unrelated donors with those of BMT or peripheral blood stem-cell transplant (PBSCT) from related donors in our hospital; all patients received essentially the same supportive care. The main purpose of this analysis was to assess the safety and efficacy of unrelated CBT compared with BMT or PBSCT from related donors in adult patients in the setting of a comparable situation regarding patient selection.
Patients, materials, and methods
Patients and controls
The study included data from 171 consecutively treated patients, 16 years of age or older, who received BMT or PBSCT from related donors (n = 71, 55 BMT and 21 PBSCT recipients) or unrelated CBT (n = 100) for acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myelogenous leukemia (CML), myelodysplastic syndrome (MDS), or malignant lymphoma (ML) between January 1997 and August 2005 at the Institute of Medical Science, University of Tokyo. T-cell depletion was not performed in either group. Patients qualified as being standard risk if they were in first or second complete remission (CR), had chronic-phase CML or refractory anemia MDS, or had no high-risk cytogenetics (eg, ALL with t(4;11) or t(9;22), or AML with complex karyotype, −5, del(5q), −7, or abnormalities of 3q). Patients in third CR, in relapse, with CML beyond chronic phase, or with high-risk cytogenetics were classified as being high risk. Patients receiving BMT, PBSCT, or CBT as a second transplant following relapse after a first allogeneic transplantation were excluded. Median follow-up was 32 months (range, 1-110 months; 39 survivors and 32 censored) for BMT and 22 months (range, 0-91 months; 72 survivors and 28 censored) for CBT recipients (P = .77). The clinical protocol was approved by the institutional review board of the Institute of Medical Science, University of Tokyo, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
HLA typing and donor selection
HLA-A and HLA-B antigens were identified by serologic typing. HLA-DRB1 alleles were determined by high-resolution molecular typing using polymerase chain reaction sequence-specific primers (PCR-SSPs). Patients without a suitable closely HLA-matched related donor, namely, with 5 of 6 or 6 of 6 matching HLA loci, were eligible for cord blood transplantation as a first treatment option, because most patients eligible for allogeneic stem-cell transplantation were thought to have insufficient time for an unrelated bone marrow donor search and early timing of transplantation was preferable. On the other hand, if those patients had any type of anti-HLA antibody, we generally attempted to locate bone marrow grafts from unrelated donors because most cord blood grafts were HLA mismatched and the predictable risk of poor engraftment results after cord blood transplantation. All cord blood grafts were evaluated by HLA-A and HLA-B typing serologically, by HLA-DRB1 typing at high resolution, and by nucleated cell counts. Preferred cord blood units matched 4 of 6 to 6 of 6 HLA loci and contained a minimal cell count of 1.5 × 107 nucleated cells/kg body weight before freezing. T-lymphocyte depletion was not performed on cord blood or bone marrow grafts.
Conditioning regimen, GVHD prophylaxis, and supportive care
All patients received a total body irradiation (TBI)–containing myeloablative pretransplantation conditioning regimen of 12 Gy, fractionated in 4 or 6 doses. The TBI + cytosine arabinoside (Ara-C: total dose 24 g/m2) combined with G-CSF (lenograstim) regimen13-15 was chosen for patients with myeloid leukemias who had an HLA-matched related donor (n = 31). Additionally, cyclophosphamide (CY) was administered to patients who received transplants from HLA-mismatched cord blood donors or HLA-mismatched related donors while reducing the Ara-C dose to a total dose of 12 g/m2 (n = 82) as reported previously.6 CY was avoided in favor of 120 mg/m2 fludarabine (n = 6) in the case of recipients who had risk of organ dysfunction, especially in the heart. Thirty-one patients received TBI + CY (n = 23) or TBI + CY + one cytotoxic drug (12 g/m2 Ara-C in 6 patients, 60 mg/kg etoposide in 1 patient, or 300 mg/m2 thiotepa in 1 patient). Fifteen patients received TBI + 60 mg/kg etoposide. Six additional patients received TBI + 90 mg/m2 fludarabine + one drug (12 g/m2 Ara-C in 3 patients or 140 mg/m2 melphalan in 3 patients) (Table 1)
One hundred and sixty-three (95%) of all 171 patients received a standard cyclosporine (CsA) and methotrexate (MTX) combination as GVHD prophylaxis. CsA was administered daily from day −1 at 3 mg/kg per day intravenously and MTX at 15 mg/m2 intravenously on day 1, followed by 10 mg/m2 on days 3 and 6. MTX on day 11 was given only to patients receiving HLA-mismatched bone marrow or peripheral blood from related donors. Six patients received only CsA, 1 received tacrolimus (FK-506) combined with a short course of MTX, and 1 received CsA plus mycophenolate mofetil (MMF). Once oral intake could be tolerated, patients were administered oral CsA at a dose ratio of 1:2.5, in 2 divided doses/d based on the last intravenous dose. In the absence of GVHD, CsA was tapered beginning between weeks 6 and 9 until it could be discontinued, depending on the degree of GVDH severity. Corticosteroid-based treatment was considered when grade II or higher severe acute GVHD occurred (1 to 2 mg/kg).
