Abstract
Ischemia/reperfusion (IR) injury is a leading cause of acute renal failure and an important contributor to allograft damage. Tissue factor (TF) is up-regulated during IR, and TF inhibition reduces renal injury. However, the underlying mechanisms by which TF contributes to injury have not been elucidated. We postulated that TF contributes to IR injury by production of coagulation proteases and subsequent signaling by protease activated receptor (PARs). We compared renal injury after 25 minutes of bilateral renal ischemia and varying periods of reperfusion in C57BL/6 mice, those expressing low levels of TF (low-TF), hirudin-treated C57BL/6, and mice lacking either PAR-1 or PAR-2. C57BL/6 mice developed severe renal failure and died within 48 hours of reperfusion. In contrast, low-TF, hirudin-treated C57BL/6, and PAR-1−/− mice were protected from renal failure and had reduced mortality, tubular injury, neutrophil accumulation, and lower levels of the chemokines KC and MIP-2. Importantly, PAR-1−/− mice had lower chemokine levels despite up-regulation of TF and fibrin deposition. In addition, treating PAR-1−/− mice with hirudin conferred no additional benefit. Somewhat surprisingly, PAR-2 deficiency did not protect from renal failure. These experiments indicate that increased TF activity after renal IR leads to increased CXC chemokine expression and subsequent neutrophil-mediated injury predominantly by thrombin-dependent PAR-1 signaling.
Introduction
Renal ischemia/reperfusion (IR) injury is an important cause of acute kidney failure. It commonly occurs in the setting of major surgery, sepsis, or trauma1,2 and is an unavoidable consequence of renal transplantation. Renal IR injury is characterized by an inflammatory cascade of endothelial cell and leukocyte activation, enhanced endothelial-leukocyte adhesion, neutrophil recruitment, and subsequent tissue damage. These processes are mediated by cytokines, adhesion molecules, and chemokines, particularly the CXC family of chemokines.3-5
Tissue factor (TF) is the main initiator of the protease coagulation cascade in vivo and is a member of the γ-interferon receptor superfamily.6 TF expression is induced by various inflammatory mediators and in turn may further escalate an inflammatory response.7-9 Such a proinflammatory role for TF has been demonstrated in a number of animal models, including sepsis,9 glomerulonephritis,10 and delayed-type hypersensitivity.11
A role for TF in IR injury has been demonstrated by a number of studies. Functional inhibition of TF significantly reduced hepatic12 and myocardial injury13 following IR. Myocardial infarct size in rabbits was also limited by the specific inhibition of thrombin but not by defibrinogenation.13 Thrombin inhibition led to a reduction of IL-8 and MCP-1 expression, whereas inhibition of either TF or thrombin reduced neutrophil infiltration. Studies in rodent models of renal IR have shown increased expression of TF and a protective effect of both TF pathway inhibitor (TFPI)14 and TF antisense oligonucleotides,15 but the mechanisms underlying these effects were not explored.
The protection afforded by inhibiting TF may be due to a reduction in fibrin deposition. In addition, blockade of the coagulation cascade would reduce signaling by the TF-VIIa complex, factor Xa, and thrombin. Both Xa and thrombin can activate protease-activated receptor 1 (PAR-1) which is constitutively expressed in human and murine kidneys16,17 and has a proinflammatory role in a mouse model of crescentic glomerulonephritis.18 PAR-2, another member of the protease-activated receptor family is also expressed in the kidney. PAR-2 can be activated by the TF-VIIa complex as well as by trypsin and tryptase but is not activated by thrombin.19,20 In an ex vivo cardiac model of IR injury PAR-2 stimulation with a PAR-2–activating peptide was protective against IR injury.21 In contrast, activation of PAR-2 in mice induces acute lung injury22 and chronic joint inflammation in a model of adjuvant arhtrits.23 A role of PAR-2 in renal IR remains to be investigated.
We sought to further explore the nature of TF-induced IR injury by comparing the effect of reduced TF levels to the effect of thrombin inhibition and the absence of either PAR-1 or PAR-2. We measured the degree of renal failure, neutrophil infiltration, chemokine expression, and fibrin deposition after renal IR in mice.
Materials and methods
Experimental animals
Male mice aged 8 to 12 weeks and weighing 20 to 30 grams were used for all experiments. The low-TF mice (mTF−/−, hTF+), generated as previously described,24 have targeted disruption of the murine TF gene but are rescued from lethality by a minigene directing low-level (0.7% of wild type) expression of human TF. They display a typical murine distribution of TF with normal growth and a normal coagulation profile.25 PAR-1 and PAR-2 knockout mice (PAR-1−/− and PAR-2−/−) were obtained from Professor S. Coughlin, University of San Francisco. Both low-TF and PAR-1−/− mice were bred 6 generations onto the C57BL/6 background. PAR-2−/− mice were on a mixed genetic background26 and were compared with appropriate PAR-2+/+ strain controls. Preliminary experiments demonstrated that strain controls (mTF+/+ mice) for low-TF mice developed renal failure with the same kinetics and to the same degree as wild-type C57BL/6 mice. Low-TF mice were therefore compared with C57BL/6 wild-type (WT) mice. WT mice were obtained from the Biological Resources Centre, University of New South Wales (UNSW). Studies were approved by the UNSW Animal Ethics Committee and complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Ischemia reperfusion protocol
The protocol for renal IR was modified from Zhou et al.27 Mice were anesthetized with intraperitoneal midazolam 3.2 mg/kg (warmed to 37°C) and inhaled 2% isofluorane (Abbott, Kurnell, NSW, Australia). Body fluid was maintained by subcutaneous administration of 300 μL 0.9% normal saline preoperatively. Rectal temperature was maintained at 35°C to 37°C using a thermostatically controlled operating platform. After a midline laparotomy, the renal pedicles were identified and bilaterally occluded for a period of 25 minutes using nontraumatic microaneurysm clamps (Kaisers Instruments, Bibra Lake, WA, Australia). This period of ischemia was found to result in reproducible injury with minimal perioperative mortality. The abdomen was sutured closed after clamp removal, and the mice received 800 μL normal saline subcutaneously and were allowed ad libitum food and water. Sham-treated mice were subjected to identical surgical procedures without application of microaneurysm clamps. Mice were allowed to recover and killed at 2, 5, 24, or 48 hours after reperfusion.
