Abstract
The capacity of mouse spleen conventional dendritic cells (cDCs) and plasmacytoid dendritic cells (pDCs) to produce interferon-γ (IFN-γ) or IFN-α was assessed, and compared with that of natural killer (NK) cells and the recently identified interferon-producing killer dendritic cells (IKDCs), both of which are frequent contaminants in DC preparations. Fully developed cDCs or pDCs, if free of NK cells or IKDCs, showed little capacity for IFN-γ production. However, an early developmental form of the CD4−8+ cDC subtype, and the Ly6C− Ly49Q− pDC subtype, both were able to produce moderate amounts of IFN-γ, although less than IKDCs. In response to toll-like receptor 9 stimuli, both the Ly6C+ Ly49Q+ and the Ly6C− Ly49Q− pDC subtypes were effective producers of IFN-α. However, IKDCs, which efficiently produced IFN-γ and showed immediate cytotoxicity on NK target cells, did not produce IFN-α un-der these conditions.
Introduction
Interferons (IFNs) are key effector cytokines of the innate and adaptive immune systems. Type 1 interferons, such as IFN-α, are produced in large quantities by activated plasmacytoid dendritic cells (pDCs), and are particularly important in resistance to virus infections.1-5 The inflammatory cytokine IFN-γ is produced in large quantities by Th1 effector CD4 T cells, by CD8 T cells, and by natural killer (NK) cells.6-8 Interferons also play crucial roles in the initiation of adaptive immune responses. Type 1 interferons help activate the conventional dendritic cells (cDCs) which are needed to initiate primary T-cell responses. IFN-γ itself is needed to initiate the differentiation of activated T cells toward the IFN-γ–producing Th1 state. Thus, the production of even relatively small amounts of interferons by even minor cell populations could be important in the early steps of immune responses.
It has been reported by several laboratories, including our own,9-12 that DCs are able to produce IFN-γ when stimulated with interleukin 12 (IL-12) and IL-18. Despite this common finding, there was a discrepancy in the cDC subtype considered responsible, it being attributed mainly to CD4−8+ cDCs by the Japanese group,10 mainly to CD4−8− cDCs by our group,9 and to both of these subsets by others.11 However, such IFN-γ production by DC preparations has since been widely attributed to NK cells, since only a trace contamination could account for the modest level of IFN-γ production found.
A further complication to the assessment of IFN production by DC subtypes is the recent recognition of a type of cell with properties overlapping those of DC and NK cells. The existence of DC with NK-like killing properties had been recognized previously in rodents.13-16 Human NK clones have been shown to be effective at antigen presentation,17 and human pDC malignancies display NK cell markers.18-21 Recent studies by 2 groups have provided sufficient information to consider these as a distinct cell type in mice, now termed interferon-producing killer dendritic cells (IKDCs).22-24 Since IKDCs are potent producers of IFN-γ, and since they express surface CD11c as do DCs, it seemed possible these were the source of the IFN-γ production in DC preparations. IKDCs have also been reported to produce IFN-α.22,23 Since IKDCs express some surface markers characteristic of pDCs and are likely to be found in pDC preparations, a further question was whether any portion of the IFN-α production by pDC preparations could be attributed to IKDCs. A capacity to produce substantial amounts of both IFN-γ and IFN-α would make IKDCs a uniquely potent cell type.
To clarify these issues we isolated spleen cDC and pDC subtypes in such a way as to segregate them from NK cells and IKDCs, then we tested them for IFN-α and IFN-γ production. We found very little IFN-γ production by fully developed cDCs or pDCs once free of NK-like cell contaminants, but a CD8− precursor of the CD8+ cDC subset and a Ly6C− form of pDCs, both routinely found in DC preparations, were able to produce moderate levels of IFN-γ. Although we obtained extensive IFN-γ production by cells resembling IKDCs, we found no IFN-α production by these cells in response to a toll-like receptor 9 (TLR-9) stimulus. In our hands, such IFN-α production was restricted to pDCs lacking NK cell markers and lacking NK-like killing activity. The IFN-α producers included pDCs that were Ly6C− as well as Ly6C+.
Materials and methods
Mice
Mice were produced at the Walter and Eliza Hall Institute (WEHI) animal facility under specific pathogen-free conditions. Female 5- to 7-week-old C57Bl/6J Wehi mice were used in most experiments.
cDC isolation and enrichment
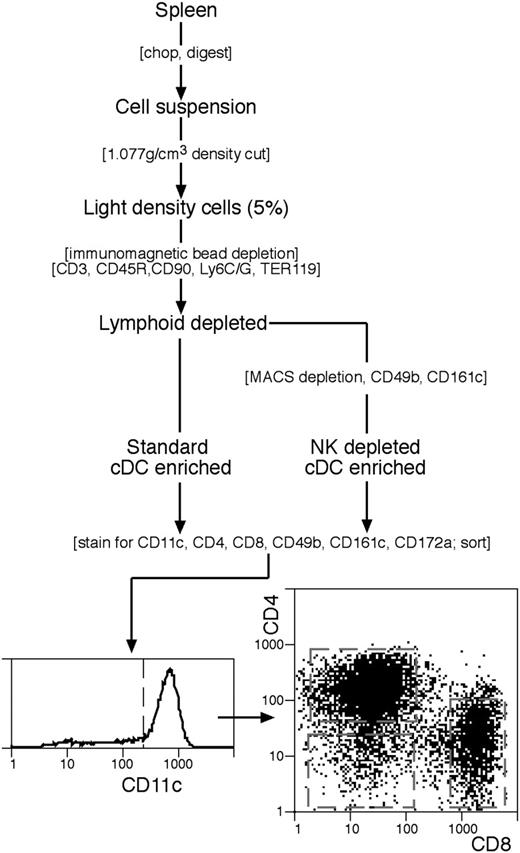
The standard procedure for isolation and enrichment of conventional DC (cDCs) was based on our earlier procedures25,26 and is summarized in Figure 1. All media were at pH 7.2 and iso-osmotic with mouse serum (308 mOs). Spleens (n=8) were cut into small fragments and digested, with frequent mixing, for 20 minutes at room temperature (22°C) in 7 mL modified RPMI-1640 medium, 2% fetal bovine serum (FCS) containing collagenase (1 mg/mL; Worthington Biochemical, Freehold, NJ; verified free of trypsinlike protease activity) and DNAase I (0.02 mg/mL; Boehringer Mannheim, Mannheim, Germany). EDTA solution (600 μL, 0.1 M, pH 7.2) was then added and mixing continued for 5 minutes. Undigested material was removed by filtration through a coarse sieve. All subsequent steps were at 0°C to 4°C, using a divalent metal–free buffered balanced salt solution containing EDTA (EDTA-BSS). Cells from the digest were centrifuged to a pellet and the pellet was resuspended in 10 mL of a 1.077 g/cm3 medium, made by dissolving Nycodenz powder (Nycomed, Oslo, Norway) in water as a dense stock (0.37 M) then diluting in EDTA-BSS to give the required density at 308 mOs. Further Nycodenz was layered below the Nycodenz cell suspension, EDTA-FCS layered above, then the tube centrifuged at 1700g for 10 minutes. The light density fraction (< 1.077 g/cm3) was collected, diluted in EDTA-BSS, recovered by centrifugation, then incubated for 30 minutes with the following monoclonal antibodies (mAbs): anti-CD3 (KT3-1.1); anti-Thy1 (T24/31.7); anti-B220 (RA3-6B2); anti-Gr1 (RB68C5); antierythrocyte (TER-119). All mAbs were titrated and used at near saturation conditions except anti-Thy1, which was used at 25% saturation. The cells were washed free of excess mAb, then antibody-coated cells were removed using anti–rat immunoglobulin (Ig)–coupled Biomag beads (Qiagen, Clifton Hill, Australia). The beads and cells at a 10:1 ratio were mixed as a concentrated slurry by slow rotation for 20 minutes, diluted with EDTA-BSS, then the beads and bound cells were removed with a magnet, using 2 removal steps. In the modified procedure to remove cells bearing NK markers, an additional depletion step was used prior to staining and sorting (Figure 1). The cells recovered after the anti–rat Ig depletion step were incubated with saturation levels of biotinylated mAb against CD49b (DX5) and against CD161c (NK1.1; clone PK136), washed, then incubated (in BSS containing 0.5% FCS) with antibiotin MACS magnetic particles (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's instructions, then passed over a Miltenyi column (Miltenyi Biotec), collecting the unbound cells. The enriched spleen cDCs obtained by both protocols were around 80% pure.