The supportive-care regimen, including prophylaxis, for infection was the same as previously reported.6 All patients after cord blood transplantation and 60 of 71 after bone marrow transplantation/peripheral blood stem-cell transplantation received G-CSF (lenograstim, 5 μg/kg per day, intravenous infusion) starting on day 1 until durable granulocyte recovery was achieved. The same supportive care, except for the G-CSF administration, was given to both groups.
End points, definitions, and assessments of hematopoietic recovery, GVHD, TRM, disease relapse, and DFS
We focused on hematologic recovery, acute and chronic GVHD, TRM, disease relapse, and DFS after unrelated cord blood transplantation compared with related bone marrow transplantation/peripheral blood stem-cell transplantation. The primary measure of hematopoietic recovery was the time required for myeloid and platelet recovery. The myeloid-cell recovery time was defined as the first of 3 consecutive days during which the absolute neutrophil count in the blood was at least 0.5 × 109/L.3 Platelet recovery time was achieved on the first of 3 days when the platelet count was higher than 2 × 109/L (or 5 × 109/L) without transfusion support. Primary engraftment failure was defined as the absence of donor-derived myeloid cells on the day of death, the day of relapse, or day 60 in patients surviving beyond day 28 after transplantation. Patients were also defined as having had primary engraftment failure when either a second allogeneic transplantation before donor-derived myeloid recovery or reconstitution with autologous cells was required. Chimerism was evaluated by fluorescence in situ hybridization for the Y chromosome or quantitative PCR analysis for microsatellite DNA markers. Acute GVHD was graded 0 to IV according to the criteria of Glucksberg et al,16 and chronic GVHD was defined as none, limited, or extensive.17 The incidence of and time to acute GVHD development were evaluated in patients surviving 21 days or longer with evidence of engraftment. Time to occurrence of any chronic GVHD disease was evaluated in patients surviving 100 days or longer after transplantation with allogeneic engraftment. TRM was defined as death from any cause except relapse. Relapse was defined by morphologic evidence of disease in peripheral blood, marrow, or extramedullary sites, or the recurrence and sustained presence of pretransplantation chromosomal abnormalities on cytogenetic analysis of bone marrow cells. Patients showing minimal residual disease (eg, the presence of bcr/abl RNA transcripts by PCR) were not classified as having relapsed. DFS was defined as survival in continuous CR.
Statistical analysis
The probability of DFS was estimated from the time of transplantation according to the Kaplan-Meier product limit method. Cumulative incidences were estimated for hematopoietic recovery, GVHD, TRM, and relapse in order to take competing risks into account. Associations between graft type and outcome were evaluated using Cox proportional hazard regression models. In addition to the hematopoietic stem-cell source, the following variables were considered as covariates: recipient age at transplantation; weight; status regarding cytomegalovirus (CMV, determined by serologic testing); recipient and donor sex; degree of ABO matching; degree of HLA matching; type (ALL, AML, CML, MDS, or malignant lymphoma) and pretransplantation duration of the underlying disease; disease status at transplantation (standard or high risk); conditioning regimen; GVHD prophylaxis used; use or nonuse of G-CSF during the first 7 days after transplantation; and time of transplantation (between 1997 and 2000, or 2001 and 2005). We used backward and stepwise procedures at a significance level of 5% to construct prognostic models, in which we tried to maintain the graft source (cord blood from an unrelated donor or bone marrow/peripheral blood from a related donor) as a variable until the final step of the procedures. The proportional hazard assumption of the Cox model was assessed essentially by a graphic approach. When groups were compared according to continuous covariates, we calculated the mean or median of each group, and the Student t test or Mann-Whitney U test was used. A Chi-square test was used to compare categoric covariates: SAS version 8.2 (SAS Institute, Cary, NC) and S Plus 2000 (Mathsoft, Seattle, WA) were used for all analyses. End points were calculated at the last contact, the date of the latest follow-up being March 1, 2006.
