Abstract
Neutrophils are critical in the inflammatory process by moving rapidly to tissue sites of inflammation. Members of the small Rho GTPase family, Rac1, Rac2, CDC42, and RhoA, are central regulators of cell migration by cytoskeleton rearrangement. The role of Rac1 in neutrophil migration related to inflammatory processes has remained elusive and has yet to be determined in physiologic in vivo models. We previously demonstrated a role for Rac1 in tail retraction. Here, we present evidence that Rac1-mediated uropod formation may be due to crosstalk with a related Rho GTPase RhoA. To assess the physiologic relevance of these findings, we used adoptive transfer of Rac1flox/flox bone marrow cells which allows postengraftment in vivo deletion of Rac1 only in blood cells. We examined the specific role of Rac1 in neutrophil migration into the lung during the inflammatory process induced by formyl-methionyl-leucyl-phenylalanine exposure. The loss of Rac1 activity in neutrophils is associated with a significant decreased neutrophil recruitment into lung alveolar and attenuation of emphysematous lesions. Overall, this study suggests that Rac1 is a physiologic integrator of signals for neutrophil recruitment into lung tissue during an inflammatory response.
Introduction
Inflammation is characterized by a rapid recruitment of neutrophils into injured tissues. Migration of neutrophils into sites of inflammation depends on a series of sequential adhesive and chemotactic events that result from the activation of signals downstream of various receptors, including adhesion molecules such as selectin and integrins, and chemokine receptors.1-3 Initially, cells interact with the endothelium by selectin molecules which promote weak adhesion and rolling of the cells on the blood vessels. Activation of G-coupled receptors in response to bacterial or host-derived chemoattractants in turn activates β2-integrins to induce firm adhesion to endothelial substrate and subsequently transmigration across the endothelial barrier. Neutrophils are then directed toward the sites of inflammation by the process of chemotaxis. Chemotaxis is the result of a coordinated rearrangement of the cytoskeleton and adhesion complexes to allow cells to migrate within an extracellular matrix toward high concentrations of chemoattractant.4 Migration is accompanied by cell polarization to form a protrusive leading edge and a contractile tail.4,5 The general mechanism of migration is conserved among mammalian cells. However, details of the molecular mechanism of these processes are cell lineage dependent and remain to be understood in physiologic settings.
The small RHO GTPases, members of the Ras superfamily, including Rac, RhoA, and CDC42, are major regulators of these events by integrating signals from chemokine receptors and adhesion molecules.6 Our group has been particularly interested in dissecting the role of Rac GTPases in hematopoietic cells which are unique with respect to the presence of all 3 Rac proteins. Rac1 is ubiquitously expressed, whereas Rac2 is expressed only in hematopoietic cells. Rac3 is expressed primarily in brain but also in most tissues examined.7-9 Using genetic approaches, we and others have demonstrated that Rac1 and Rac2 regulate both distinct and overlapping cell functions in hematopoietic cells.10-12 In neutrophils, Rac2 regulates migration by F-actin polymerization and polarization in vitro, whereas Rac1 regulates cell spreading and plays an ill-defined role in tail retraction during cell movement.10-13 Recently, Rac1 has also been implicated in gradient sensing.14 Rac2−/− mice demonstrate enhanced susceptibility to the opportunistic agent, Aspergillus fumigatus.10 A patient with a mutation in Rac2 has been described with a severe phagocytic immunodeficiency that mimics the neutrophil phenotype seen in Rac2−/− mice.13,15,16 Thus, the physiologic role of Rac2 in neutrophils has been well documented. However, the role of Rac1 in neutrophil migration still remains to be fully understood. Importantly, the physiologic relevance of in vitro studies has yet to be defined in in vivo inflammatory-related models. Knowledge of the molecular mechanism of Rac1-mediated migration may result in identifying new therapeutic targets that could be useful for the development of specific inhibitors of inflammation.
In this report, we present evidence that Rac1 activity plays an important role in cell-body contraction and uropod formation during neutrophil migration. This Rac1 effect appears to be related, at least in part, to crosstalk with RhoA. Importantly, Rac1 deficiency in hematopoietic cells reduces fMLP-induced neutrophil migration into the lung and the subsequent emphysematous lesions. Therefore, our study suggests an unexpected role for Rac1 in neutrophil migration in a physiologically relevant model.
Materials and methods
Generation of Rac1-deficient mice
We used Rac1 (flox) allele (Rac1flox/flox) crossed with transgenic mice expressing Cre under interferon-γ–inducible Mx1 promoter (CreTg+;Rac1flox/flox) and CreTg+;WT littermate control derived from N > 7 generations.12 Bone marrow cells from CreTg+;WT or CreTg+;Rac1flox/flox were transplanted into lethally irradiated C57Bl/6 recipients (1175 cGy in split dose). Five weeks after bone marrow reconstitution, CreTg+;WT and CreTg+;Rac1flox/flox reconstituted animals were treated with 4 injections of 300 μg polyI:polyC (polyI:C; Amersham Pharmacia Biotech, Piscataway, NJ) to delete floxed Rac1 alleles.12 Animals were used for experiments 7 days after polyI:C treatment. Blood was harvested, and white blood cell count and neutrophil count were enumerated with a hematologic analyzer for mouse blood sample (Hemavet System; Drew Scientific, Oxford, CT). All animals were bred in the Cincinnati Children's Research Foundation pathogen-free animal facility. All experimental procedures were approved by the institutional animal committee IACUC.
Retrovirus vector construction and transduction of bone marrow cells
MIEG3 retroviruses expressing wtRac1 tagged with HA (hemagglutinin) epitope at the N-terminus and enhanced green fluorescence protein (EGFP)17 or modified yellow fluorescence protein (YFP) were used. To generate the chimeric protein EGFP-RhoA, RhoA cDNA was cloned into pEGFP-C1 vector (Clontech, Palo Alto, CA). The resulting fusion was sequenced and subcloned into a murine stem cell virus (MSCV)–based retrovirus vector as previously described.17
To analyze in vitro RhoA localization, WT and Rac1−/− cells were transduced twice with MSCV-EGFP-RhoA on fibronectin fragment CH296 (kindly provided by Takara Bio, Otsu, Japan).13 EGFP+ were sorted and neutrophils were generated.12,17 To analyze rescue of Rac1 functions, Rac1−/− cells were transduced with both MSCV-EGFP-RhoA and MIEG3-HARac1-YFP, and cells positive for both EGFP and YFP were sorted (fluorescence-activated cell sorting [FACS]Vantage; Becton Dickinson, Mountain View, CA).