The procedure for isolating spleen cDC subtypes. A cDC-enriched preparation was prepared by either the standard procedure, or with an additional immunomagnetic particle depletion step to eliminate cells bearing NK cell markers. Final purification by flow cytometric cell sorting involved selection for CD11chi cDCs then sorting into CD4+8−, CD4−8− or CD4−8+ subtypes. Full details are in “Materials and methods.”
The procedure for isolating spleen cDC subtypes. A cDC-enriched preparation was prepared by either the standard procedure, or with an additional immunomagnetic particle depletion step to eliminate cells bearing NK cell markers. Final purification by flow cytometric cell sorting involved selection for CD11chi cDCs then sorting into CD4+8−, CD4−8− or CD4−8+ subtypes. Full details are in “Materials and methods.”
Staining, sorting, and analysis of cDC subsets
To effect the final purification of cDCs and segregate them into subtypes, the enriched cDC preparation was normally stained with mAbs against CD11c (N418-FITC), CD4 (GK1.5-Alexa 594), and CD8α (YTS 169.4-APC) and with CD49b (DX5-biotin with PE-streptavidin second stage) for analysis. Propidium iodide (1 μg/mL) was included in the final wash to label dead cells. When the CD4−8− cDC subset was isolated for further subdivision, the anti-CD11c was conjugated to APC, the anti-CD4 and anti-CD8 mAbs were both conjugated to Alexa 594, and the cells were also stained with mAb against CD172a (Sirp-α; P84-FITC). For further analysis the cells were also stained for CD24 (M1/69-PE) or for MHC II (M5/114-PE). Sorting was carried out on a DIVA instrument (Becton Dickinson, San Jose, CA) and analysis on a FACStar Plus instrument (Becton Dickinson), with exclusion of dead cells, autofluorescent cells, and cell doublets.
pDC isolation and enrichment
The procedure was based on our previous study,27 and is summarized in Figure 2A. The production of a spleen cell suspension and isolation of the light density fraction was identical with the procedure for cDCs. The immunomagnetic bead depletion step was also similar, except that the cells were coated with a different cocktail of mAbs, which spared pDCs but allowed some depletion of cDCs. These were mAbs against: CD3 (KT3-1.1); CD90 (T24/31.7); CD19 (1D3); Ly6G (1A8); erythrocytes (TER-119); F4/80 antigen (F4/80); CD11b (M1/70); and CD205 (NLDC145). Note that the mAb against CD205 allowed only partial depletion of the CD205+ cDC subtypes. No additional depletion for cells bearing NK markers was used, although the procedure used for cDCs was effective if predepletion of NK cells and IKDCs was required.
Subpopulations within pDC preparations. (A) The procedure for isolating pDCs and IKDCs. The initial steps were similar to those used for cDCs (Figure 1) except the immunomagnetic bead depletion mAb cocktail differed. No depletion of cells bearing NK cell markers was used. Rather, the pDCs were isolated by flow cytometric cell sorting (CD45RAhi CD11cint) and segregated into Ly6C+ and Ly6C− subsets, and the IKDCs were segregated as CD49b+ and CD11cint cells, either CD45RAhi or CD45RAint. Full details are in “Materials and methods.” (B) The correlation between Ly6C and Ly49Q expression by pDC preparations. pDCs were isolated as in panel A and gated on sorting as CD11cint CD45RAhi cells.
Subpopulations within pDC preparations. (A) The procedure for isolating pDCs and IKDCs. The initial steps were similar to those used for cDCs (Figure 1) except the immunomagnetic bead depletion mAb cocktail differed. No depletion of cells bearing NK cell markers was used. Rather, the pDCs were isolated by flow cytometric cell sorting (CD45RAhi CD11cint) and segregated into Ly6C+ and Ly6C− subsets, and the IKDCs were segregated as CD49b+ and CD11cint cells, either CD45RAhi or CD45RAint. Full details are in “Materials and methods.” (B) The correlation between Ly6C and Ly49Q expression by pDC preparations. pDCs were isolated as in panel A and gated on sorting as CD11cint CD45RAhi cells.
pDC purification and analysis
To effect final purification and analysis of pDC subsets (Figure 2A), the pDC-enriched preparation was normally stained for CD11c (N418-Alexa 594), CD45RA (14.8-APC), Ly6C (5075-3.6-FITC), and CD49b (DX5-biotin with PE-streptavidin second stage). When the cells were stained for Ly49Q (2E6-FITC), the mAb against Ly6C was a biotin conjugate with PE-streptavidin second stage, and CD49b staining was omitted. Dead cells were stained with propidium iodide as for cDCs. The sorting and analysis was as specified for cDCs.
NK cell isolation
Splenic cell suspensions were generated using collagenase digestion as in the DC isolation procedure. Red cells and dead cells were removed using a 1.091 g/cm3 Nycodenz density cut. The viable light density cells were incubated with anti-CD4 (GK1.5), anti-CD8 (53.6-7), anti-CD24 (M1/69), and anti-MHC II (M5/114) mAbs and coated cells were removed using anti–rat Ig magnetic beads. NK cells were then isolated by sorting for CD49b+(DX5+) CD3− cells.