Results
Characteristics of patients and donors
The patients' age, sex, CMV serology, diagnosis, the ratio of standard-risk versus high-risk, duration from diagnosis to transplantation, and GVHD prophylaxis regimen were almost the same between the BMT/PBSCT and CBT recipients. Overall rates of high-risk patients were 62% for BMT/PBSCT recipients and 57% for CBT recipients (P = .57). On the other hand, there were significant differences in the following variables (Table 1). Patients receiving CBT had lower body weight and received transplants in a later calendar year. Sixty-seven percent of BMT/PBSCT recipients were administered a conditioning regimen without CY, and 91% of CBT recipients were administered a conditioning regimen with CY. The 6 possible matches between the recipient and the donor were scored serologically for HLA-A and HLA-B and genetically for DRB1 alleles, and the results showed 54 (76%) matched grafts in BMT/PBSCT recipients and no complete matches in CBT recipients. Details of HLA disparities between CBT recipients and grafts are described in the footnote to Table 1. Although the number of leukocytes for CBT recipients was 1 log lower than in BMT/PBSCT recipients, 93 of 100 cord blood grafts contained more than 2.0 × 107 cells/kg. The median number of CD34+ progenitor cells was 0.93 × 105/kg (range, 0.15 × 105 to 8.97 × 105/kg) before freezing of cord blood grafts.
Engraftment, hematopoietic recovery, GVHD, and length of hospitalization
Four patients (4%) died within 28 days of cord blood transplantation, and primary graft failure occurred in 5 of the surviving 96 in the CBT recipient group. There was one early death (2%) on day 7 due to multiple organ failure in the BMT/PBSCT recipient group, but no patients had primary graft failure.
Patients receiving CBT had significantly slow neutrophil and platelet recovery in multivariate analysis (Table 2), in contrast with almost comparable recoveries in hematopoietic engraftment with longer-term follow-up. The overall myeloid engraftment rates on day 60 were 91% (95% confidence interval [CI], 85% to 97%) for CBT recipients and 96% (95% CI, 91% to 100%) for BMT/PBSCT recipients. Platelet counts of more than 2 × 109/L on day 100 were 85% (95% CI, 78% to 92%) and 94% (95% CI, 89% to 100%), and platelet counts of more than 5 × 109/L on day 180 were 83% (95% CI, 75% to 91%) and 92% (95% CI, 85% to 98%) for CBT and BMT/PBSCT recipients, respectively. One hundred percent of donor chimerisms were confirmed in all recipients after hematopoietic recovery by the techniques described in “Patients, materials, and methods.”
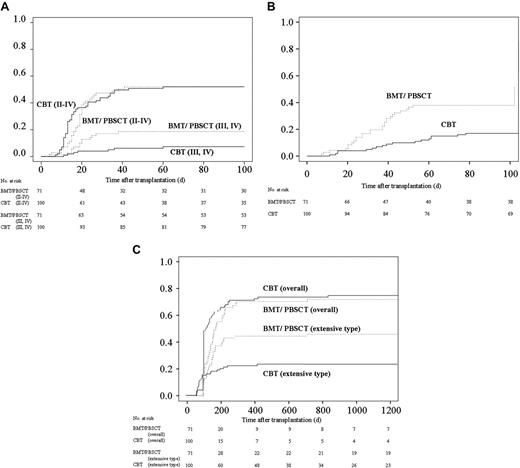
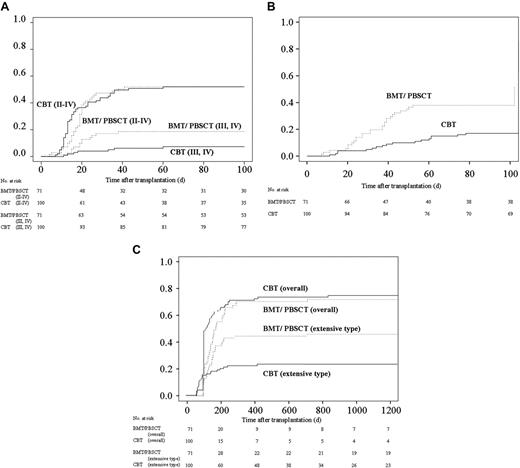
More than 90% of patients in both groups received CsA plus a short-term MTX regimen as GVHD prophylaxis. The tapering rate of immunosuppressant drugs differed among individual patients due to variations in GVHD severity, renal function, and primary disease risk, although the protocol was assigned as described previously.6 Consequently, the rate of decreasing immunosuppressants and discontinuation for CBT recipients was faster than those for BMT/PBSCT recipients (Table 3)The cumulative incidence of grades II to IV acute GVHD in both groups was almost equivalent (Figure 1A). On the other hand, despite the rapid tapering of prophylactic drugs for GVHD and the high degree of HLA disparity among CBT recipients, the cumulative incidence of grades III and IV acute GVHD was significantly lower in multivariate analysis (hazard ratio: 0.38; 95% CI: 0.15 to 0.95; P = .04; Table 2 and Figure 1B). The cumulative incidence of requiring steroids for treating acute GVHD among CBT recipients was significantly lower than among BMT/PBSCT recipients (hazard ratio: 0.25; 95% CI: 0.13 to 0.50; P < .01; Table 2 and Figure 1C).