To analyze in vivo rescue of Rac1 functions, Rac1flox/flox was transduced with MIEG3-HARac1 or empty MIEG3. EGFP+ cells were sorted 2 days after transduction, and 5 × 105 cells were injected into lethally irradiated WT animals. Five weeks after bone marrow reconstitution, all animals were treated with polyI:C to induce deletion of endogenous floxed Rac1 alleles, and animals were used for experiments.
Neutrophil isolation
Neutrophils were isolated from bone marrow cells using a discontinuous Percoll (Pharmacia) gradient.10 Cells were mixed with 45% Percoll and layered onto Percoll gradient consisting in 3 mL 81%, 2 mL 62%, 2 mL 55%, 2 mL 50% in Hanks Balanced Salt Solution (HBSS; Invitrogen, Carlsbad, CA). Neutrophils were separated by centrifugation at 600g for 30 minutes at 10°C. Red cells were then depleted using ficoll (1119) density centrifugation. Neutrophils were washed with HBSS, resuspended into HBSS, 1% BSA, and kept on ice. Neutrophils were also generated after culture of low-density bone marrow cells as previously described.12,17 In both cases, neutrophil purity, assessed after cytospin preparations of the cells and Diff Quick (Dade Berhing, Deerfield, IL) staining, was similar between genotypes and estimated between 65% and 75% depending on experiments. No differences in the phenotype of the cells were observed between the 2 methods of neutrophil isolation.
Neutrophil chemotaxis
Neutrophil chemotaxis was performed using Boyden chamber assay as described previously.10,12,17 Neutrophil migration was also assessed using transwell (3 μm; Costar, Cambridge, MA) coated with fibrinogen (25 μg/mL; Sigma, Louis, MO). The cells (4 × 105) were diluted in 100 μL HBSS, 1 mM Ca 2+, 1 mM Mg2+ with or without 10 μM formyl-methionyl-leucyl-phenylalanine (fMLP; Sigma), and migration toward fMLP was allowed for 3 hours. The migrated cells recovered from the bottom well were counted using hemocytometer.
Immunofluorescence
To characterize F-actin assembly on integrin ligation and subcellular localization of EGFP-RhoA protein, slides were coated with fibrinogen (25 μg/mL) or with streptavidin and biotin anti-CD18 (clone C71/16, 10 μg/mL; PharMingen, San Diego, CA). Neutrophils (5 × 104) were prestimulated with 10 μM fMLP in HBSS containing Ca2+ and Mg2+ and seeded onto coated slides for 30 minutes at 37°C. The cells were then fixed with 2% paraformaldehyde (Sigma), stained with rhodamine-phalloidin (Molecular Probe, Eugene, OR), and mounted with reagent containing DAPI (Slowfade Gold Antifade reagent with DAPI; Molecular Probe). Z series of fluorescence images were captured with similar time exposure per sample as previously described.17 Images were acquired using a Leica DMIRB fluorescence microscope (Leica, Wetzlar, Germany) equipped with an ORCA-ER camera (Hamamatsu, Bridgewater, NJ) and a 63×/0.7 numerical aperture (NA) air objective. Images were analyzed using Volocity and Openlab (Improvision, Lexington, MA) software. Quantifications were performed by region measurement using Openlab. Cell spreading was analyzed by measuring cell-surface area. Cell-body contraction was analyzed by measuring the width of the tail at two thirds of the cell opposite to the lamellipodia. For cells displaying little lamellipodia, as in Rac2-deficient cells, cell-body contraction was analyzed by measuring the width of the cells perpendicular to plasma membrane extension. To analyze F-actin polarity, the cells were divided into 2 equal sections along the transverse axis, and the mean fluorescence intensity (MFI) of F-actin was measured in each section. The transverse axis was defined as perpendicular to the leading edge lamellipodia and uropod. The results are expressed as fold-increased MFI of leading lamellipodia compared with the tail. For cells that do not show an obvious leading edge, as in Rac2-deficient cells, the transverse axis was defined as perpendicular to plasma membrane extension. The results are expressed as fold-increased MFI of one section compared with the opposite section. EGFP-RhoA was analyzed by measuring the mean fluorescence intensity in the tail as defined for cell-body contraction. At least 30 cells were examined per conditions.
Analysis of Rho GTPase activity
Rho GTPase activity was analyzed using the “pull down” assay which consists in using the binding domain of RHO GTPase effectors to immunoprecipitate the activated GTP-bound form of RHO. The binding domain of Rhotekin is used to pull down RhoA. Neutrophils (106) were stimulated with 10 μM fMLP in HBSS at the indicated time. The reactions were stopped by placing the tubes on ice. The cells were lysed with 300 μL Mg2+-based lysis buffer for Rho GTPase pull down assay (Upstate, Charlottesville, VA) for 10 minutes, and the lysates were clarified by high-speed centrifugation for 10 minutes. The cell lyses were then incubated with GST-Rhotekin–binding domain beads (30 μg; Upstate) for 60 minutes at 4°C. After incubation, the lysis is kept and used as loading control. The beads were washed 3 times with lysis buffer. The amount of immunoprecipitated GTP-RhoA was then analyzed by immunoblot.12 The blots were quantified by densitometry and normalized to the loading control, and the results were expressed as fold-increased density compared with unstimulated cells.
Immunoblot
Specific antibodies against Rac1 proteins (Upstate), Rac2 proteins (Santa Cruz Biotechnology, Santa Cruz, CA) and RhoA (Santa Cruz Biotechnology) were used.
Challenge with fMLP
The mice were challenged with 20 μg fMLP by oral-tracheal intubation. Mice were anesthetized using isoflurane and placed in a supine position on their back at a gentle angle. Mice were then intubated with a bent-tipped cannula which is inserted through the mouth while keeping gentle pressure upward toward the ventral surface of pharynx and avoiding the tongue as not to occlude the tracheal opening. Solution was then instilled.