Cytokine production culture conditions
Sorted cells (105) were cultured in 200 μL modified RPMI 1640 medium that contained 10% FCS, in 96-well round-bottom plates at 37°C under 10% CO2-in-air. After culture, supernatants were collected and stored for a short time at −20°C prior to analysis. To determine IFN-γ production, cells were cultured for 20 to 65 hours with 5 ng/mL IL-12p70 (R&D Systems, Minneapolis, NJ) and 20 ng/mL IL-18 (Peprotech, Rocky Hill, NJ), and where indicated with addition of CpG1668 (Geneworks, Hindmarsh, Australia) at a final concentration of 0.5 μM. To determine IFN-α production cells were cultured for 48 hours with CpG2216 (Geneworks) or CpG1668 at a final concentration of 0.5 μM. When determining IL-12p70 production cells were cultured for 40 hours with 20 ng/mL IL-4 (Immunex, Seattle, WA), 20 ng/mL IFN-γ (Peprotech), 50 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF) (Cytolab, Rehovot, Israel), and CpG1668 at a final concentration of 0.5 μM.
Analysis of cytokine production by ELISA
Culture supernatants were assayed for the presence of cytokine by 2-site enzyme-linked immunosorbent assay (ELISA). Purified capture monoclonal antibodies to IFN-γ (R4-6A2), IFN-α (RMMA-1; PBL Biomedical Laboratories, Piscataway, NJ), or IL-12p70 (R2-9A5) were coated onto 96-well polyvinyl chloride microtiter plates (Dynatech Laboratories, Chantilly, VA). Binding of cytokine was quantitated using the appropriate detection antibodies: biotin-conjugated XMG1.2 (IFN-γ) or C17.8 (IL-12p70) or a polyclonal rabbit anti–mouse IFN-α (PBL Biomedical Laboratories). A streptavidin–horseradish peroxidase (HRPO) conjugate (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) or a F(ab′)2 fragment donkey anti–rabbit HRPO conjugate (Jackson Immunoresearch Laboratories, West Grove, PA) and a substrate solution containing 548 μg/mL ABTS (Sigma-Aldrich, Castle Hill, Australia) and 0.001% hydrogen peroxide (Ajax Chemicals, Auburn, Australia) in 0.1 M citric acid, pH 4.2, were used to provide a readout. The optical density of each sample was read at 405 nm to 490 nm using an ELISA plate reader.
Cytotoxicity assays
The YAC-1 target cells were harvested in exponential growth phase, washed, and labeled by incubating 106 cells with 100 μL (51Cr) sodium chromate in 100 mL medium for 1.5 hours at 37°C then washed 4 times. Target cells (104) and various levels of effector cells were plated in 0.2 mL modified RPMI-1640 10% FCS medium in 96-well round-bottom plates, centrifuged for 1 minute at 335g, then incubated 4 hours at 37°C. After centrifugation (5 minutes, 400g) 100 μL supernatant was removed and the chromium release (E) was measured by γ-emission counting. Spontaneous chromium release (S) was determined by incubating target cells alone. Maximum release (M) was determined after target cell lysis with 10% Triton-X. Specific lysis was calculated according to the formula: % lysis = 100 × (E-S)/(M-S). Spontaneous release was always below 10%.
Antigen cross-presentation assays
For the in vitro presentation assay the cDC subsets were freshly isolated from unstimulated mice. For the in vivo presentation assay, as described previously,28 mice were given injections of 30 μg lipopolysaccharide (Sigma-Aldrich), then 1 hour later with 3 mg ovalbumin (Sigma-Aldrich), then the cDC subsets were isolated 19 hours later. Cross-presentation was assessed by the ability to stimulate ovalbumin-specific TCR-transgenic naive CD8+ OT-I T cells, isolated as described previously28 from lymph nodes. Cultures were set up in V-bottom 96-well plates in 0.2 mL modified RPMI-1640/10% FCS medium at 37°C in 10% CO2-in-air. The cDC subsets were plated at 0.5 × 103 to 5 × 103 cells per well and the OT-I T cells were added at 2 × 104 cells per well. For the in vitro presentation only, ovalbumin was added to the cultures at 250 μg/mL. After 2 days in culture, the wells were pulsed with 1 μCi (37 (kBq)/well (3H) thymidine for 8 hours, the cells harvested onto glass fiber filters, and the incorporation into cellular DNA determined by liquid scintillation counting.
Results
Spleen cDC preparations, cDC subsets, and IKDC contaminants
Our standard protocol for isolation of cDCs is given in Figure 1. The DCs were first enriched by selecting the 3% to 5% lightest density cells, which removed the bulk of B cells, T cells, and NK cells. Subsequent immunomagnetic bead depletion of the light density fraction then eliminated most pDCs, and residual B cells, T cells, and other lineages. Finally, sorting for cells bearing high levels of CD11c strongly selected for cDCs and against any residual pDCs, T cells, or NK cells. Additional sorting for CD4 or CD8 expression allowed segregation of the 3 main cDC subtypes, namely those that were CD4+8−, CD4−8+, and CD4−8− (also termed CD4+, CD8+, and DN, respectively; Figure 1). Recently we have shown,29 using the newer markers CD172a (Sirp-α) and CD24 (heat stable antigen), that the CD4+ and DN cDC subsets are both CD172ahi CD24lo, whereas the CD8+ cDC subset is CD172alo CD24hi. This provides another approach to segregating these cDC subtypes.
This standard protocol produced cDC subtypes that were around 99% pure on reanalysis, but we could not eliminate the possibility of contamination below the 1% level. It became clear that our enriched cDC preparations, prior to sorting, contained around 0.5% of cells that were light in density, expressed moderate levels of CD11c, and that also expressed the NK marker CD49b, revealed by the mAb DX5 (Figure 3A). These resemble IKDCs, the recently described “hybrid” NK-DCs.22,23 Most but not all of these IKDCs were eliminated by our gating for CD11chi cDCs (Figure 3A). As an approach to eliminating all IKDCs, as well as any residual conventional NK cells, we introduced an additional MACS depletion step using antibodies selective for NK cells (Figure 1). As shown in Figure 3A, this resulted in a very marked reduction of cells bearing the NK marker CD49b in both the cDC-enriched preparation and the sorted cDC fractions. Particularly important was the elimination of NK cells and/or IKDCs from the CD4−8− cDC fraction, since selection against CD4 and CD8 expression would concentrate any NK-like cells that had not been removed by the CD11c gating.