Cumulative incidences of acute and chronic GVHD after transplantation and kinetics of immunosuppressant use after transplantation. (A) Cumulative incidence of acute GVHD. The values of grades II to IV acute GVDH on day 100 were 52% (95% CI, 42% to 62%) for CBT and 52% (95% CI, 40% to 64%) for BMT/PBSCT recipients (P = .69). The values of grades III and IV acute GVDH on day 100 were 7% (95% CI, 2% to 13%) for CBT and 19% (95% CI, 19% to 28%) for BMT/PBSCT recipients (P = .04). (B) The cumulative incidence of requiring steroid therapy in patients after cord blood transplantation and bone marrow transplantation/peripheral blood stem-cell transplantation. The values on day 100 were 17% (95% CI, 10% to 24%) for CBT and 38% (95% CI, 27% to 49%) for BMT/PBSCT recipients (P < .01). (C) Cumulative incidence of chronic GVHD in patients surviving more than 100 days. The values for overall chronic GVHD were 71% (95% CI, 62% to 80%) at 1 year and 74% (95% CI, 40% to 64%) at 3 years after cord blood transplantation, in contrast to 68% (95% CI, 56% to 79%) at 1 year and 69% (95% CI, 58% to 80%) at 3 years after bone marrow transplantation/peripheral blood stem-cell transplantation (P = .09). The values of the cumulative incidence of extensive-type GVDH were 22% (95% CI, 14% to 30%) at 1 year and 25% (95% CI, 15% to 32%) at 3 years after cord blood transplantation, in contrast to 44% (95% CI, 32% to 55%) at 1 year and 45% (95% CI, 33% to 57%) at 3 years after bone marrow transplantation/peripheral blood stem-cell transplantation (P = .01).
Cumulative incidences of acute and chronic GVHD after transplantation and kinetics of immunosuppressant use after transplantation. (A) Cumulative incidence of acute GVHD. The values of grades II to IV acute GVDH on day 100 were 52% (95% CI, 42% to 62%) for CBT and 52% (95% CI, 40% to 64%) for BMT/PBSCT recipients (P = .69). The values of grades III and IV acute GVDH on day 100 were 7% (95% CI, 2% to 13%) for CBT and 19% (95% CI, 19% to 28%) for BMT/PBSCT recipients (P = .04). (B) The cumulative incidence of requiring steroid therapy in patients after cord blood transplantation and bone marrow transplantation/peripheral blood stem-cell transplantation. The values on day 100 were 17% (95% CI, 10% to 24%) for CBT and 38% (95% CI, 27% to 49%) for BMT/PBSCT recipients (P < .01). (C) Cumulative incidence of chronic GVHD in patients surviving more than 100 days. The values for overall chronic GVHD were 71% (95% CI, 62% to 80%) at 1 year and 74% (95% CI, 40% to 64%) at 3 years after cord blood transplantation, in contrast to 68% (95% CI, 56% to 79%) at 1 year and 69% (95% CI, 58% to 80%) at 3 years after bone marrow transplantation/peripheral blood stem-cell transplantation (P = .09). The values of the cumulative incidence of extensive-type GVDH were 22% (95% CI, 14% to 30%) at 1 year and 25% (95% CI, 15% to 32%) at 3 years after cord blood transplantation, in contrast to 44% (95% CI, 32% to 55%) at 1 year and 45% (95% CI, 33% to 57%) at 3 years after bone marrow transplantation/peripheral blood stem-cell transplantation (P = .01).