Bronchoalveolar lavage
Bronchoalveolar lavages (BALs) were performed 24 hours after fMLP instillation.18 Blood was harvested, and white blood cell count and neutrophil count were enumerated as described in “Generation of Rac1-deficient mice” before the animal was killed. Mice were killed by peritoneal pentobarbital injection. The trachea was visualized through midline ventral incision and blunt dissection, cannulated with 20 gauge Luer stub adapter, and secured by ligature. Three aliquots of 1 mL PBS (phosphate buffered saline; Invitrogen) were instilled and slowly withdrawn. The total number of cells recovered in BALs was counted using hemocytometer. Neutrophil content was evaluated after cytospin preparation of the cells and Diff Quick staining. Images were acquired using a Nikon Optiphot-2 (Nikon, Melville, NY) microscope equipped with an RT ColorSpot camera (Diagnostic Instruments, Sterling Heights, MI) and a 40×/0.7 NA air objective. Images were analyzed using Adobe Photoshop Element 2.0 (Adobe Systems, San Jose, CA).
Lung histology
Lung histology was performed 24 hours or 5 weeks after fMLP instillation. Mice were killed as described in “Bronchoalveolar lavage,” and the chest was opened by midline incision followed by exsanguinations. The trachea was cannulated, and the lungs were fixed by instillation of 4% paraformaldehyde (Sigma) in PBS for 1 minute under 25 cm H2O pressure. The lung was removed, washed in PBS, embedded in paraffin at 60°C. Sections (5 μm) were cut. To analyze neutrophil infiltration into the lung 1 day after challenge, lung sections were sent to the pathology laboratories of Cincinnati Children's Hospital Medical Center and stained for neutrophil esterase with Leder stain.19 Images were acquired and analyzed as described in “Bronchoalveolar lavage.” Neutrophils per high-power ×40 magnification were counted. Five fields per section were analyzed. The experiment was analyzed in a blinded manner.
To analyze emphysematous lesions 5 weeks after challenge, the lung sections were stained with hematoxylin and eosin. Images were acquired as described in “Bronchoalveolar lavage,” except that a 20×/0.4 NA air objective was used. Morphometric measurement of overall proportion of the respiratory parenchyma and air space was determined by using a point counting method.20 Measurements were performed on sections taken throughout the left, right upper, and right lower lobes using Metamorph imaging software (Universal Imaging, West Chester, PA). A computer-generated, 121-point lattice grid was superimposed on each field, and the number of intersections (points) falling over respiratory parenchyma (alveoli and alveolar duct) or airspace was counted. Points falling over bronchioles, large vessel, and smaller arterioles and venules were excluded from the study. Fractional area (% fx area) was calculated by dividing the number of points for each compartment (n) by the total number of points contained within the field (N), and then multiplying by 100: % fx area = n/N × 100. The fields were randomly chosen. Ten fields per section were analyzed.
Results
Loss of Rac1 activity is associated with abnormal neutrophil transmigration and uropod formation
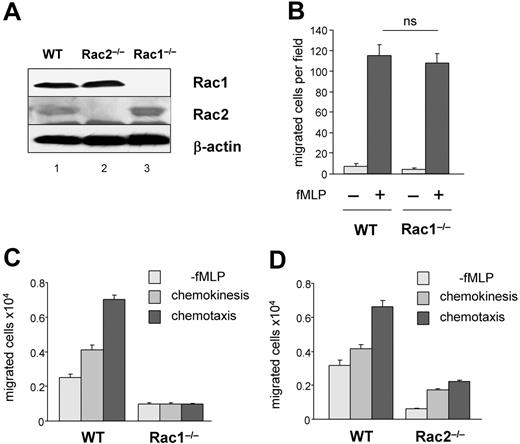
To examine the role of Rac1 in neutrophil migration, we used Rac1flox/flox mice crossed with transgenic mice expressing Cre recombinase by interferon-γ–inducible Mx1 promoter (CreTg+;Rac1flox/flox), as previously described.12 Figure 1A showed complete loss of Rac1 expression in bone marrow–derived neutrophils after poly:IC treatment. In vitro, using Boyden chamber, the chemotactic response of Rac1−/− neutrophils to fMLP was normal as well as the formation of a polarized lamellipodia of F-actin in uniform concentration of stimulation.12 However, Rac1−/− neutrophils demonstrated increased spreading on β2-integrin ligation and abnormal tail retraction.12 Interestingly, Rac1−/− animals demonstrated impaired neutrophil recruitment into peritoneal cavities after thioglycollate challenge (M.-D.F., unpublished data, April 2004; Glogauer et al11), suggesting that Rac1 may play a physiologic role in neutrophil migration in vivo. Thus, we further examined the role of Rac1 in neutrophil migration. As previously described, no significant defect in migration toward gradient of fMLP was observed in Rac1−/− neutrophils using Boyden chamber chemotaxis assay (Figure 1B). However, in this assay, the cells do not migrate completely through the membrane. Thus, to mimic a complete transmigration process, we examined neutrophil migration using transwell assay. In addition, because Rac1−/− neutrophils demonstrated increased spreading on β2-integrin ligation12 and β2-integrin engagement is relevant to neutrophil/endothelial interaction, the transwells were coated with fibrinogen, a β2-integrin ligand. Because β2-integrins are not constitutively active, the assays were performed in the presence of Ca2+ and Mg2+ to preactivate the integrin molecules. Migration was assessed either without chemokine stimulation or with uniform concentration of stimulus (fMLP) to measure chemokinesis (motility) or directed migration (chemotaxis) in response to fMLP gradient. WT cells responded to fMLP gradient with significantly higher numbers of cells migrating than in chemokinesis assays (Figure 1C). In contrast, the numbers of migrated Rac1−/− neutrophils were dramatically lower compared with WT cells in each condition (Figure 1C). This result was not due to reduced adhesion or integrin expression because no differences in the numbers of Rac1−/− neutrophils adherent to fibrinogen nor the levels of expression of CD11b and CD18 integrins have been reported between these cells and WT neutrophils.12 Consistent with the known role of Rac2 in F-actin assembly and chemotaxis, Rac2-deficient cells also demonstrated poor migration compared with WT cells (Figure 1D). These results indicate that Rac1−/− neutrophils showed defective transmigration through a membrane and suggest that Rac1 plays a role in cell motility.