The production of IFN-γ by cDC subset preparations in relation to contamination with cells bearing NK markers. cDCs were isolated as outlined in Figure 1, either by standard procedure or with additional NK cell marker depletion. (A) Analysis of the content of CD49b+ CD11cint IKDCs within different cDC preparations, beginning with the enriched standard cDCs, the enriched cDCs with additional NK-marker depletion, the CD4−8− fraction of the NK-depleted cDC preparation without the normal gating for CD11c, and the final sorted, NK-marker–depleted and CD11chi-selected CD4−8− cDC preparation. (B) The production of IFN-γ in the supernatant following culture of 105 cells of each fraction in 0.2 mL medium with IL-12, IL-18, and CpG 1668, as in Table 1. The cDC samples were as analyzed in panel A, with contamination of NK-marker–depleted CD4−8− cDCs being less than 0.001%. IKDCs were isolated and sorted from enriched pDCs as CD45RA+ CD11cint CD49b+ cells, as shown in Figure 2. NK cells were CD49b+ cells directly sorted from a spleen suspension. Results are a single experiment typical of 3 showing the mean of 4 cultures plus or minus the standard deviation (SD). The broken line gives the threshold for detecting the production of IFN-γ above the background.
The production of IFN-γ by cDC subset preparations in relation to contamination with cells bearing NK markers. cDCs were isolated as outlined in Figure 1, either by standard procedure or with additional NK cell marker depletion. (A) Analysis of the content of CD49b+ CD11cint IKDCs within different cDC preparations, beginning with the enriched standard cDCs, the enriched cDCs with additional NK-marker depletion, the CD4−8− fraction of the NK-depleted cDC preparation without the normal gating for CD11c, and the final sorted, NK-marker–depleted and CD11chi-selected CD4−8− cDC preparation. (B) The production of IFN-γ in the supernatant following culture of 105 cells of each fraction in 0.2 mL medium with IL-12, IL-18, and CpG 1668, as in Table 1. The cDC samples were as analyzed in panel A, with contamination of NK-marker–depleted CD4−8− cDCs being less than 0.001%. IKDCs were isolated and sorted from enriched pDCs as CD45RA+ CD11cint CD49b+ cells, as shown in Figure 2. NK cells were CD49b+ cells directly sorted from a spleen suspension. Results are a single experiment typical of 3 showing the mean of 4 cultures plus or minus the standard deviation (SD). The broken line gives the threshold for detecting the production of IFN-γ above the background.
IFN-γ production by cDC subtypes and by IKDCs
We examined the possibility that the IFN-γ production by our cDC preparations, and in particular by the CD4−8− cDC subset,9 was due to trace contamination by NK cells or by IKDCs. Indeed, the CD49b+ cells present in our standard cDC-enriched preparations produced almost as much IFN-γ in response to IL-12 and IL-18 as isolated NK cells (confirming their likely identification as IKDCs) and this was 50 to 100 times higher on a per cell basis than our most active cDC subset (Figure 3B). We initially tried intracellular staining and flow cytometric analysis to determine if the IFN-γ production by our standard cDC preparations was due to a few contaminating cells making a lot of IFN-γ, or to many cDCs each making a little. However, the high noise of the assay rendered attempts to count a few high producers inconclusive, and the IFN-γ production on a per cell basis was too low to detect a significant shift above the background staining if most cells were IFN-γ producers. Accordingly, in subsequent studies we used an ELISA to determine IFN-γ levels in the supernatant of cDC cultures, since this was a low background, low noise assay allowing measurements over a wide range of IFN-γ secretion.
Using the ELISA, we confirmed the production of IFN-γ by the CD4−8− cDC subset isolated by our standard procedure, although the level was low compared with NK cells (Table 1). The CD4+8− cDC preparation also produced some IFN-γ, but this may have represented overlap with the CD4−8− cDCs, since the sorting boundary between these was not distinct. Two lines of evidence from preliminary experiments made it unlikely that this IFN-γ production was due to cells resembling NK cells or T cells. First, the production of IFN-γ was slower than the rapid output from NK cells or T cells; thus around 80% of the production of IFN-γ from NK cells was achieved within a 40-hour culture, whereas only 15% to 20% of the total production by cDCs was found in the supernatant at this time. Second, the production of IFN-γ was still obtained by cDCs isolated from Rag-1–null mice or from Il2rg-null mice, deficient in T cells or NK cells, respectively (data not shown). However, since IKDCs are apparently present even in Il2rg−/−Rag-2−/− mice,23 IKDCs were not excluded as a source of IFN-γ in these experiments.
To determine in a direct way the possible contribution of IKDCs to the IFN-γ production by our isolated cDC subpopulations, we carefully analyzed the level of cells bearing CD49b in the various fractions, comparing the standard cDC subset preparations with those from cDCs predepleted of cells bearing NK markers (Figure 3A). Although our standard enriched cDCs contained around 0.5% putative IKDCs, most but not all of these were depleted by the CD11chi gate we routinely used to select cDCs. Predepletion of cells bearing NK markers reduced this initial level of putative IKDCs in the cDC preparation around 250-fold (Figure 3A). This predepletion of cells bearing NK markers did result in some reduction (around 2-fold) in the final IFN-γ production by the cDC subsets, suggesting that traces of contaminating IKDCs had made some contribution to the measured IFN-γ production by the original cDC subset preparations (Figure 3B). However, significant IFN-γ production was still obtained when IKDCs or NK cells were below detectable levels. Importantly, the CD4−8− cDC subset was still a significant producer of IFN-γ when not even a single CD49b-bearing cell could be detected on analysis of 105 cells (Figure 3A-B). Thus, even though NK cells and IKDCs were around 100-fold more efficient as IFN-γ producers, they could not explain this level of IFN-γ production. These results support our previous conclusion,9 and the conclusion from Table 1, that the major source of IFN-γ by spleen cDC preparations is the CD4−8− cDC fraction.
Developing cDCs within the CD4−8− cDC preparation
We have recently demonstrated that the spleen contains a substantial pool of immediate precursors of cDCs.30 Most of these are of medium rather than low buoyant density, lack surface expression of MHC II, and express only moderate levels of CD11c. However, some of these cDC precursors appear to be in transit toward the fully developed steady-state spleen cDCs, having already acquired the light density and higher surface CD11c characteristics, and having already begun surface MHC II expression. These cDC precursors have not yet acquired surface expression of CD4 or CD8, although some have already demonstrated commitment toward particular cDC subtypes by the expression of the CD172a− (Sirpα−) CD24+ phenotype of CD8+ cDCs.29 Since any of these “in transit” cDC precursors in a cDC preparation would be found within the CD4−8− (DN) cDC fraction, we checked for their presence.