Chronic GVHD affected 73 of 82 CBT and 49 of 55 BMT/PBSCT recipients surviving more than 100 days. Twenty-three CBT and 30 BMT/PBSCT recipients developed extensive GVHD. The incidence of overall chronic GVHD in CBT recipients tended to be higher than that in BMT/PBSCT recipients (Table 2; Figure 1D); however, the cumulative incidence of extensive-type GVHD among CBT recipients was significantly lower than that among recipients using grafts from related donors (hazard ratio: 0.49; 95% CI: 0.29 to 0.85; P = .01; Table 2 and Figure 1E).
Eighty-three CBT recipients and 59 BMT or PBST recipients were discharged from the hospital. The median number of days of hospitalization for CBT recipients was 121 and tended to be longer than that for BMT/PBSCT recipients, which was 89 days after transplantation (hazard ratio: 0.73; 95% CI: 0.450 to 1.06; P = .10; Table 2).
In CBT recipients, HLA disparities did not have any significant effects on engraftment, hematopoietic recovery, GVHD, or length of hospitalization.
TRM, relapse, and DFS
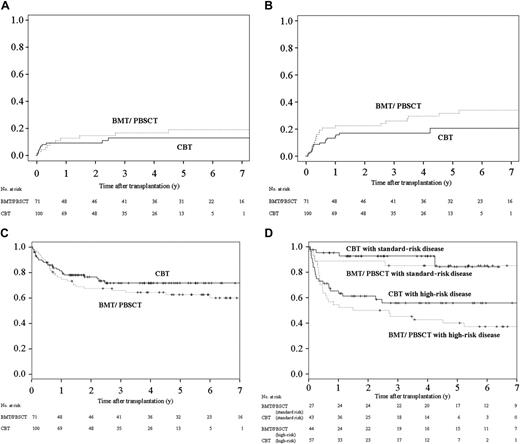
The respective 100-day and 1-year cumulative incidences of TRM were 8% (95% CI, 3% to 14%) and 9% (95% CI, 3% to 15%) among CBT recipients, and 4% (95% CI, 0 to 9%) and 13% (95% CI, 4% to 21%) among BMT/PBSCT recipients (Figure 2A). Higher age and higher risk of disease had significant impacts, as shown in Table 4, but the source of graft did not. Higher risk of disease and the diagnosis of ALL were significantly poor factors for relapse. Higher risk of disease was also a significant risk factor on DFS results in the multivariate analysis, as shown in Table 4.
Outcomes among CBT and BMT/PBSCT recipients. (A) The 1-year and 3-year cumulative incidences of TRM were 8% (95% CI, 3% to 14%) and 9% (95% CI, 3% to 15%) among CBT recipients, respectively, in contrast to 4% (95% CI, 0 to 9%) and 13% (95% CI, 4% to 21%) among BMT/PBSCT recipients, respectively. The differences between the 2 groups were not significant (P = .13). (B) The 3-year cumulative incidences of relapse among recipients were 17% (95% CI, 9% to 25%) after cord blood transplantation and 26% (95% CI, 15% to 37%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant (P = .34). (C) The 3-year Kaplan-Meier estimate of DFS was 70% (95% CI, 61% to 80%) after cord blood transplantation and 60% (95% CI, 49% to 72%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant (P = .26). (D) The 3-year Kaplan-Meier estimate of DFS in patients with standard-risk disease was 93% (95% CI, 85% to 100%) after cord blood transplantation and 85% (95% CI, 71% to 99%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant by nonadjusted comparison (P = .72). The 3-year Kaplan-Meier estimate of DFS in patients with high-risk disease was 56% (95% CI, 42% to 70%) after cord blood transplantation and 45% (95% CI, 30% to 60%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant by nonadjusted comparison (P = .26).