The loss of Rac1 activity leads to impaired neutrophil transmigration. Neutrophils were derived from WT, Rac1−/−, or Rac2−/− bone marrow cells. (A) Rac protein expression analyzed by immunoblot using antibodies specific for Rac1 or Rac2. (B) Neutrophil migration was analyzed using Boyden chamber assay in response to 1 μM fMLP. The result represents the number of migrated cells per field. (C-D) Neutrophil migration using transwell coated with fibrinogen. Migration was evaluated without fMLP or in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD, representative experiment in triplicate from 3 independent experiments.
The loss of Rac1 activity leads to impaired neutrophil transmigration. Neutrophils were derived from WT, Rac1−/−, or Rac2−/− bone marrow cells. (A) Rac protein expression analyzed by immunoblot using antibodies specific for Rac1 or Rac2. (B) Neutrophil migration was analyzed using Boyden chamber assay in response to 1 μM fMLP. The result represents the number of migrated cells per field. (C-D) Neutrophil migration using transwell coated with fibrinogen. Migration was evaluated without fMLP or in uniform concentration or in a gradient of 10 μM fMLP. The histogram represents the total number of migrated neutrophils recovered from the bottom well, mean ± SD, representative experiment in triplicate from 3 independent experiments.
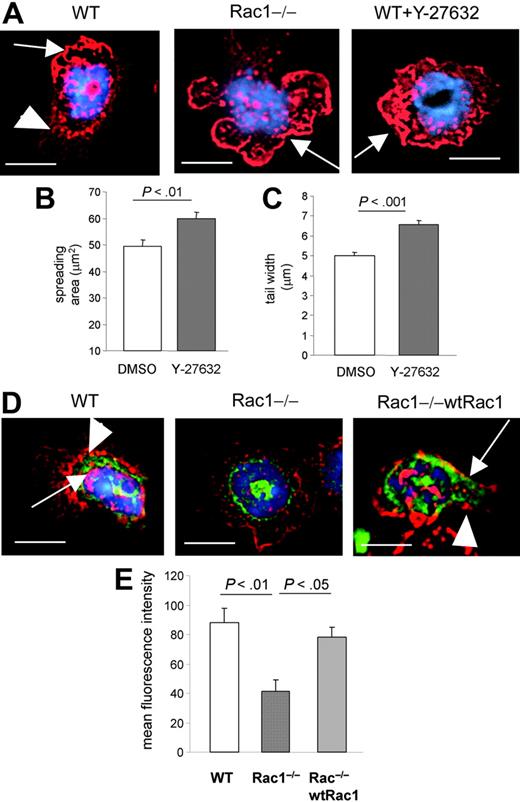
Cell motility requires neutrophils to polarize and rearrange F-actin. This can occur in uniform concentration of stimulation (ie, intrinsic cell polarity). Therefore, to further explore the mechanism of Rac1-mediated neutrophil motility, we analyzed β2-integrin–mediated F-actin assembly using slides coated with either anti-CD18 or fibrinogen and stimulated in uniform concentration of fMLP. In comparison with unstimulated cells, β2-integrin ligation induced lamellipodia, contraction of the cell body, and formation of a uropod in WT cells (Figure 2A). In contrast, Rac1−/− cells showed abnormally large lamellipodia and an absence of cell-body contraction and uropod formation (Figure 2A-B). The width of the tail was significantly increased in the absence of Rac1 compared with WT cells (Figure 2B), associated with increased spreading (Figure 2C) (as previously reported12 ). Similar results were observed during adhesion to fibrinogen (not shown). In contrast but consistent with our previous report,10,12,17 Rac2-deficient cells appeared smaller than WT cells with little lamellipodia formation (Figure 2A-D). Of note, F-actin polymerized and polarized at one pole of the cell in the absence of Rac1, whereas Rac2-deficient cells demonstrated defective F-actin assembly in neutrophils (Figure 2D), in uniform concentration of fMLP stimulation and on β2-integrin ligation substratum. Thus, these results further suggest that Rac1 and Rac2 regulate neutrophil migration by distinct mechanism(s) in which Rac2 but not Rac1 plays a role in intrinsic F-actin polarity. Importantly, this study suggests that Rac1 plays a role in migration by regulating cell contraction and uropod formation.
The loss of Rac1 activity leads to impaired uropod formation in neutrophils. WT, Rac1−/−, and Rac2−/− neutrophils were prestimulated with fMLP and seeded on anti-CD18–coated slides for 30 minutes. Some cells were seeded on noncoated slides for 30 minutes as negative control. The cells were then fixed and stained with rhodamine-phalloidin and mounted with reagent containing DAPI for nucleus. (A) Representative pictures of fluorescent images of F-actin (in red) for each genotype from 3 independent experiments. The nucleus is visualized in blue. The arrows point out the leading edge. The arrowhead points out the width of the tail. Note the presence of narrow lamellipodia and tail in WT cells, whereas Rac1−/− cells display a large lamelipodia and tail. Scale bar = 5 μm. (B) Quantification of body contraction by measuring the width of the cell (see “Materials and methods”). (C) Spreading area. (D) Cell polarity. The cells were divided into 2 equal transverse sections, and fluorescent intensity of F-actin was measured in arbitrary units. The results are expressed as fold-increased level of fluorescence of one pole of the cells compared with the opposite pole. Results show mean ± SEM.
The loss of Rac1 activity leads to impaired uropod formation in neutrophils. WT, Rac1−/−, and Rac2−/− neutrophils were prestimulated with fMLP and seeded on anti-CD18–coated slides for 30 minutes. Some cells were seeded on noncoated slides for 30 minutes as negative control. The cells were then fixed and stained with rhodamine-phalloidin and mounted with reagent containing DAPI for nucleus. (A) Representative pictures of fluorescent images of F-actin (in red) for each genotype from 3 independent experiments. The nucleus is visualized in blue. The arrows point out the leading edge. The arrowhead points out the width of the tail. Note the presence of narrow lamellipodia and tail in WT cells, whereas Rac1−/− cells display a large lamelipodia and tail. Scale bar = 5 μm. (B) Quantification of body contraction by measuring the width of the cell (see “Materials and methods”). (C) Spreading area. (D) Cell polarity. The cells were divided into 2 equal transverse sections, and fluorescent intensity of F-actin was measured in arbitrary units. The results are expressed as fold-increased level of fluorescence of one pole of the cells compared with the opposite pole. Results show mean ± SEM.