To examine the role of thrombin, wild-type mice were administered 4 mg/kg subcutaneous hirudin (Pharmion, Melbourne, VIC, Australia) 2 hours prior to surgery and every 12 hours depending on the period of reperfusion.18 Thrombin inhibition after hirudin administration was confirmed by measuring activated partial thromboplastin time (APTT) from blood collected into tubes containing 3.8% sodium citrate in a 9:1 blood-citrate ratio, and adequate hirudin treatment was defined by a greater than 4 times increase in APTT compared with the mean APTT measured in control mice. At least 6 mice from each experimental group were included in the analysis of each time point and compared with a similar number of sham-operated mice.
Plasma creatinine
Blood was collected by cardiac puncture into a heparinized syringe. Plasma creatinine was measured by the South Eastern Laboratory Services, Prince of Wales Hospital, Sydney, using an automated Jaffe method.
Histopathology
Histological damage of the kidneys was determined by a semiquantitative assessment of tubular injury. Kidneys were fixed in 4% paraformaldehyde for 24 hours and then embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin (H&E). Tubular injury was identified by the presence of any of flattened tubular cells, loss of brush border, and cast formation. Ten high-powered fields (hpf's; × 400 magnification) from the outer medulla and corticomedullary junction were examined from each animal to determine the percentage of tubules showing evidence of injury and scored according to the following scale: 0, no injury; 1, less than 10%; 2, 10% to 25%; 3, 26% to 75%; 4, greater than 75%.28 The 10 scores were averaged to give the tubular injury score for each specimen.
Infiltrating neutrophils (polymorphonuclear leukocytes) were quantified by counting polymorphonuclear cells (PMNs) from 10 consecutive hpf's along the outer medulla and corticomedullary junction. The sum of the 10 counts was ascribed as the neutrophil score, measured as PMN per hpf for each specimen. A single investigator, blinded to the mouse genotype and surgical procedure, performed all scoring.
Myeloperoxidase activity
Myeloperoxidase (MPO) activity was assayed as a measure of neutrophil activity in kidneys using a modified protocol.29 A quarter of each kidney was homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 6.0) and centrifuged at 15 600g at 4°C for 30 minutes. The pellet was resuspended in 1 mL 5 mM potassium phosphate and recentrifuged under the same conditions. This process was repeated. After washing, the pellets were resuspended in 1 mL extraction buffer (50 mM potassium phosphate buffer [pH 6.0] containing 0.5% hexadecyltrimethylammoniumbromide), followed by 3 rounds of freeze-thawing. The suspension was incubated at 4°C for 20 minutes then centrifuged at 15 600g at 4°C for 15 minutes. The supernatant (100 μL) was mixed with 100 μL reaction buffer (50 mM potassium phosphate buffer [pH 6.0] containing 0.6 mg/mL O-dianisidine dihydrochloride and 0.03% hydrogen peroxide). Absorbance was measured at 450 nm after 5 minutes of incubation. After normalization for protein concentration, the MPO content was expressed as units of MPO activity per milligram of protein. Protein concentrations were determined using the DC Protein Assay Kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol.
Relative reverse transcriptase polymerase chain reaction (RT-PCR)
Expression of mRNA was evaluated by relative RT-PCR with an 18S ribosomal RNA internal standard. Kidney tissue was harvested into RNAlater (Ambion, Austin, TX) and stored at −80°C. RNA was extracted using TRIZOL (Invitrogen, Mt Waverley, VIC, Australia), and cDNA was transcribed with the RevertAid cDNA synthesis kit (MBI Fermentas, Hanover, MD) using random hexamer primers. Transcribed cDNA was amplified in a 20-μL reaction containing 1.5 to 2.5 mM MgCl2, 150 μM each dNTP, 1.0 μM each primer (KC, 5′-CATGGCTGGGATTCACCTC-3′, 5′-TCTGTCTTCTTTCTCCGTTACTTG-3′; MIP-2, 5′-TGCCGGCTCCTCAGTGCTG-3′, 5′-AAACTTTTTGACCGCCCTTGA-3′; TF, 5′-AGACCAACTTGTGATTCA-3′, 5′-GTGCTTGAGCCTTTCCGATA-3′), 18S rRNA internal standard primers, and competimers (Ambion) and 0.4 U DNA polymerase. Initial experiments were performed for each PCR to determine the exponential phase of the reaction. Amplification consisted of a denaturation period of 90 seconds at 94°C followed by 30 sequential cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds and extension at 72°C for 30 seconds. RT-PCR products were electrophoresed on 1.5% agarose gels containing ethidium bromide, and band intensities for both the target gene and 18S were quantified using Kodak Digital Science 1D software (Kodak, New Haven, CT). Results are expressed as the ratio of the target gene band intensity to the 18S internal standard band intensity.
Enzyme-linked immunosorbent assay
The CXC chemokines KC and MIP-2 were quantified in the kidney by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Frozen tissue was homogenized in an extraction buffer containing protease inhibitor mix (Roche Diagnostics, Mannheim, Germany). Chemokines were expressed as picogram per milliliter per milligram of total protein. Protein concentrations were determined using the DC Protein Assay Kit (Bio-Rad, Hercules, CA) according to the manufacturer's protocol.
Measurement of tissue factor activity
Functional TF activity was measured by a 1-stage prothrombin assay.24 Half of a frozen kidney was homogenized in 15 mM octyl-β-d-glucopyranoside (Sigma, St Louis, MO) made up in 25 mM HEPES-buffered normal saline (pH 7.0). Following centrifugation at 15 600g for 5 minutes, TF activity in the supernatant was measured using pooled mouse plasma. Clotting time was measured in seconds using a Start 4 automatic coagulation analyser (Diagnostica Stago, Asnieres Cedex, France). TF activity was expressed as arbitrary units per milligram of total protein, by reference to a standard curve of rabbit thromboplastin (Sigma, Castle Hill, NSW, Australia).
Fibrin immunofluorescence
Fibrin deposition was assessed as previously described.13 Briefly, kidneys were snap frozen in liquid nitrogen. Frozen sections were fixed in acetone for 1 to 2 minutes before blocking with 1% bovine serum albumin (BSA) and 1% horse serum in PBS for 1 hour at room temperature and then incubating at room temperature for a further hour with 1:100 sheep anti–rabbit fibrinogen, crossreactive to mouse fibrin (gift from Dr P. Tipping, Centre for Inflammatory Diseases, Monash University, Melbourne, VIC, Australia). Bound antibody was detected with a FITC-conjugated donkey anti–goat IgG (1:100 in 1% BSA in PBS; Jackson ImmunoResearch, West Grove, PA), and the section was counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The area of FITC staining was averaged over 10 consecutive hpf's and quantified in square micrometers using SlideBook 4.0 software (Intelligent Imaging, Denver, CO).