Outcomes among CBT and BMT/PBSCT recipients. (A) The 1-year and 3-year cumulative incidences of TRM were 8% (95% CI, 3% to 14%) and 9% (95% CI, 3% to 15%) among CBT recipients, respectively, in contrast to 4% (95% CI, 0 to 9%) and 13% (95% CI, 4% to 21%) among BMT/PBSCT recipients, respectively. The differences between the 2 groups were not significant (P = .13). (B) The 3-year cumulative incidences of relapse among recipients were 17% (95% CI, 9% to 25%) after cord blood transplantation and 26% (95% CI, 15% to 37%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant (P = .34). (C) The 3-year Kaplan-Meier estimate of DFS was 70% (95% CI, 61% to 80%) after cord blood transplantation and 60% (95% CI, 49% to 72%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant (P = .26). (D) The 3-year Kaplan-Meier estimate of DFS in patients with standard-risk disease was 93% (95% CI, 85% to 100%) after cord blood transplantation and 85% (95% CI, 71% to 99%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant by nonadjusted comparison (P = .72). The 3-year Kaplan-Meier estimate of DFS in patients with high-risk disease was 56% (95% CI, 42% to 70%) after cord blood transplantation and 45% (95% CI, 30% to 60%) after bone marrow transplantation/peripheral blood stem-cell transplantation. The differences between the 2 groups were not significant by nonadjusted comparison (P = .26).
There was no apparent difference between the risk of relapse and DFS in both groups in multivariate analysis (Table 4). The 3-year cumulative incidence of relapse was 17% (95% CI, 9% to 25%) in CBT recipients and 26% (95% CI, 15% to 37%) in BMT/PBSCT recipients (Figure 2B). The 3-year probabilities of Kaplan-Meier–estimated DFS were 70% (95% CI, 61% to 80%) after cord blood transplantation and 60% (95% CI, 49% to 72%) after bone marrow transplantation/peripheral blood stem-cell transplantation (Figure 2C). Because the ratio of high-risk disease in the BMT/PBSCT recipient group (62%) was slightly higher than in the CBT recipient group (57%, P = .57), we compared DFS rates of both groups for each disease risk. DFS of both groups was also equivalent in standard-risk patients and high-risk patients. The 3-year probabilities of DFS were 93% (95% CI, 85% to 100%) after cord blood transplantation and 85% (95% CI, 71% to 99%) after bone marrow transplantation/peripheral blood stem-cell transplantation in recipients in the standard-risk disease category (Figure 2D), and those in recipients in the high-risk disease category were 56% (95% CI, 42% to 70%) after cord blood transplantation and 45% (95% CI, 30% to 60%) after bone marrow transplantation/peripheral blood stem-cell transplantation.
The proportion of causes of death was equivalent between CBT and BMT recipients (Table 5): almost 10% of deaths in both groups were GVHD related. The major cause of death in both recipient groups was relapse.
Discussion
Recently, 2 registration-based retrospective studies comparing the results of unrelated cord blood transplantation and unrelated bone marrow transplantation in adults have been published.10,11 The investigators concluded that unrelated cord blood is an acceptable alternative source of hematopoietic stem cells for adults with acute leukemia who do not have an HLA-matched marrow donor. We have also reported on a comparative analysis of CBT versus BMT from unrelated donors for adult patients at our institute.6 The overall results for CBT recipients were better than for BMT recipients in terms of GVHD, TRM, and DFS; moreover, our results for CBT recipients were better than those reported in European or US studies.
In this analysis, we compared updated results of unrelated cord blood transplantation with those of related transplantations in our hospital and demonstrated an equivalent safety and efficacy between both. Hematologic recovery was slower after cord blood transplantation, although the overall engraftment rates were not significantly different, with more than 80% of patients in both groups achieving myeloid and platelet engraftment. Incidences of severe acute GVHD and extensive-type chronic GVHD were significantly lower in CBT recipients.
Patients receiving related grafts were treated at earlier dates, whereas CBT recipients were treated more recently. We used almost the same supportive care during the period for both recipients of BMT/PBSCT and CBT. On the other hand, with the anticipated improved survival rate overall in allogeneic transplantation patients over time, we thought this factor of treatment dates may have in part contributed to the equivalent survival observed in patients receiving related versus cord blood allogeneic grafts. However, this factor did not affect any clinical results in our multivariate analysis.