Loss of Rac1 activity is associated with abnormal RhoA subcellular localization in neutrophils
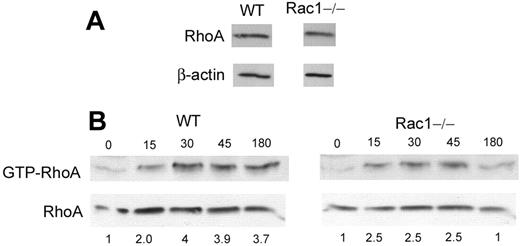
Cell-body contraction and tail retraction are cell functions previously attributed to the Rho GTPase, RhoA.4,21 Indeed, in cell lines, RhoA has been shown to localize at the rear of the cells22,23 and, by its effector ROCK, to regulate cell spreading and uropod formation by antagonizing membrane protrusion.24,25 RhoA is also known to regulate tail retraction during cell movement.24 We, therefore, examined the effect of the inhibition of ROCK on neutrophil cell shape on integrin ligation. WT neutrophils treated with the ROCK inhibitor, Y-27632, demonstrated a cell shape very similar to Rac1−/− cells with minimal cell-body contraction and increased spreading (Figure 3A-C). We next examined whether the loss of Rac1 activity could have a direct effect on RhoA functions in these cells. Because RhoA is known to localize at the rear of migrating cells,22,23 we examined whether Rac1 regulates cellular localization of RhoA. The localization of EGFP-RhoA was examined on integrin ligation and compared with F-actin structure. In WT cells, EGFP-RhoA demonstrated a polarized subcellular localization, mainly concentrated near the uropod (Figure 3D-E). In contrast, in Rac1−/− cells, RhoA was found mainly in the perinuclear membrane domain with no polarization (Figure 3D-E). Reintroduction of Rac1 into Rac1−/− cells restored the localization of RhoA as seen in WT cells (Figure 3D-E). These results suggest that the loss of Rac1 activity leads to defective RhoA subcellular localization in neutrophils. We also examined whether RhoA activity was deregulated in absence of Rac1. RhoA expression was similar in WT and Rac1−/− cells (Figure 4A). The amount of GTP-bound RhoA, which represents the active form of RhoA, was slightly lower in Rac1−/− cells compared with WT cells in response to fMLP (Figure 4B). These results suggest the existence of crosstalk between Rac1 and RhoA in which Rac1 appears to be necessary for proper RhoA localization at the cell rear.
The loss of Rac1 activity leads to defective RhoA subcellular localization. (A) Effect of ROCK inhibition on F-actin structure of WT neutrophils. WT cells, pretreated with the ROCK inhibitor Y-27632, were stimulated and stained with rhodamine-phalloidin and mounted with reagent containing DAPI for nucleus, as in Figure 2, and F-actin structure (in red) was compared with WT and Rac1−/− neutrophils. The nucleus is visualized in blue. The arrows point out the leading edge. The arrowheads point out the uropod in WT cells. Note the presence of narrow lamellipodia and uropod formation in WT cells, whereas cells treated with Y-27632 or Rac1−/− cells display a large lamellipodia without uropod formation. (B) Spreading area. (C) Width of the uropod. (D) WT, Rac1−/−, and Rac1−/− (wtRac1) polymorphonuclear neutrophils (PMNs) expressing EGFP-RhoA (in green) were stimulated and stained as above. Arrows point out EGFP-RhoA. Arrowheads point out the tail. Note the presence of RhoA in the uropod of WT cells and Rac1−/− (wtRac1) cells, whereas RhoA remained in a perinuclear domain in Rac1−/− neutrophils. (E) EGFP-RhoA in the tail. Fluorescent intensity of EGFP-RhoA in the tail was measured in arbitrary units. Representative images from at least 2 independent experiments, scale bar = 5 μm. Results show mean ± SEM.
The loss of Rac1 activity leads to defective RhoA subcellular localization. (A) Effect of ROCK inhibition on F-actin structure of WT neutrophils. WT cells, pretreated with the ROCK inhibitor Y-27632, were stimulated and stained with rhodamine-phalloidin and mounted with reagent containing DAPI for nucleus, as in Figure 2, and F-actin structure (in red) was compared with WT and Rac1−/− neutrophils. The nucleus is visualized in blue. The arrows point out the leading edge. The arrowheads point out the uropod in WT cells. Note the presence of narrow lamellipodia and uropod formation in WT cells, whereas cells treated with Y-27632 or Rac1−/− cells display a large lamellipodia without uropod formation. (B) Spreading area. (C) Width of the uropod. (D) WT, Rac1−/−, and Rac1−/− (wtRac1) polymorphonuclear neutrophils (PMNs) expressing EGFP-RhoA (in green) were stimulated and stained as above. Arrows point out EGFP-RhoA. Arrowheads point out the tail. Note the presence of RhoA in the uropod of WT cells and Rac1−/− (wtRac1) cells, whereas RhoA remained in a perinuclear domain in Rac1−/− neutrophils. (E) EGFP-RhoA in the tail. Fluorescent intensity of EGFP-RhoA in the tail was measured in arbitrary units. Representative images from at least 2 independent experiments, scale bar = 5 μm. Results show mean ± SEM.
The loss of Rac1 activity leads to impaired RhoA activity. (A) RhoA expression in WT and Rac1−/− PMNs by immunoblot. (B) WT and Rac1−/− PMNs were stimulated with fMLP at the indicated time (second) and subjected to the “pull down” assay for RhoA activity which consists of using the binding domain of the RhoA effector rhotekine which binds RhoA only in the GTP-bound activated form. The amount of RhoA visualized by immunoblot represents the amount of GTP bound. Total cell lysis was analyzed for RhoA expression as loading control. The ratio between GTP-RhoA and total RhoA was analyzed by densitometry of the blot and is expressed in arbitrary units. The numbers represent fold-increased density compared with unstimulated cells. Representative blot from 3 independent experiments.