Statistical analysis
Statistical analysis was performed using JMP statistical software package for Macintosh version 5 (SAS, Cary, NC). Normally distributed data were expressed as the mean ± standard error of the mean (SEM), and groups were compared using a 2-tailed Student t test. Nonparametric data from histological scoring were compared using the Mann-Whitney U test. Survival was compared using Kaplan-Meier methods. A P value below .05 was considered significant.
Results
TF–thrombin–PAR-1 signaling contributes to renal failure and mortality following renal IR injury
Our hypothesis was that the TF-coagulation protease-PAR signaling system would contribute to renal IR injury. To test this hypothesis, WT (C57BL/6), low-TF, PAR-1−/−, and PAR-2−/− mice were subjected to 25 minutes of bilateral renal ischemia followed by up to 48 hours of reperfusion. To further assess the functional role of thrombin, WT and PAR-1−/− mice were treated with the specific thrombin inhibitor hirudin and then subjected to renal IR injury. Mice subjected to sham surgery were used as controls.
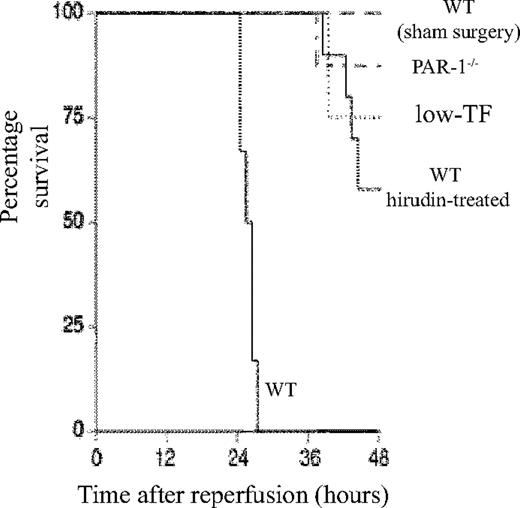
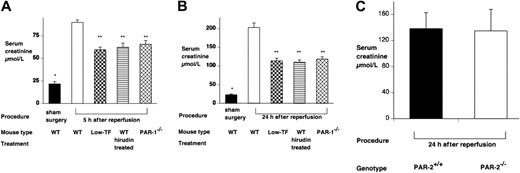
WT mice developed severe renal failure after IR with elevated plasma creatinine compared with sham-operated mice at 2 hours (60.8 ± 2.4 μM versus 19.5 ± 2.7 μM), 5 hours (89.8 ± 2.6 μM versus 21.8 ± 2.6 μM), and 24 hours (203.1 ± 12.0 μM versus 22.9 ± 2.1 μM) after reperfusion (P < .001 at each time point). By 48 hours all WT mice had died (Figure 1). The mean plasma creatinine of sham-operated WT mice did not differ from control mice or sham-operated mice of other genotypes or treatments (data not shown), and there were no deaths by 48 hours.
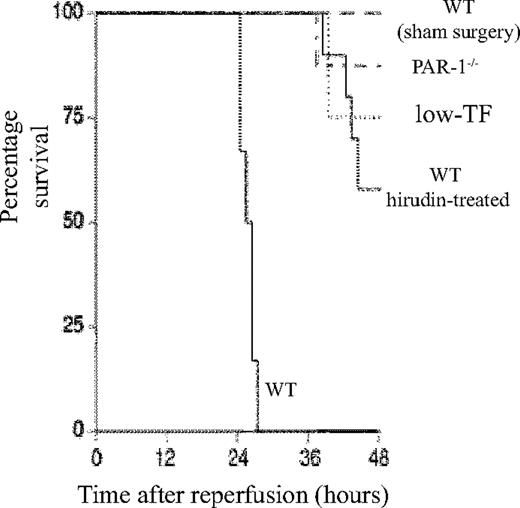
Role of TF–thrombin–PAR-1 system in renal ischemia/reperfusion injury. Kaplan-Meier plot of the survival of WT (n = 6), low-TF (n = 4), and WT mice treated with hirudin (n = 12) and PAR-1−/− (n = 8) mice undergoing 25 minutes of bilateral renal ischemia and WT sham-operated mice (n = 4) 48 hours after surgery. Low-TF, WT-hirudin treated, and PAR-1−/− mice all had significantly improved survival (P < .01).
Role of TF–thrombin–PAR-1 system in renal ischemia/reperfusion injury. Kaplan-Meier plot of the survival of WT (n = 6), low-TF (n = 4), and WT mice treated with hirudin (n = 12) and PAR-1−/− (n = 8) mice undergoing 25 minutes of bilateral renal ischemia and WT sham-operated mice (n = 4) 48 hours after surgery. Low-TF, WT-hirudin treated, and PAR-1−/− mice all had significantly improved survival (P < .01).
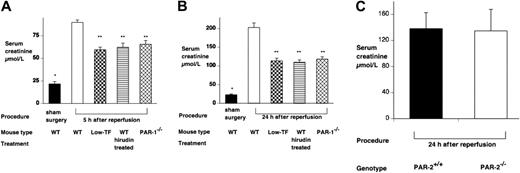
A functional role for TF and PAR-1 in IR was demonstrated, because both low-TF and PAR-1−/− mice had reduced mortality (P < .001 compared with WT mice undergoing renal IR) (Figure 1) and less serum creatinine at 5 and 24 hours (Figure 2). The functional importance of thrombin was confirmed, because hirudin treatment gave similar protection to renal function as PAR-1 deficiency or reduced TF levels (Figures 1–2). Hirudin treatment of WT mice significantly reduced the mortality of injured mice at 48 hours but trended to be less effective than PAR-1 deficiency or low-TF. There was, however, no statistically significant difference in mortality between low-TF, hirudin-treated WT, and PAR-1−/− mice. PAR-2 deficiency afforded no protection from renal failure compared with controls at 24 hours of reperfusion (Figure 2C). These data indicated that PAR-1 activation appeared to be the predominant mechanism by which thrombin provided proinflammatory signaling, because hirudin treatment of PAR-1−/− mice gave no additional benefit over PAR-1 deficiency (data not shown).