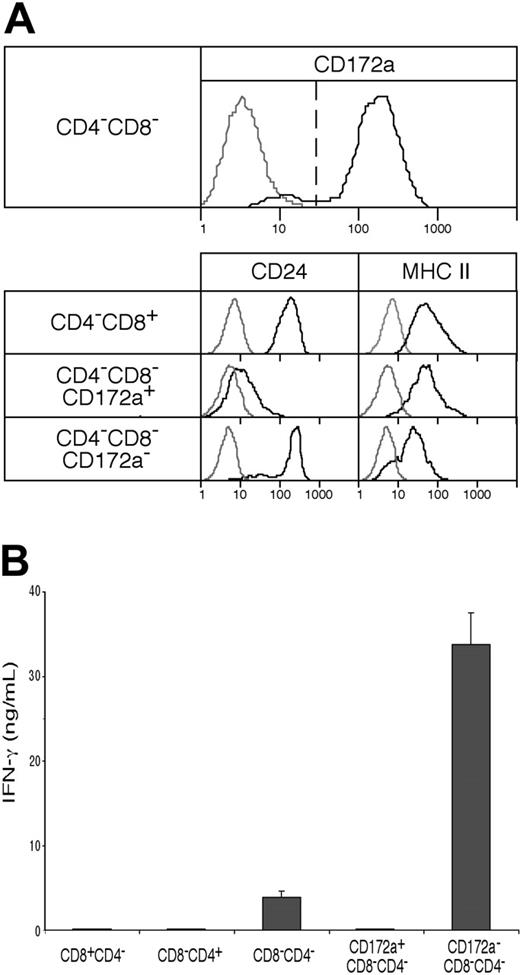
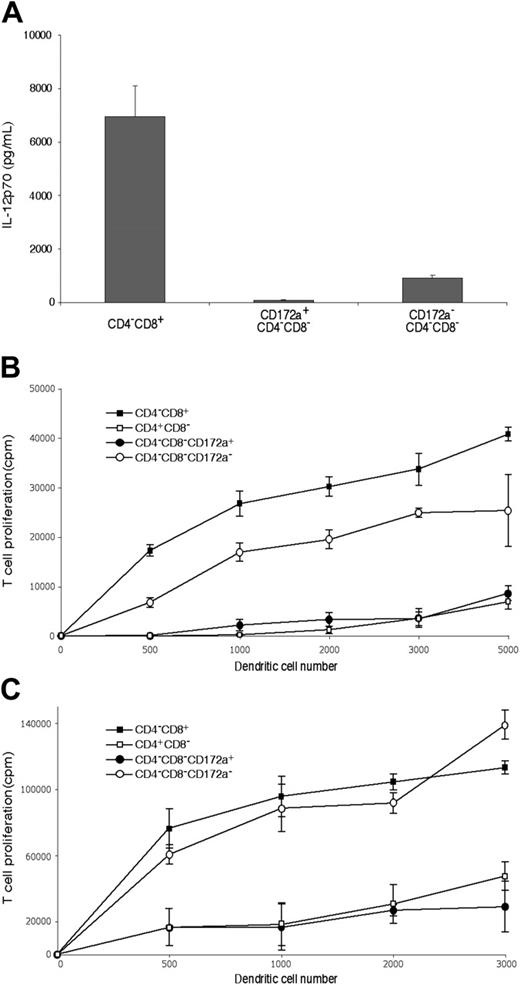
As shown in Figure 4A, although fully developed CD4−8− cDCs should be CD172a+, the CD4−8− cDC preparation did include a minor group of cells (10%-11%) that were CD172a−. Further analysis showed these CD172a− cells were lower in surface expression of MHC II, and were positive for CD24 expression (Figure 4A). This suggested they were indeed earlier cells en route to the CD4−8+ cDC subtype. Indeed, when these were cultured overnight in a conditioned medium, 42% then expressed CD8α (data not shown). Functionally, they appeared to be developing the properties characteristic of CD8α+ cDCs. Thus, when stimulated in culture with CpG and the appropriate cytokines, the CD172a− CD4−8− cDC subset produced some IL-12p70, like CD8+ cDCs,9 whereas the CD172a+ CD4−8− subset showed only marginal IL-12p70 production (Figure 5A). In addition, the minor CD172a− CD4−8− cDC subset was almost as effective as CD8+ cDCs in taking up exogenous soluble antigen and presenting it on MHC I (“cross-presentation”11,28,31 ) (Figure 5B). When the CD172a− CD4−8− cDC subset was isolated from mice that had been given injections of ovalbumin and lipopolysaccharide a day earlier,28 it was as efficient as CD8+ cDCs in activating the ovalbumin-specific CD8+ T cells (Figure 5C). In both these in vitro and in vivo tests, the major CD172a+ CD4−8− cDC subset was poor at cross-presentation, as expected of CD8− cDCs (Figure 5B-C). In conclusion, the CD4−8− cDC fraction as isolated included a small proportion of early forms of the CD8+ cDC sublineage.
The presence of an early form of the CD8+ cDC lineage within the CD4−8− cDC preparations, and its capacity to produce IFN-γ. (A) CD4−8− cDCs, sorted from NK-marker–depleted cDC preparations as in Figure 1 and analyzed as in Figure 3A, were segregated into CD172a+ (Sirpα+), typical CD4−8− cDCs and into a minor subset of CD172a− (Sirpα−) cells, which when analyzed were less developed (by surface MHC II expression) and which had the CD172a− CD24+ phenotype of the typical CD4−8+ cDCs. Gray lines represent background fluorescence from samples with only the relevant stain omitted; black lines represent the fluorescence of the positively stained sample. (B) IFN-γ production (in ng/mL) was measured in the supernatant 65 hours after culture with IL-12 and IL-18 of 105 cells (in 0.2 mL) of the fractions from panel A. Results are the means plus or minus SD of 4 cultures from the one experiment, typical of 3.
The presence of an early form of the CD8+ cDC lineage within the CD4−8− cDC preparations, and its capacity to produce IFN-γ. (A) CD4−8− cDCs, sorted from NK-marker–depleted cDC preparations as in Figure 1 and analyzed as in Figure 3A, were segregated into CD172a+ (Sirpα+), typical CD4−8− cDCs and into a minor subset of CD172a− (Sirpα−) cells, which when analyzed were less developed (by surface MHC II expression) and which had the CD172a− CD24+ phenotype of the typical CD4−8+ cDCs. Gray lines represent background fluorescence from samples with only the relevant stain omitted; black lines represent the fluorescence of the positively stained sample. (B) IFN-γ production (in ng/mL) was measured in the supernatant 65 hours after culture with IL-12 and IL-18 of 105 cells (in 0.2 mL) of the fractions from panel A. Results are the means plus or minus SD of 4 cultures from the one experiment, typical of 3.