The loss of Rac1 activity leads to impaired RhoA activity. (A) RhoA expression in WT and Rac1−/− PMNs by immunoblot. (B) WT and Rac1−/− PMNs were stimulated with fMLP at the indicated time (second) and subjected to the “pull down” assay for RhoA activity which consists of using the binding domain of the RhoA effector rhotekine which binds RhoA only in the GTP-bound activated form. The amount of RhoA visualized by immunoblot represents the amount of GTP bound. Total cell lysis was analyzed for RhoA expression as loading control. The ratio between GTP-RhoA and total RhoA was analyzed by densitometry of the blot and is expressed in arbitrary units. The numbers represent fold-increased density compared with unstimulated cells. Representative blot from 3 independent experiments.
Loss of Rac1 activity induces a defect in neutrophil migration into the lung
To examine the physiologic relevance of Rac1 in neutrophil functions in vivo, we used a model of lung inflammation. Because Rac1 is expressed in endothelial and interstitial cells, we used adoptive transfer of marrow cells from CreTg+;Rac1flox/flox into lethally irradiated C57BL/6 WT mice. This experimental design allows postengraftment in vivo deletion of Rac1 in blood cells and thus analysis of the specific role of Rac1 in neutrophil migration into the lung during the inflammation response. As controls, we engrafted bone marrow from WT littermates (CreTg+;Rac1wt/wt). Five weeks after bone marrow reconstitution, both WT and Rac1flox/flox-reconstituted mice were treated with 4 injections of polyI:C. This treatment resulted in a complete loss of Rac1 protein in bone marrow–derived neutrophils as assessed by immunoblot (Figure 5A). The total white blood cell count, including neutrophils, was normal in WT and Rac1−/−-reconstituted animals (Figure 5B). Neutrophils freshly isolated from polyI:C-treated CreTg+;Rac1flox/flox mice demonstrated in vitro fMLP-induced chemotaxis similar to WT cells (Figure 5C) as shown in Figure 1 using in vitro bone marrow–derived neutrophils, suggesting that polyI:C treatment does not affect neutrophil function in vivo.
The loss of Rac1 activity induces a defect in neutrophil migration into the lung alveolar spaces. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP in the lung, and bronchoalveolar lavage was performed 24 hours after challenge. (A) Rac protein expression in bone marrow cells from WT or Rac1−/−-reconstituted mice was analyzed by immunoblot using antibodies specific for Rac1 or Rac2. (B) Blood was harvested, and white blood cell (WBC) count and neutrophil (PMN) count were enumerated with a hematologic analyzer for mouse blood sample. The histogram represents the total number of cells per microliter of blood. (C) Neutrophils were isolated from peripheral blood of mice reconstituted with WT or Rac1−/− hematopoietic cells after treatment with polyI:C. Neutrophil migration was analyzed using Boyden chamber in response to 1 μM fMLP. The result represents the number of migrated cells per field. (D) White blood cell counts and neutrophil counts at the time of killing, after fMLP challenge. (E) Total number of cells recovered in the BAL. (F) Total number of PMNs recovered in the BAL. Mean ± SEM from 3 independent experiments. (G) Mice reconstituted with WT, Rac1−/−, and Rac1−/− bone marrow cells expressing exogenous wtRac1 were challenged with fMLP. Cells recovered in BAL were counted by hemocytometer. Representation of individual mice; horizontal bars represent average. (H) Representative images of cytospin preparation of BAL of each group, ×400.
The loss of Rac1 activity induces a defect in neutrophil migration into the lung alveolar spaces. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP in the lung, and bronchoalveolar lavage was performed 24 hours after challenge. (A) Rac protein expression in bone marrow cells from WT or Rac1−/−-reconstituted mice was analyzed by immunoblot using antibodies specific for Rac1 or Rac2. (B) Blood was harvested, and white blood cell (WBC) count and neutrophil (PMN) count were enumerated with a hematologic analyzer for mouse blood sample. The histogram represents the total number of cells per microliter of blood. (C) Neutrophils were isolated from peripheral blood of mice reconstituted with WT or Rac1−/− hematopoietic cells after treatment with polyI:C. Neutrophil migration was analyzed using Boyden chamber in response to 1 μM fMLP. The result represents the number of migrated cells per field. (D) White blood cell counts and neutrophil counts at the time of killing, after fMLP challenge. (E) Total number of cells recovered in the BAL. (F) Total number of PMNs recovered in the BAL. Mean ± SEM from 3 independent experiments. (G) Mice reconstituted with WT, Rac1−/−, and Rac1−/− bone marrow cells expressing exogenous wtRac1 were challenged with fMLP. Cells recovered in BAL were counted by hemocytometer. Representation of individual mice; horizontal bars represent average. (H) Representative images of cytospin preparation of BAL of each group, ×400.
These reconstituted mice were then exposed to fMLP by intratracheal instillation. To determine the level of neutrophils which migrated into alveolar spaces, bronchoalveolar lavages (BALs) were performed 24 hours after fMLP exposure. On fMLP exposure into the lung, peripheral blood neutrophil counts remained similar in both genotypes (Figure 5D). BALs from Rac1−/−-reconstituted mice showed significantly reduced numbers of total cells (Figure 5E), including a significant reduction in neutrophils (Figure 5F) compared with WT-reconstituted mice. To confirm the specificity of Rac1 function, Rac1flox/flox bone marrow cells were transduced with retrovirus vector carrying HA-tagged wtRac1 cDNA and EGFP. As controls, WT and Rac1flox/flox bone marrow cells were transduced with empty vectors. EGFP+ cells were sorted and transplanted into lethally irradiated C57BL/6 recipient mice. Five weeks after transplantation, 90% of granulocytes in the peripheral blood expressed EGFP as assessed by flow cytometry, indicating that the recipient mice were reconstituted with the transduced donor cells (not shown). The animals were treated with polyI:C to induce the excision of the endogenous Rac1 allele, then exposed to fMLP into the lung, and BALs were performed 1 day later. As assessed by immunoblot, exogenous Rac1 protein was expressed in the transduced Rac1−/− bone marrow–derived neutrophils (not shown). The number of migrated cells was significantly higher in BALs from recipient mice reconstituted with Rac1−/− (wtRac1) cells in comparison with Rac1−/−-reconstituted mice and not significantly different from WT-reconstituted mice (Figure 5G), as illustrated in Wright-Giemsa staining of cytospin preparation of the cells of BALs from each of the animals (Figure 5H).