Effect of renal ischemia reperfusion injury on renal function. Serum creatinine of WT (n = 6), low-TF (n = 6), and WT (n = 6) mice treated with hirudin and PAR-1−/− (n = 6) mice undergoing 25 minutes of bilateral renal ischemia. Treatment was followed by (A) 5 or (B) 24 hours of reperfusion or, for WT mice, sham surgery. IR resulted in significant renal failure in WT versus sham-operated mice. *P < .001 at both 5 and 24 hours of reperfusion. Low-TF, hirudin-treated WT mice and PAR-1−/− mice developed significantly less severe renal failure compared with WT mice (**P < .001). (C) Serum creatinine of PAR-2−/− (n = 8) or background strain control WT (PAR-2+/+) (n = 8) mice undergoing 25 minutes of bilateral renal ischemia followed by 24 hours of reperfusion. PAR-2−/− mice developed similar degree of renal failure compared with the WT controls. Data are expressed as mean ± SEM.
Effect of renal ischemia reperfusion injury on renal function. Serum creatinine of WT (n = 6), low-TF (n = 6), and WT (n = 6) mice treated with hirudin and PAR-1−/− (n = 6) mice undergoing 25 minutes of bilateral renal ischemia. Treatment was followed by (A) 5 or (B) 24 hours of reperfusion or, for WT mice, sham surgery. IR resulted in significant renal failure in WT versus sham-operated mice. *P < .001 at both 5 and 24 hours of reperfusion. Low-TF, hirudin-treated WT mice and PAR-1−/− mice developed significantly less severe renal failure compared with WT mice (**P < .001). (C) Serum creatinine of PAR-2−/− (n = 8) or background strain control WT (PAR-2+/+) (n = 8) mice undergoing 25 minutes of bilateral renal ischemia followed by 24 hours of reperfusion. PAR-2−/− mice developed similar degree of renal failure compared with the WT controls. Data are expressed as mean ± SEM.
IR injury results in increased TF mRNA and functional activity
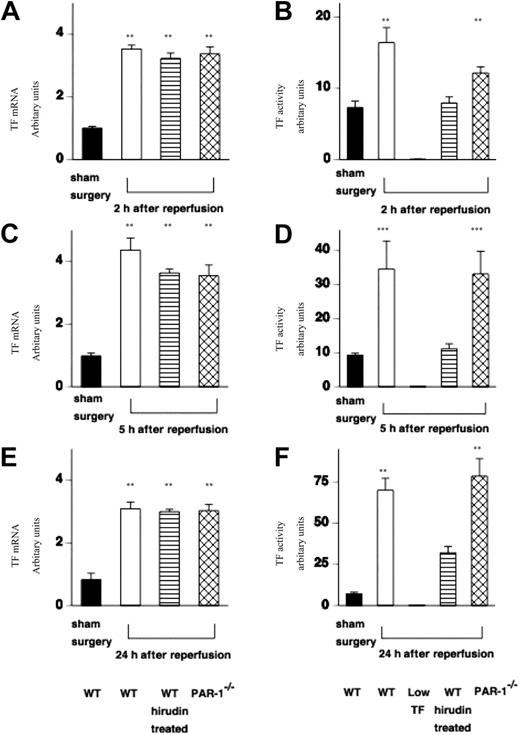
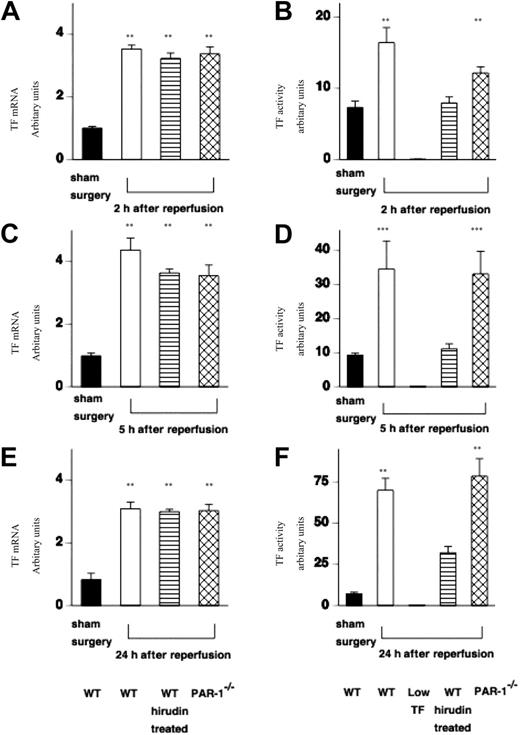
Having demonstrated a functional role for TF–thrombin–PAR-1 signaling in renal failure and mortality, we examined the molecular and cellular events that were likely contributors to renal injury. First, we examined the effect of IR injury on TF mRNA expression in WT (C57BL/6) mice, WT mice treated with hirudin, and PAR-1−/− mice.
In all cases, TF mRNA was increased at 2 hours and remained elevated throughout the 24-hour period of study (Figure 3A,C,E). Sham surgery had no effect on renal TF mRNA expression at any time point in any of the mice (Figure 3A,C,E). This result was the same for the other genotypes (data not shown). Low-TF mice do not express mouse TF mRNA. TF functional activity in WT and PAR-1−/− mice was increased in a time-dependent manner (Figure 3B,D,F). TF activity was reduced in hirudin-treated mice, which is likely because of residual levels of hirudin being present in renal tissue extracts that inhibit thrombin activity in the one-stage prothrombin assay used to measure renal TF activity. As expected renal TF activity from low-TF mice was very low (0.24 ± 0.1 au/mg protein at 24 hours of reperfusion).
Effect of IR injury on renal TF mRNA expression and functional activity. (A,C,D) TF mRNA was measured in extracts of kidney tissue from WT (n = 12), WT hirudin-treated (n = 7-9/time point), and PAR-1−/− mice (n = 12) at varying times (2, 5, and 24 hours) following renal IR injury or sham surgery (n = 6). (All values are expressed relative to 18S ribosomal RNA, and data are shown as mean ± SEM.) TF mRNA was increased at 2 hours after reperfusion and remained elevated for 24 hours (at all points **P < .001). TF mRNA was not increased in any sham-operated WT or PAR-1−/− animals (WT only shown). (B,D,F) TF functional activity (measured from extracts of kidney tissue using a 1-stage prothrombin assay) was increased in WT mice (n = 16) or PAR-1−/− mice (n = 16) at varying times (2, 5, and 24 hours) following renal IR injury compared with sham surgery (n = 6) (at 2 and 24 hours, **P < .005; at 5 hours, ***P < .05). Renal TF activity was very low from low-TF mice at all time points, and WT hirudin-treated mice had relatively reduced levels of TF activity (n = 7-9/group). IR injury resulted in no significant difference in TF expression between WT and PAR-1−/− mice. (All values are expressed in relative units compared with a standard curve of rabbit thromboplastin in which a 1 in 10 dilution of standard was designated 100 units.)