The functional properties of the CD172a− CD24+ cells within the CD4−8− cDC subset preparations. The cDC subsets were isolated as in Figure 1 using the NK-marker depletion protocol, then the CD172a positive and negative fractions of the CD4−8−CD11chi subset separated as in Figure 4. (A) Production of IL-12p70 following stimulation with CpG 1668, IL-4, GM-CSF, and IFN-γ. Production is from 105 cells in 0.2 mL medium for 40 hours. The mean plus or minus SD of 3 cultures is given for one experiment, typical of 2. (B) Ability to process and cross-present on MHC I soluble antigen in vitro, assayed by culture of freshly isolated cDC fractions with ovalbumin and OT-I CD8 T cells. Points are the mean of 4 cultures plus or minus SD. One experiment typical of 2 is shown. (C) Ability to process and cross-present soluble antigen on MHC I in vivo, assayed by isolation of cDC fractions 20 hours after injection of mice with lipopolysacchride followed by ovalbumin, then culture of the cDC fractions with OT-I CD8 T cells without addition of ovalbumin. The result is a single experiment. Points are the mean of 3 to 4 cultures plus or minus SD.
The functional properties of the CD172a− CD24+ cells within the CD4−8− cDC subset preparations. The cDC subsets were isolated as in Figure 1 using the NK-marker depletion protocol, then the CD172a positive and negative fractions of the CD4−8−CD11chi subset separated as in Figure 4. (A) Production of IL-12p70 following stimulation with CpG 1668, IL-4, GM-CSF, and IFN-γ. Production is from 105 cells in 0.2 mL medium for 40 hours. The mean plus or minus SD of 3 cultures is given for one experiment, typical of 2. (B) Ability to process and cross-present on MHC I soluble antigen in vitro, assayed by culture of freshly isolated cDC fractions with ovalbumin and OT-I CD8 T cells. Points are the mean of 4 cultures plus or minus SD. One experiment typical of 2 is shown. (C) Ability to process and cross-present soluble antigen on MHC I in vivo, assayed by isolation of cDC fractions 20 hours after injection of mice with lipopolysacchride followed by ovalbumin, then culture of the cDC fractions with OT-I CD8 T cells without addition of ovalbumin. The result is a single experiment. Points are the mean of 3 to 4 cultures plus or minus SD.
Production of IFN-γ by the cDC precursors within the CD4−8− cDC fraction
A preparation of spleen cDCs, once free of IKDCs or NK cell contaminants, showed only a low capacity to produce IFN-γ. However, since much of this capacity was concentrated in the CD4−8− cDC fraction, we checked whether this was due to the CD172a+, fully developed CD4−8− cDCs, or due to the CD172a−, CD8+ cDC precursors within the CD4−8− cDC preparation. As shown in Figure 4B, it was clear that all of the capacity of CD4−8− cDCs to form IFN-γ was concentrated within the minor CD172a− subfraction, and this fraction now showed a more impressive capacity on a cell-for-cell basis. An important conclusion was that all the fully developed spleen cDC subtypes, now including the CD172a+ CD4−8− cDCs, showed only a very low capacity for IFN-γ production.
Lack of cytotoxic activity by the cDC precursors within the CD4−8− cDC fraction
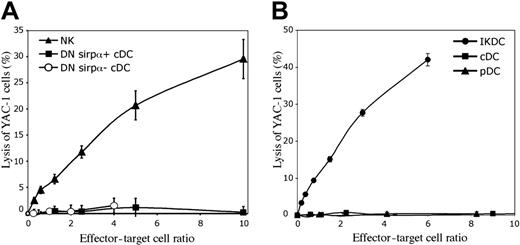
Since the CD172a− precursors of CD4−8+ cDCs within the CD4−8− population produced IFN-γ, as do NK cells, we tested if they possessed an NK-like cytotoxic activity on the NK target cell YAC-1. However, when compared with NK cells, they had no ability to kill YAC-1 cells (Figure 6A). In this respect they gave results identical to the major CD172a+ subset of CD4−8− cDCs, which as expected also lacked cytotoxic activity. In both cases this was in line with their lack of expression of the NK cell marker CD49b.
The lysis of YAC-1 target cells by components of DC preparations. The freshly sorted cells were cultured at various concentrations for 4 hours at 37°C with 10451Cr-labeled YAC-1 cells. (A) The lack of cytolytic activity by the CD172a− cells within the CD4−8− cDC preparations (DN Sirpα−). These cells were isolated as in Figure 4A, and compared with the CD4−8− CD172a+ cDCs (DN Sirpα+) and to NK cells. The mean plus or minus SD of 3 experiments is presented. (B) The cytolytic activity of IKDCs, sorted from the pDC preparations as CD49b+ CD11cint CD45RA+ cells (both CD45RAhi and CD45RAint), as in Figure 2A. One experiment is presented; a second experiment gave similar results.
The lysis of YAC-1 target cells by components of DC preparations. The freshly sorted cells were cultured at various concentrations for 4 hours at 37°C with 10451Cr-labeled YAC-1 cells. (A) The lack of cytolytic activity by the CD172a− cells within the CD4−8− cDC preparations (DN Sirpα−). These cells were isolated as in Figure 4A, and compared with the CD4−8− CD172a+ cDCs (DN Sirpα+) and to NK cells. The mean plus or minus SD of 3 experiments is presented. (B) The cytolytic activity of IKDCs, sorted from the pDC preparations as CD49b+ CD11cint CD45RA+ cells (both CD45RAhi and CD45RAint), as in Figure 2A. One experiment is presented; a second experiment gave similar results.
Spleen pDC preparations, pDC subsets, and IKDC contaminants
Since IKDCs appear closer to pDCs than to cDCs in surface phenotype,22,23 we examined their contribution to pDC preparations. Our protocol for the isolation of pDCs is given in Figure 2A. The approach was similar to that used for cDCs, but the mAbs used for immunomagentic bead depletion differed. B cells were depleted with anti-CD19, rather than with anti-CD45R (B220), which also depletes pDCs. Granulocytes were depleted with a mAb specific for Ly6G that has no reactivity with Ly6C, to avoid any possible pDC depletion. Some mAbs reactive with cDCs were included to reduce the load of cDCs, although they did not give complete removal. However, since we wished to examine the relationship of IKDCs to the pDC subsets, no depletion of cells bearing NK markers was applied. The pDCs were finally sorted and separated from residual cDCs based on their high expression of CD45RA and intermediate rather than high levels of CD11c (Figure 2A). Very similar results were obtained if anti-CD45R (B220) was used as a pDC\E marker instead of CD45RA (data not shown). Since cells bearing intermediate levels of CD45RA can overlap the CD45RAhi fraction, and since IKDCs are found with intermediate as well as high levels of CD45R,22,23 CD45RAint cells were also sorted for analysis.