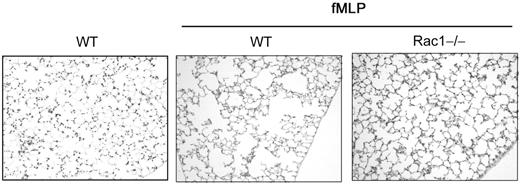
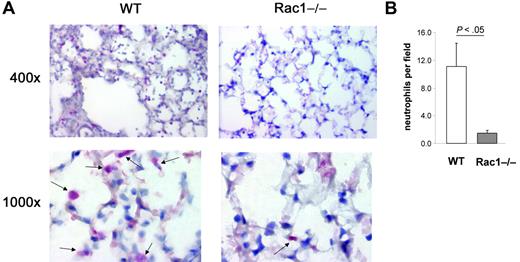
Finally, to determine the level of neutrophils infiltrated into the lung tissue of challenged mice, lung histology was performed 1 day after challenge with fMLP. Lung tissues were stained with Leder stain for neutrophil esterase.19 WT-challenged animals demonstrated numerous neutrophils in lung interstitial tissues and in alveolar spaces (Figure 6A). In contrast, lung tissues of Rac1−/−-reconstituted mice were poorly infiltrated with neutrophils (Figure 6A). The number of neutrophils infiltrated into the lung of Rac1−/−-reconstituted mice was significantly lower than WT animals (Figure 6B). These results strongly suggest that Rac1 plays a physiologic role during neutrophil migration into the lung.
The loss of Rac1 activity induces a defect in neutrophil migration into the lung tissues. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP in the lung. Lungs were harvested 24 hours after challenge and fixed, and lung tissue was stained with Leder stain for neutrophil esterase. (A) Representative pictures of esterase-positive (pink) cells (see arrows) infiltrated into the lung. (B) Number of esterase-positive cells per field. Mean ± SD, n = 5.
The loss of Rac1 activity induces a defect in neutrophil migration into the lung tissues. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP in the lung. Lungs were harvested 24 hours after challenge and fixed, and lung tissue was stained with Leder stain for neutrophil esterase. (A) Representative pictures of esterase-positive (pink) cells (see arrows) infiltrated into the lung. (B) Number of esterase-positive cells per field. Mean ± SD, n = 5.
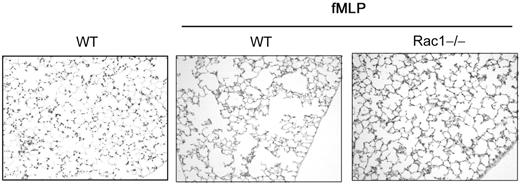
Loss of Rac1 activity reduces emphysema development
Emphysema can result from neutrophil infiltration into the lung that is associated with increased neutrophil elastase release.26 To assess the potential role of Rac1 in long-term inflammatory cell–mediated tissue destruction, we examined the lung histopathology of mice 5 weeks after fMLP treatment in the mouse model described in Figure 5. WT-reconstituted mice exposed to fMLP displayed typical emphysematous lesions with enlargement of the alveolar spaces (Figure 7). In contrast, Rac1−/−-reconstituted mice showed dramatic and significant reduction in the development of fMLP-induced emphysematous lesions compared with WT controls. The degree of enlargement of the alveolar spaces was quantified by morphometric measurement.20 The alveolar spaces measured in Rac1−/−-reconstituted mice were similar to unchallenged WT mice (57.6 ± 7.8 versus 52.9 ± 2.8, NS) and significantly less than WT (73.3 ± 3.04, P < .001) (Figure 7).
The loss of Rac1 activity reduces emphysema development. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP (20 μg per mouse) into the lung. WT non-exposed mice were used as control. Five weeks after exposure, lung histology was performed. Sections were stained with hematoxylin and eosin, ×200 (n = 16 from 2 independent experiments).
The loss of Rac1 activity reduces emphysema development. Mice reconstituted with WT or Rac1−/− hematopoietic cells were exposed to fMLP (20 μg per mouse) into the lung. WT non-exposed mice were used as control. Five weeks after exposure, lung histology was performed. Sections were stained with hematoxylin and eosin, ×200 (n = 16 from 2 independent experiments).
Overall, this study presents evidence that Rac1 is a physiologic regulator of neutrophil migration into the lung during an inflammatory response by controlling, at least in part, the formation of a uropod at the rear of the cell during neutrophil movement.
Discussion
Hematopoietic cells are unique with respect to the expression of Rac2 in addition to the ubiquitous Rac1. Rac2 has been extensively studied and appears to be the main regulator of F-actin polymerization and polarization at the leading edge in primary neutrophils.10-12,17 The role of Rac1 in cell migration still remains to be fully understood in hematopoietic cells. We and others have shown that Rac1-deficient cells fail to efficiently transmigrate into peritoneal cavities on thioglycollate challenge (M.-D.F., unpublished data, April 2004; and Glogauer et al11 ), indicating that Rac1 may play a physiologic role in neutrophil migration in vivo. Cell migration is a multiple step process which requires a coordinated rearrangement of F-actin and adhesion complexes, cell polarity, and gradient sensing.27 During random movement (motility), cells undergo intrinsic polarization and form a protrusive leading edge and a contractile tail. During directed migration (chemotaxis), the leading edge of F-actin specifically orients toward the source of stimulation.4,5,21 Intrinsic polarity and oriented polarity appear to be separately regulated. The in vivo migration defect associated with loss of Rac1 functions could result from defective chemotaxis, defective motility, or both. Using transwell migration or time-lapsed video microscopy, Rac1 was suggested to play a role in neutrophil chemotaxis and orientation of F-actin polarity toward the fMLP gradient by Pi3K-dependent gradient sensing.11,14 This Rac1-mediated chemotaxis may likely contribute to the lack of in vivo migration. We did not detect any chemotactic defects in Rac1-deficient neutrophils using the Boyden chamber assay (Figures 1 and 5), which may be attributed to poor sensitivity of this technique to detect some chemotactic events. However, because we have previously implicated Rac1 in β2-integrin spreading and tail retraction,12 we postulated that Rac1 could also regulate other cellular functions than chemotaxis during neutrophil migration.