Effect of IR injury on renal TF mRNA expression and functional activity. (A,C,D) TF mRNA was measured in extracts of kidney tissue from WT (n = 12), WT hirudin-treated (n = 7-9/time point), and PAR-1−/− mice (n = 12) at varying times (2, 5, and 24 hours) following renal IR injury or sham surgery (n = 6). (All values are expressed relative to 18S ribosomal RNA, and data are shown as mean ± SEM.) TF mRNA was increased at 2 hours after reperfusion and remained elevated for 24 hours (at all points **P < .001). TF mRNA was not increased in any sham-operated WT or PAR-1−/− animals (WT only shown). (B,D,F) TF functional activity (measured from extracts of kidney tissue using a 1-stage prothrombin assay) was increased in WT mice (n = 16) or PAR-1−/− mice (n = 16) at varying times (2, 5, and 24 hours) following renal IR injury compared with sham surgery (n = 6) (at 2 and 24 hours, **P < .005; at 5 hours, ***P < .05). Renal TF activity was very low from low-TF mice at all time points, and WT hirudin-treated mice had relatively reduced levels of TF activity (n = 7-9/group). IR injury resulted in no significant difference in TF expression between WT and PAR-1−/− mice. (All values are expressed in relative units compared with a standard curve of rabbit thromboplastin in which a 1 in 10 dilution of standard was designated 100 units.)
Both PAR-1 deficiency and hirudin treatment were associated with renoprotection despite up-regulation of TF mRNA. This suggests that TF contributes to injury via thrombin generation and the activation of PAR-1.
Reduced TF expression or inhibition of thrombin–PAR-1 signaling results in reduced renal injury, neutrophil infiltration, and MPO activity
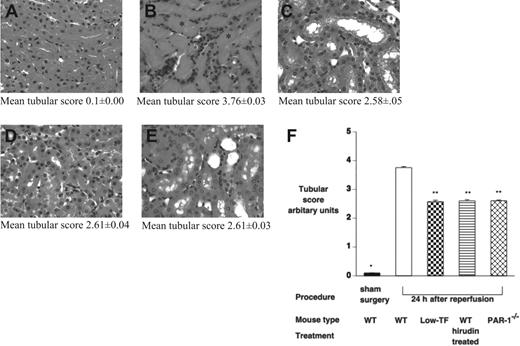
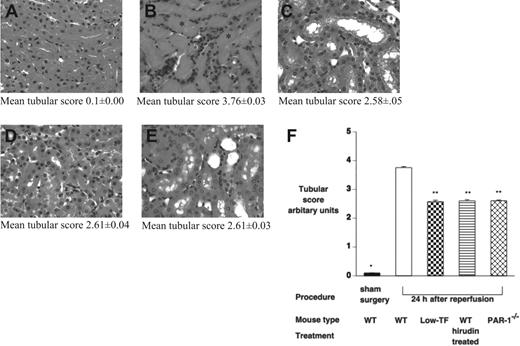
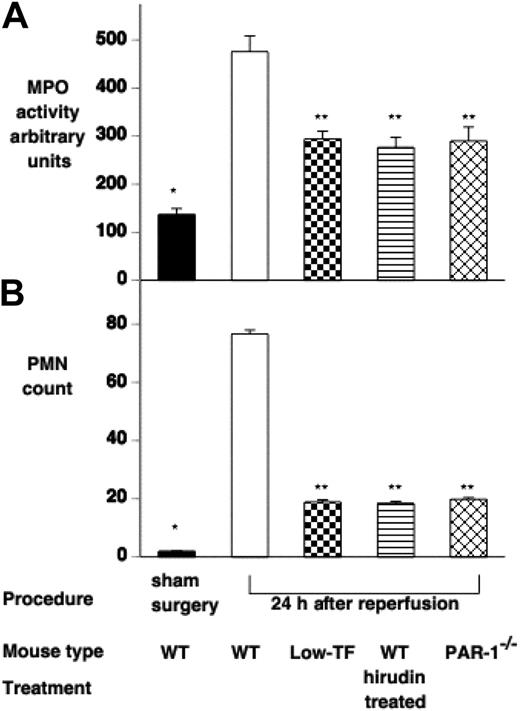
To further assess the effect of IR injury on renal tissue, tubular damage was assessed by a histological scoring system, and neutrophil infiltration was assessed by both counting of PMNs on tissue sections and by tissue MPO activity 24 hours after ischemia. Renal architecture in all sham-operated mice was normal (Figure 4A). WT mice displayed significant injury 24 hours after ischemia with tubular cast formation, loss of brush boarder, and tubular necrosis (Figure 4B). Low-TF, hirudin-treated WT mice, and PAR-1−/− mice 24 hours after ischemia showed qualitatively similar histological changes to each other with relatively much less severe injury compared with WT mice (Figure 4B-E). Tubular injury was significantly less in low-TF, hirudin-treated WT mice, and PAR-1−/− mice, compared with WT mice (mean tubular score: low-TF, 2.58 ± 0.10, hirudin-treated WT, 2.60 ± 0.04, and PAR-1−/−, 2.61 ± 0.03 versus WT 3.76 ± 0.00; P < .001 for each pair; Figure 4F). Renal neutrophil infiltration and MPO activity were significantly reduced in low-TF, PAR-1−/−, and hirudin-treated WT mice compared with WT mice undergoing renal IR injury (Figure 5).
Effect of IR on renal tubular injury. (A-E) Hematoxylin and eosin–stained tissue sections of (A) WT mice undergoing sham surgery followed by 24 hours of reperfusion and of (B) WT, (C) low-TF, (D) C57BL/6 hirudin-treated, and (E) PAR-1−/− mice undergoing 25 minutes of renal ischemia followed by 24 hours of reperfusion. In panel B, tubular cast formation is indicated by *. (F) Tubular injury scores of WT (n = 16), low-TF (n = 7), and WT (n = 7) mice treated with hirudin and PAR-1−/− (n = 16) mice undergoing 25 minutes of bilateral ischemia followed by 24 hours of reperfusion or WT mice undergoing sham surgery. IR injury resulted in significant tubular injury in WT mice compared with sham-operated animals (*P < .001). Low-TF, WT mice treated with hirudin and PAR-1−/− mice had significantly reduced tubular injury compared with WT (**P < .001). Images were captured using an Olympus BX51 microscope equipped with a uPlanFl 40 ×/0.75 numerical aperture (NA) air objective (Olympus, North Ryde, Australia) connected to a Retiga EXi digital camera, and captured with Q capture for Apple Macintosh (QImaging, Burnaby, BC, Canada). Data are expressed as mean ± SEM.