The pDC fraction, CD45RAhi CD11cint, could be further subdivided based on Ly6C expression into a major group (91% ± 4%) of Ly6C+ cells, considered as the typical spleen pDCs, and a minor (9% ± 4%) subgroup of Ly6C− cells (Figure 2A). The Ly6C+ cells also stained weakly with the anti-Ly6G mAb Gr-1, which is believed to cross-react with Ly6C; however, in our hands the staining was too weak to allow the clear segregation seen with mAb directed to Ly6C itself. In contrast to these results with spleen pDCs, when the equivalent CD45RAhi CD11cint pDC fraction was isolated from bone marrow (BM), it consisted of 57% Ly6C+ cells and 43% Ly6C− cells (data not shown). In addition, cultures of BM stimulated by Flt-3 ligand29 produced CD45RAhi pDCs, which consisted of 32% Ly6C+ and 68% Ly6C− cells (data not shown). This segregation of pDCs based on Ly6C expression recalled recent studies in which segregation of pDCs using Ly49Q expression produced Ly49Q− and Ly49Q+ subtypes, the Ly49Q− subtype being predominant in BM and the Ly49Q+ subtype being predominant in spleen.32,33 The Ly49Q− pDCs have been reported to develop into Ly49Q+ pDCs in culture.33 Accordingly, we stained spleen pDC preparations for both Ly6C and Ly49Q and cross-correlated the expression of the 2 markers. Indeed, there was an extensive correlation, the Ly6C− pDCs being mainly Ly49Q−, and the Ly6C+ pDCs being mainly Ly49Q+ (Figure 2B).
To determine if any IKDCs were present within our spleen pDC preparations, we stained for CD49b using the mAb DX5. As shown in Figure 2A, a small proportion of cells in the CD45RAhi CD11cint pDC fraction were strongly positive for CD49b. These were entirely within the Ly6C− subset, which could be clearly separated into Ly6C− CD49b+ cells and Ly6C− CD49b− cells. The CD49b+, putative IKDCs showed a wider range of CD45RA expression than pDCs and some were also present in the CD45RAint fraction of the enriched pDC preparation (Figure 2A). Such cells would not readily be depleted using anti-CD45R mAb, so probably contributed to the few IKDCs that contaminated our standard cDC preparations.
NK-like killing by IKDCs
To check that the CD49b+ cells isolated from our pDC preparations were equivalent to the IKDCs described by others,22,23 we tested their cytotoxic activity on the NK target cell YAC-1. Indeed, these CD49b+ Ly6C− CD11c+ CD45RA+ putatative IKDCs showed an immediate strong cytotoxic activity on YAC-1 cells in a 4-hour assay (Figure 6B). This was similar to the activity of NK cells isolated from spleen suspensions (Figure 6A). In contrast, the CD49b-depleted pDC and cDC preparations showed no killing of YAC-1 targets (Figure 6B). The extent of killing was not notably increased by including CpG1668 in the cytotoxic assays to activate the IKDCs (data not shown). When these putative IKDCs were first preactivated by overnight culture with CpG1668 together with GM-CSF, duplicating the approach of others,22 the viable cells that survived were also cytotoxic to YAC-1; however, under these conditions viable cell survival was only 10% to 30% (data not shown). By the criterion of NK-like killing, the CD49b+ cells in our pDC preparations could be classified as IKDCs.
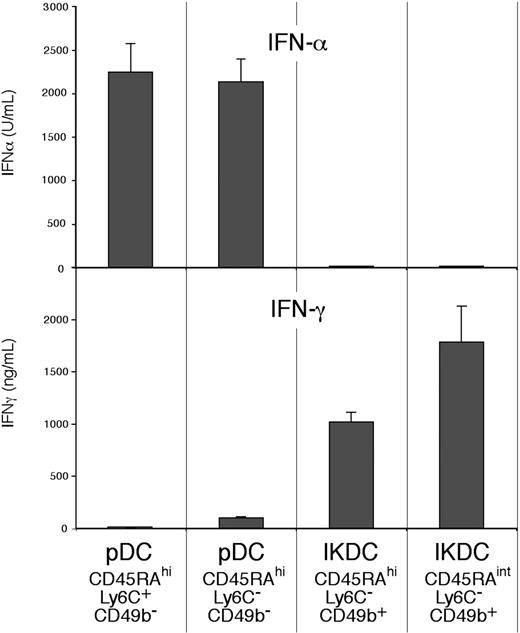
Production of interferons by pDCs and IKDCs
To determine which interferons were produced by the “true” pDCs and which were produced by the putative IKDC “contaminants,” the sorted fractions of Figure 2A were assayed for the production in culture of IFN-α in response to the TLR-9 stimuli CpG2216 or CpG1668, and for the production of IFN-γ in response to IL-12 and IL-18.
IFN-α was produced in substantial amounts by those CD45RAhi CD11cint pDCs that did not express the NK marker CD49b (Figure 7). The results with CpG1668 (not shown) were similar to those obtained with CpG2216. The IFN-α–producing cells included both the Ly6C+ CD49b− and the Ly6C− CD49b− fractions. IFN-α was also produced in comparable amounts by both the Ly6C− and the Ly6C+ fractions of the pDCs produced by cultures of BM stimulated by Flt-3 ligand (data not shown). However, no IFN-α was produced by the CD49b+ cells, the putative IKDCs within the spleen pDC preparations, either by the CD45RAhi Ly6C− CD49b+ cells or by the CD45RAint Ly6C− CD49b+ cells (Figure 7). Thus, all the IFN-α produced in response to TLR-9 stimulation was produced by the pDCs, not by the IKDC contaminants.
Comparison of the capacity to produce IFN-α and IFN-γ by subsets of pDC preparations. The pDC preparation and the subsets considered as pDCs (CD49b−) or as IKDCs (CD49b+) were isolated as in Figure 2. The sorted cells (105) were cultured in 0.2 mL medium for 48 hours (IFN-α) or for 65 hours (IFN-γ). The stimulus used was CpG2216 for IFN-α production, IL-12 and IL-18 for IFN-γ production. Results are the means plus or minus SD of 5 individual cultures, from one experiment typical of 3.
Comparison of the capacity to produce IFN-α and IFN-γ by subsets of pDC preparations. The pDC preparation and the subsets considered as pDCs (CD49b−) or as IKDCs (CD49b+) were isolated as in Figure 2. The sorted cells (105) were cultured in 0.2 mL medium for 48 hours (IFN-α) or for 65 hours (IFN-γ). The stimulus used was CpG2216 for IFN-α production, IL-12 and IL-18 for IFN-γ production. Results are the means plus or minus SD of 5 individual cultures, from one experiment typical of 3.