In the multivariate analysis, older age and high risk of disease had significant impacts in terms of TRM. High risk of disease was also a significant risk factor for relapse and DFS. The diagnosis of ALL was another significantly poor indicator of relapse. However, there was no apparent difference in the risks of TRM, relapse, and rate of DFS between the CBT and BMT/PBSCT recipient groups. DFS in both groups was also comparable among standard-risk and high-risk groups.
We speculated on several reasons for our favorable results in CBT recipients. One of the reasons might be the availability of grafts containing sufficient numbers of cells and because Japanese body size is relatively small. In fact, there were only 7 patients who received cord blood grafts containing less than 2.0 × 107 nucleated cells/kg body weight among our 100 CBT recipients. Secondly, Japanese patients might have some advantages in the setting of HLA-mismatched transplantation due to HLA or non-HLA immune genetics.7-9,18 In particular, there is mounting evidence indicating that polymorphisms in non-HLA immune mediators and host defense genes, such as tumor necrosis factor, interleukin-10, or their receptor genes, could affect the severity of GVHD.19-21 The immunogenetics in Japanese7-9,22 may also have contributed to the favorable results in related stem-cell transplantation23,24 and unrelated bone marrow transplantation compared with reports from Western countries. This racial advantage might be significantly observed in the setting of allogeneic transplantation using HLA-mismatched grafts such as cord blood transplantation. Thirdly, the preparative conditioning and GVHD prophylaxis regimens used in our study might also have been favorable factors.
In addition to the just-mentioned reasons, the timing of transplantation is a very important factor relating to clinical results. The quick availability of cord blood as a stem-cell source is thought to be one of the most important advantages compared with unrelated bone marrow grafts.6,25 If the patient was eligible for allogeneic transplantation but had no related donor, we generally selected a cord blood graft first, rather than waiting for the results of an unrelated marrow donor search. In fact, duration from diagnosis to transplantation was almost the same between the BMT/PBSCT and CBT recipients in the study. This might be one of the reasons for our favorable results in adult CBT recipients, especially in terms of TRM, compared with most previously published studies. The earlier timing contributes to better clinical outcomes in both patients with stable or advanced-stage disease at transplantation because disease progression can be prevented and accumulation of chemotherapy-induced tissue toxicity can be decreased. The rapid preparation of grafts may also increase the likelihood of achieving successful transplantation in patients.
Once a patient was considered eligible for allogeneic transplantation and did not have a related donor, we performed cord blood transplantation at the same timing as bone marrow transplantation/peripheral blood stem-cell transplantation from a related donor. On the other hand, in most institutes, including those in Japan, a cord blood graft was not selected as a primary graft for patients who did not have a family donor, which might be a reason for the different clinical results for cord blood transplantation among centers in Japan.26
In this analysis, we compared the clinical outcomes of CBT from unrelated donors with BMT/PBSCT from related donors including HLA-matched siblings and also HLA-mismatched relatives, the latter 2 types of donors being quickly available. We took this approach because HLA closely-matched relatives were previously considered as acceptable donors in some clinical settings in patients without HLA-matched sibling donors.24,27-29
Our clinical results suggest that cord blood from unrelated donors could be as safe and effective a stem-cell source as bone marrow or mobilized peripheral blood from related donors for adult patients when used as a primary unrelated stem-cell source.
Authorship
Contribution: S.T. and S.A. designed the study; J.O., A.T., T.K., N.T., K.F., M.U., K.T., T.I., and A.T. performed patients' care; M.O.M. performed data management; T.Y. analyzed data statistically; and S.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satoshi Takahashi, Division of Molecular Therapy, The Advanced Clinical Research Center, The Institute of Medical Science, The University of Tokyo, 6-1, Shirokanedai-4, Minatoku, Tokyo 108-8639, Japan; e-mail: radius@ims.u-tokyo.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by The Kobayashi Foundation.
We are indebted to the medical and nursing staff of the Hematopoietic Stem Cell Transplant Program at the Research Hospital, Institute of Medical Science, University of Tokyo for taking care of the patients; the Tokyo CBB, Hokkaido CBB, Hyogo CBB, Tokai CBB, Metro Tokyo Red Cross CBB, Chushikoku CBB, and the Caitlin Raymond International Registry for processing the cord blood units; and especially to Dr Tokiko Nagamura in Tokyo CBB for processing the cord blood grafts.