Effect of IR on renal tubular injury. (A-E) Hematoxylin and eosin–stained tissue sections of (A) WT mice undergoing sham surgery followed by 24 hours of reperfusion and of (B) WT, (C) low-TF, (D) C57BL/6 hirudin-treated, and (E) PAR-1−/− mice undergoing 25 minutes of renal ischemia followed by 24 hours of reperfusion. In panel B, tubular cast formation is indicated by *. (F) Tubular injury scores of WT (n = 16), low-TF (n = 7), and WT (n = 7) mice treated with hirudin and PAR-1−/− (n = 16) mice undergoing 25 minutes of bilateral ischemia followed by 24 hours of reperfusion or WT mice undergoing sham surgery. IR injury resulted in significant tubular injury in WT mice compared with sham-operated animals (*P < .001). Low-TF, WT mice treated with hirudin and PAR-1−/− mice had significantly reduced tubular injury compared with WT (**P < .001). Images were captured using an Olympus BX51 microscope equipped with a uPlanFl 40 ×/0.75 numerical aperture (NA) air objective (Olympus, North Ryde, Australia) connected to a Retiga EXi digital camera, and captured with Q capture for Apple Macintosh (QImaging, Burnaby, BC, Canada). Data are expressed as mean ± SEM.
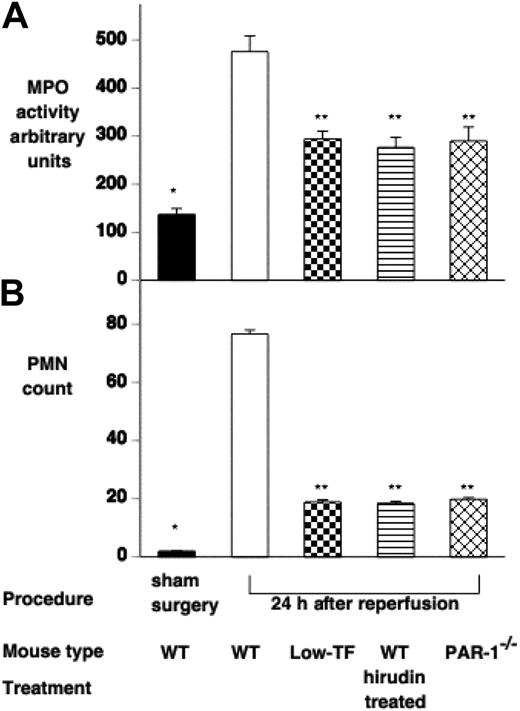
Effect of IR injury on renal polymorphonuclear (PMN) cell infiltration. PMN infiltration was assessed by (A) measuring renal myeloperoxidase activity and (B) counting PMN cells on H&E-stained tissue sections of WT (n = 16), low-TF (n = 7), WT mice treated with hirudin (n = 7) and PAR-1−/− (n = 16) mice undergoing 25 minutes of bilateral ischemia followed by 24 hours of reperfusion or WT mice undergoing sham surgery. WT mice undergoing IR had significantly increased MPO and PMN counts compared with sham-operated mice (*P < .001). Low-TF, WT mice treated with hirudin and PAR-1−/− mice had significantly reduced renal PMN accumulation compared with WT (**P < .001). Data are expressed as mean ± SEM.
Effect of IR injury on renal polymorphonuclear (PMN) cell infiltration. PMN infiltration was assessed by (A) measuring renal myeloperoxidase activity and (B) counting PMN cells on H&E-stained tissue sections of WT (n = 16), low-TF (n = 7), WT mice treated with hirudin (n = 7) and PAR-1−/− (n = 16) mice undergoing 25 minutes of bilateral ischemia followed by 24 hours of reperfusion or WT mice undergoing sham surgery. WT mice undergoing IR had significantly increased MPO and PMN counts compared with sham-operated mice (*P < .001). Low-TF, WT mice treated with hirudin and PAR-1−/− mice had significantly reduced renal PMN accumulation compared with WT (**P < .001). Data are expressed as mean ± SEM.
Reduced PAR-1 signaling results in reduced CXC chemokine response
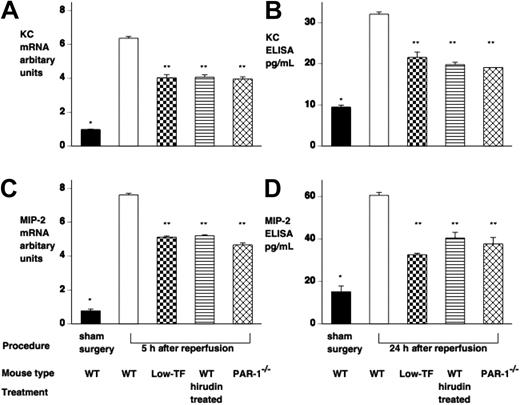
We then examined the effect of reduced PAR-1 signaling on renal chemokine expression. Analysis of the expression of various inflammatory mediators by RNase protection assay indicated that KC and MIP-2 were strongly up-regulated (data not shown). Subsequent RT-PCR and ELISA confirmed that KC and MIP-2 mRNA and protein were both increased following renal IR injury (Figure 6), with up-regulation of mRNA by 2 hours and protein by 5 hours, both of which persisted for 24 hours. Sham-operated mice showed no significant change in renal chemokine expression. Low-TF mice, hirudin-treated WT, and PAR-1−/− mice showed similar degrees of reduction of chemokine mRNA and protein expression (Figure 6).
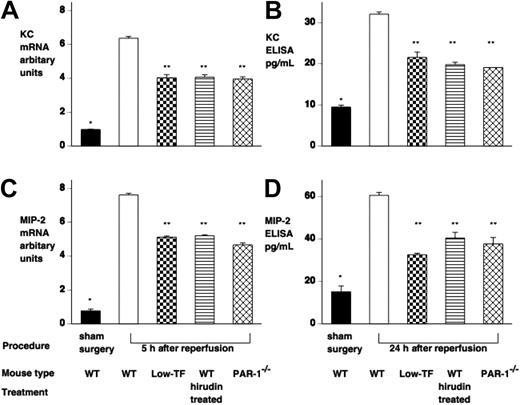
IR injury induces both renal KC and MIP-2 mRNA and protein. Mice underwent renal ischemia followed by 5 to 24 hours of reperfusion or sham surgery. (A,C) KC and MIP-2 mRNA was assessed by RT-PCR and levels expressed relative to 18S ribosomal RNA. (B,D) KC and MIP-2 protein was assessed by ELISA, and levels were expressed as picogram per milliliter (samples were loaded equally for total protein). WT mice undergoing IR injury had significantly elevated KC and MIP-2 mRNA and protein compared with sham-operated WT mice (*P < .001). Low-TF WT mice treated with hirudin and PAR-1−/− mice had significantly reduced KC and MIP-2 mRNA and protein compared with WT mice (**P < .001). Data are expressed as mean ± SEM.