In contrast to the results on IFN-α production, IFN-γ was produced at high levels by the CD49b+ cells within the pDC preparations (Figure 7), indicating that these could indeed be called IKDCs. However, no IFN-γ was produced by the typical CD45RAhi CD11cint Ly6C+ spleen pDCs, and only moderate amounts were produced by the presumed earlier pDC form, the Ly6C− CD49b− subset. Thus, there was little overlap in the capacity to produce these 2 interferons in response to the stimuli used, the pDCs producing IFN-α, the IKDCs producing IFN-γ.
Discussion
The main objective of this study was to determine the extent of production of interferons by fully developed, steady-state DCs of the type found in mouse spleen. The existence of multiple subtypes of DCs and of other cells with properties that overlap those of DCs, made this a demanding exercise. The problems are emphasized by the recent description of a cell type termed IKDC22-24 which, like DCs, is light in density and expresses surface CD11c, CD45R (B220), and MHC II, but which also has the cytotoxic potential and surface markers of NK cells. The true lineage origins of these IKDCs are yet to be determined. Such IKDCs are likely to be a contaminant in most cDC preparations where expression of CD11c is the main selection criterion. Because many IKDCs express relatively high levels of CD45R and CD45RA, they will be a component of pDC preparations selected by these criteria. Stringent exclusion of cells bearing NK markers, such as CD49b (DX5), is required to eliminate these cells.
Since IKDCs are capable of producing large amounts of IFN-γ, much of the modest production obtained from conventional cDC and pDC preparations is likely to derive from a small number of IKDC contaminants. Even though most IKDCs were eliminated by our previous multistep cDC purification procedure, trace amounts may have contributed to the IFN-γ production we obtained. However, using very stringent criteria to exclude all cells bearing NK markers, our cDC preparations still were capable of a low but significant level of IFN-γ production. We have now tracked down the minor cDC subset responsible for this IFN-γ production.
We have found that spleen contains a reservoir of precursors of cDCs, but not of pDCs.30 Most of these pre-cDCs lack surface MHC II, express only intermediate levels of CD11c and moderate buoyant density, so are separable from fully developed steady-state cDCs. However, because of the rapid turnover of spleen cDCs,34 spleen must contain cells in transit between pre-cDCs and fully developed cDCs. We have now identified such a minor population of cDCs within the CD4−8− fraction of our cDC preparations. Although these cells lack surface CD8α, we believe they are of the CD8+ lineage, because of their CD172a− CD24+ surface phenotype, their capacity to produce IL-12p70, and their ability to cross-present exogenous antigens. They are not cytotoxic, and so differ from both NK cells and IKDCs in this respect. The presence of low numbers of these early CD8+ cDC forms within the CD4−8− cDC preparations may explain why CD4−8− cDCs have sometimes appeared to develop, with low efficiency, into CD4−8+ cDCs.35,36 We now find it is these “in transit” early forms of the CD8+ cDC lineage which produce all the IFN-γ we had attributed to the CD4−8− cDC population.9 This reconciles our finding with that of others10 who found the CD8+ cDCs rather than the CD4−8− cDCs to be the cDC subset producing IFN-γ. Depending on the rate of surface CD8α acquisition, the gatings used to select the cDC subsets, and the status of the mice used, these IFN-γ–producing early CD8+ cDCs might fall into one or another sorting gate.
The immunologic significance of the potential of splenic early CD8+ cDCs to produce moderate amounts of IFN-γ is not clear. These cells do have the potential to produce the IL-12p70 needed to induce IFN-γ secretion. It is of interest that a presumed early form of pDCs, the Ly6C− Ly49Q− subset, also has a similar moderate capacity to produce IFN-γ. This IFN-γ production capacity may provide an early “priming” function to allow Th1 T-cell development. Alternatively, many precursor cells may have some capacity to produce IFN-γ and the physiologically important aspect may be the switching off of this capacity once cDCs and pDCs are fully developed. Whatever the interpretation, it is important to note that the fully developed, steady-state cDCs and pDCs that interact with naive T cells have little capacity for IFN-γ production.
Amongst DCs, the pDCs are considered to be the major producers of type 1 interferons in response to viral and other infections and in response to the TLR-9 stimulus CpG.1-5 We have noted that CD4−8+ cDC preparations are capable of some IFN-α production in response to a combination of poly I:C and CpG stimulation.9 The interpretation of this finding is now uncertain, since activated forms of pDCs express CD8α and down-regulate CD45RA,27 so traces of activated pDCs may have contributed to this IFN-α production. However, it is now clear that all cDCs have a capacity to produce IFN-α if appropriately stimulated, as shown by intracellular stimulation of cDC subtypes with double-stranded RNA,29 so it is important to specify the nature of the stimulus used. We now demonstrate that the Ly6C− CD45RAhi CD11cint cells in spleen and BM pDC preparations are fully capable of IFN-α production in response to the TLR-9 stimulus CpG, so they can be classified as pDCs. They correspond to the Ly49Q− pDCs described by others32,33 and considered to be early forms of pDCs. Ly49Q− pDCs have already been reported to produce IFN-α in response to CpG.32,33 Although Ly6C− cells in BM pDC preparations have been reported to be poor producers of IFN-α,37 these preparations were apparently dominated by the Ly6C− CD49b+ cells, which we now find to be inactive. By our criteria only the CD49b− cells within pDC preparations can be classified as IFN-α–producing pDCs, but these will include the Ly6C− Ly49Q− and Ly6C+ Ly49Q+ components.
A striking finding in our study is the dichotomy between IFN-γ and IFN-α production among the cells we isolate from pDC preparations. The putative IKDCs, CD45RA+ CD11cint Ly6C− CD49b+, although being potent producers of IFN-γ, failed to produce IFN-α in response to CpG stimulation. This finding is in line with an earlier study showing similar cells from BM were poor producers of IFN-α,37 but contrasts with the 2 recent reports claiming that IKDCs produce both IFN-γ and IFN-α.22,23 Some subtle differences in the status of the mice or in stimulation conditions could possibly explain this discrepancy, although the use of CpG as a TLR-9 stimulus was a common factor, and we tested both the 1668 and the 2216 forms of CpG. However, since the IFN-α production was lower than for pDCs and was variable in the previously published report,22 the discrepancy may have been due to the presence of a proportion of Ly6C− CD49b− cells in their IKDC preparations. Since we find that IKDCs are directly cytotoxic to NK targets and produce IFN-γ but not IFN-α, they appear functionally closer to NK cells than to pDCs. Since IKDCs have been shown to have a potent antitumor activity and so to have possible medical applications, both their lineage origin and their capacity to produce IFN-α under different conditions of stimulation now needs closer investigation.
Authorship
Contribution: D.V. performed research and analysis; M.O. performed and designed research and assisted in writing; H.H. performed research; M.F. and M.L. performed research and contributed reagents; I.C. performed research; K.S. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ken Shortman, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria 3050, Australia; e-mail: shortman@wehi.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.