Here, we present evidence that Rac1 plays a role in cell motility by regulating cell-body contraction and uropod formation in response to fMLP in combination with integrin ligation. We used transwells coated with a β2-integrin ligand, fibrinogen, to measure Rac1-mediated neutrophil transmigration in response to fMLP. In this assay, Rac1-deficient cells fail to migrate not only in chemotaxis settings, as previously reported by others using noncoated transwells,11 but also in chemokinesis settings, indicating that, in addition to its role in directional migration, Rac1 regulates motility. The abnormal migration seen in the absence of Rac1 was associated with significant defect in β2-integrin–induced cell-body contraction with a lack of uropod formation, whereas F-actin polarity was normal, in uniform concentration of fMLP. These results suggest that intrinsic F-actin polarity in response to fMLP combined with integrin ligation may not require Rac1 activity, whereas orientation of F-actin toward a source of stimulation may require Rac1 activity.14 Because Rac1-deficient neutrophils previously demonstrated defective F-actin polymerization in response to fMLP alone,11 these studies also suggest that Rac1 functions may be receptor specific. In high-speed migrating cells, such as neutrophils and lymphocytes, uropod formation is an active process resulting from the localization at the cell rear of proteins implicated in contraction and adhesion, including myosin II.28-31 In this context, the uropod may provide contractile forces necessary for effective body translocation and tail retraction. The Rho-ROCK pathway is known to regulate cell contraction and tail retraction.24,25,32 In response to chemoattractant or adhesion molecules, RhoA localizes at the rear of the cells and generates an uropod via myosin II function.22,23 We have shown here that, in Rac1-deficient cells, RhoA failed to properly localize in the tail, suggesting the existence of crosstalk between Rac1 and RhoA where RhoA localization depends on Rac1 activity. Failure in RhoA localization in the tail may account for the absence of uropod formation. These observations may also provide mechanistic explanation for the defect in tail retraction during cell movement in the absence of Rac1, previously reported by us12 and others.14 Although in response to fMLP RhoA activity was modestly lower in Rac1-deficient cells compared with WT cells, RhoA activity was not significantly different from WT cells on integrin ligation as measured by the pull down assay (M.-D.F., unpublished data, January 2006). This may indicate that Rac1 is merely required for RhoA localization but does not control its activity on integrin ligation. For example, CDC42 activity is required for proper localization of Akt to the membrane but not for its phosphorylation,33 suggesting that the level of protein activity may not reflect its function. However, the measurement of RhoA activity in specific subcellular compartment may provide important information on the crosstalk between Rac1 and RhoA. RhoA activity in Rac1-deficient cells by fluorescence resonance energy transfer analysis is under investigation. Our model is consistent with the hypothesis that Rho GTPases interact in a time- and space-dependent manner.4,5,21,34 For example, CDC42 activity is deregulated in Rac2-deficient hematopoietic stem cells.35 RhoA localization in the tail can also be dependent on CDC42 activity.23 Because Rac, CDC42, and Rho are known to work antagonistically, Rho GTPases may control each other's subcellular localization to counteract their functions. The mechanisms by which Rac1 regulates RhoA localization remain to be determined. Overall, our study and others suggest a dual role for Rac1 in which Rac1 regulates cell chemotaxis by Pi3K-dependent F-actin polarization11,14 and cell motility by RhoA-dependent uropod formation. While this manuscript was under revision, Rac1 was shown to regulate uropod formation by RhoA also during chemotaxis, suggesting a global role for the crosstalk between Rac1 and RhoA during neutrophil migration.36
In this report, we also provide evidence that Rac1 plays a physiologic role in neutrophil migration in vivo in an inflammation model. Using a model of fMLP-induced neutrophil recruitment into the lung, we demonstrate that Rac1 is important for neutrophil transmigration into the pulmonary tissue and interstitial and alveolar spaces. In our adoptive bone marrow transfer model, the peripheral neutrophil count is unchanged in the absence of Rac1 before and after fMLP challenge into the lung. Therefore, the lack of neutrophil egress into the lung cannot merely be due to lack of neutrophil availability from the circulation. Neutrophils have been well established to be key mediators of lung inflammation. Indeed, lung emphysematous lesions result from an excess of neutrophil elastase in the lung leading to an enzymatic degradation of elastin and eventually to destruction of the lung parenchyma.26 In this context, 5 weeks after fMLP challenge, emphysema poorly developed in the absence of Rac1, which may be attributed to a lack of neutrophil recruitment in the absence of Rac1. Emphysema is a complex and multiple parameter process. It is therefore possible that other unstudied effector cells of emphysema, such as macrophages, or other neutrophil functions, such as degranulation, may be deregulated in the absence of Rac1 and also contribute to the absence of emphysema in these animals.26,37,38 However, this study clearly indicates the physiologic role of Rac1 in inflammatory-related diseases. Neutrophil migration into the lung appears to be regulated by both β2-integrin–dependent and –independent mechanisms.39-42 Our study suggests that Rac1 integrates, at least in part, signals from β2-integrins necessary for uropod formation. These observations may provide relevant information in the molecular mechanism of neutrophil migration specifically into the pulmonary tissue.
Overall, this study suggests that Rac1 plays a role in uropod formation maybe by regulating the spatial distribution of effector proteins. Our results suggest that Rac proteins could be potential molecular targets to block neutrophil functions and prevent the development of abnormal inflammatory activity. In this regard, soluble small molecule inhibitors of Rac43 may prove to be useful therapeutically in the future.
Authorship
Contribution: K.S. and C.E.H. performed the research; M.-D.F. and P.-Y.B. designed and performed the research, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Dr Berclaz is now affiliated with Eli Lilly and Company, Indianapolis, IN 46285.
Correspondence: Marie-Dominique Filippi, Division of Experimental Hematology, Cincinnati Children's Hospital, 3333 Burnet Ave, Cincinnati OH 45229; e-mail: Marie-Dominique.Filippi@cchmc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank our colleagues of the Division of Experimental Hematology for helpful comments on the manuscript. We thank Dan Marmer and the Flow Analysis Core at Cincinnati Children's Hospital Medical Center for assistance with cell sorting. We thank Shelli Homan and Victoria Summey-Harmer for animal husbandry and Jude Hayden for computer assistance. We also thank Amgen, Inc, and Takara Bio for reagents.
This work was supported by the Alpha 1 Anti-Trypsin Foundation and Cincinnati Children's Hospital Medical Center (CCHMC) Translational Research Initiative (P.-Y.B.).