IR injury induces both renal KC and MIP-2 mRNA and protein. Mice underwent renal ischemia followed by 5 to 24 hours of reperfusion or sham surgery. (A,C) KC and MIP-2 mRNA was assessed by RT-PCR and levels expressed relative to 18S ribosomal RNA. (B,D) KC and MIP-2 protein was assessed by ELISA, and levels were expressed as picogram per milliliter (samples were loaded equally for total protein). WT mice undergoing IR injury had significantly elevated KC and MIP-2 mRNA and protein compared with sham-operated WT mice (*P < .001). Low-TF WT mice treated with hirudin and PAR-1−/− mice had significantly reduced KC and MIP-2 mRNA and protein compared with WT mice (**P < .001). Data are expressed as mean ± SEM.
Fibrin deposition does not correlate with renoprotection
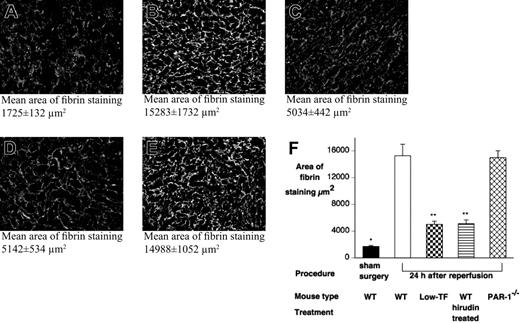
Inhibition of TF or thrombin will reduce the generation of fibrin. To analyze fibrin deposition in this model, levels of fibrin in kidney specimens of WT (n = 12), low-TF (n = 8), hirudin-treated WT mice (n = 12), and PAR-1−/− mice (n = 12), obtained after 24 hours of reperfusion, were assessed by immunohistochemical staining and compared with sham-operated and control mice. Significant levels of fibrin deposition were not detected in control mice and sham-operated mice (Figure 7). In contrast, fibrin staining was detected in the WT and PAR-1−/− mice after reperfusion (Figure 7). Low levels of fibrin were also detected in injured low-TF mice and hirudin-treated mice. These results indicated that levels of fibrin deposition did not correlate with markers of renal injury.
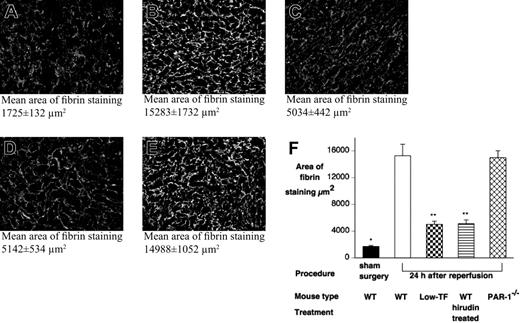
Renal fibrin deposition does not correlate with renal failure or mortality. Renal fibrin deposition was quantified, following 24 hours' reperfusion after 25 minutes of renal ischemia or after sham surgery, by indirect immunofluorescence with an antifibrin/fibrinogen antibody detected with a FITC-labeled secondary antibody. Tissue sections (10 fields per slide, 400×) were viewed with an Olympus BX51 epifluorescence microscope equipped with a uPlanFl 40 ×/0.75 NA air objective, and digital images were captured with a Retiga EXi digital camera. To determine the area of fibrin staining, images were processed with SlideBook 4.0 for Macintosh (Intelligent Imaging, Denver, CO). Representative micrographs of (A) WT sham-operated, (B) WT, (C) low-TF, (D) C57BL6 hirudin-treated, and (E) PAR-1−/− mice 24 hours after renal IR. Mean tubular score ± SEM indicated below each panel. (F) Quantitation of renal fibrin deposition 24 hours after renal IR from WT (n = 12), low-TF (n = 8), WT (n = 12) mice treated with hirudin, PAR-1−/− (n = 12) mice, and sham-operated mice. WT and PAR-1−/− mice undergoing IR had significantly more fibrin staining compared with sham-operated WT mice (*P < .001). Low-TF, and WT mice treated with hirudin had significantly reduced fibrin staining compared with WT mice after IR (**P < .001). Data are expressed as mean ± SEM.
Renal fibrin deposition does not correlate with renal failure or mortality. Renal fibrin deposition was quantified, following 24 hours' reperfusion after 25 minutes of renal ischemia or after sham surgery, by indirect immunofluorescence with an antifibrin/fibrinogen antibody detected with a FITC-labeled secondary antibody. Tissue sections (10 fields per slide, 400×) were viewed with an Olympus BX51 epifluorescence microscope equipped with a uPlanFl 40 ×/0.75 NA air objective, and digital images were captured with a Retiga EXi digital camera. To determine the area of fibrin staining, images were processed with SlideBook 4.0 for Macintosh (Intelligent Imaging, Denver, CO). Representative micrographs of (A) WT sham-operated, (B) WT, (C) low-TF, (D) C57BL6 hirudin-treated, and (E) PAR-1−/− mice 24 hours after renal IR. Mean tubular score ± SEM indicated below each panel. (F) Quantitation of renal fibrin deposition 24 hours after renal IR from WT (n = 12), low-TF (n = 8), WT (n = 12) mice treated with hirudin, PAR-1−/− (n = 12) mice, and sham-operated mice. WT and PAR-1−/− mice undergoing IR had significantly more fibrin staining compared with sham-operated WT mice (*P < .001). Low-TF, and WT mice treated with hirudin had significantly reduced fibrin staining compared with WT mice after IR (**P < .001). Data are expressed as mean ± SEM.
Discussion
This study provides evidence that TF contributes to renal IR via thrombin-dependent activation of PAR-1. Disruption of the key stages of this pathway led to decreased renal injury, lower mortality, and reduced expression of proinflammatory chemokines.
We demonstrated that TF plays a prominent role in IR-related inflammation and tissue injury by showing that mice with low levels of TF were protected from renal injury. These mice had a significant survival advantage compared with WT mice and a striking reduction in renal PMN accumulation after IR. We have documented up-regulation of the TF gene in renal tissue 2 hours after ischemia and a progressive increase in functional activity up to 24 hours after ischemia. Frank et al30 also showed up-regulation of TF mRNA and activity in a similar murine model of renal IR. After prolonged renal ischemia and contralateral nephrectomy in a rat model, TF was up-regulated in the kidney with increased TF staining in glomeruli, blood vessels, and stimulated monocytes.14 In a separate study, this group also demonstrated a survival advantage and reduction in histological injury in rats treated with TFPI. The same group, using a less severe model, has subsequently shown a survival advantage and protection from tubular necrosis by the use of TF antisense oligonucleotides.15 The mechanism underlying TF-mediated injury was not explored, although it was postulated that necrosis was caused by microcirculatory incompetence and microthrombus formation. Our data, however, suggest that thrombus formation, as assessed by fibrin deposition, is not the principal pathway of TF-mediated injury in renal IR.
Thrombin may induce TF expression in a variety of cells. However, TF up-regulation in this model appeared not to be thrombin dependent because both hirudin-treated and PAR-1−/− mice displayed equivalent TF mRNA expression.
In the current study, TF deficiency was associated with reduced up-regulation of MIP-2 and KC after IR. Inhibiting these chemokines with neutralizing antibodies has been shown to protect mice from renal IR via reduced neutrophil infiltration.5 TF may regulate chemokine expression and consequent neutrophil recruitment directly via TF cytoplasmic tail-dependent NF-κB activation31 or in combination with FVIIa by a protease-activated receptor mechanism (reviewed by Rao and Pendurthi32 ). However, we found that WT mice treated with hirudin had a similar degree of protection from renal IR injury as low-TF mice, despite up-regulation of TF mRNA. This suggests that a major pathway for TF proinflammatory signaling in IR injury is by the activation of the coagulation cascade and thrombin production. Therefore, our results suggest that direct cell signaling by TF alone or in complex with VIIa may not be the major mediator of TF-related IR injury. Similarly, Frank et al30 found that treatment with the factor Xa inhibitor, fondaparinux, was protective against renal IR without altering the expression of TF. However, because hirudin treatment may also reduce factor Xa levels, we cannot rule out a role of Xa activation of PAR-1 in the injury.33,34
Hirudin, a specific inhibitor of thrombin, will inhibit all protease-dependent actions of thrombin, including procoagulant effects, PAR signaling, and activation of other molecules (eg, protein C). We therefore used mice deficient in the main cellular receptor of thrombin, PAR-1, to further elucidate the pathway of TF-dependent IR injury. We found similar degrees of renal protection in PAR-1−/− mice to low-TF and hirudin-treated WT mice. PAR-1−/− mice were also found to have lower CXC chemokine expression and less neutrophil infiltration than WT mice after IR, suggesting a proinflammatory role for PAR-1 in renal IR. The contribution of thrombin-regulated signaling by non–PAR-1 pathways (eg, PAR-4) also appears to be minimal in renal IR because no further protection was obtained by treating PAR-1−/− mice with hirudin. Matrix metalloproteinase 1 (MMP-1) is another activator of PAR-1.35 In addition, thrombin has been shown to induce MMP-1 expression.36 However, the fact that hirudin treatment, low-TF, and PAR-1 deficiency all gave a similar degree of protection of renal function suggests that, if MMP-1 does have a major role in the injury, it is downstream of the TF thrombin pathway.
In vitro studies have shown that TF-VIIa-FXa complex activates PAR-2.19,20 The absence of any benefit or more severe renal injury in PAR-2–deficient mice suggests PAR-2 signaling was not a significant pathway in this model. In contrast, activation of PAR-2 in mice contributes to mortality following administration of LPS,37 induces acute lung injury,22 and is pivotal in mediating chronic joint inflammation following adjuvant arthritis.23
Regulation of renal hemodynamics is a further potential mechanism by which PARs may contribute to renal function following renal IRI. In the isolated perfused rat kidney PAR-1 activation results in vasoconstriction and PAR-2 vasodilation.38 Hence, in our model at least some of the benefit of PAR-1 deficiency or reduced PAR-1 signaling may derive from improved glomerular hemodynamics.
Fibrin, like thrombin, may potentially induce chemokine signaling.39 Further, some studies suggest that fibrin is important in macrophage accumulation in inflammatory diseases such as glomerulonephritis and cardiac IR.40,41 In the latter case, fibrinopeptide B binding to VE-cadherin was shown to be the important mechanism regulating monocyte trans-endothelial cell migration.41 However, Frank et al30 found that defibrinogenated mice were not protected from lethality in renal IR injury. A potential explanation of this discrepancy is that residual levels of fibrinogen in that model were sufficient to form fibrin and the fibrinopeptide B breakdown product, thereby promoting leukocyte trans-endothelial cell migration. Alternatively, there may be differences in mechanisms of inflammation between cardiac and renal IR models. However, our finding of similar levels of interstitial fibrin in the WT and PAR-1−/− mice after 24 hours of reperfusion, suggests that any contribution of fibrin to renal IR is downstream of the TF–thrombin–PAR-1 axis. It is possible that PAR-1–dependent chemokine production may be required for fibrin to express a proinflammatory role in renal reperfusion injury. As with cardiac reperfusion, it is possible that binding of the β chain of fibrin to VE-cadherin may also be important for leukocyte accumulation in renal IR; however, thrombin-mediated PAR-1 signaling and chemokine expression are critical upstream events.
In conclusion, we have shown that deficiency of TF or lack of PAR-1 signaling is protective in murine renal IR. The predominant mechanism appears to be by reduced CXC chemokine expression and consequent neutrophil infiltration. Inhibition of PAR-1 may therefore provide an important new strategy for reducing reperfusion injury.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Contribution: J.S. performed the majority of experiments and participated in experimental design and data analysis; S.E.K. performed some experiments and participated in experimental design and analysis as well as manuscript preparation; D.R.D. performed some experiments and participated in data analysis and manuscript preparation; M.S. performed some experiments and participated in data analysis; P.W.P. provided experimental input and assisted with manuscript preparation; J.A.C. provided critical comment and assisted with manuscript preparation; N.M. provided key reagents and assisted with manuscript preparation; and J.H.E. led the project and was responsible for experimental design, analysis, and manuscript preparation.
Acknowledgments
This work was supported by postgraduate medical research scholarships from the Australian Kidney Foundation and the National Health and Research Council of Australia.
D.R.D. holds a National Health and Medical Research Council Peter Doherty Fellowship (ID no. 222974).