Abstract
Therapeutic vaccination against idiotype is a promising strategy for immunotherapy of B-cell malignancies. Its feasibility, however, is limited by the requirement for a patient-specific product. Here we describe a novel vaccine formulation prepared by simply extracting cell-membrane proteins from lymphoma cells and incorporating them together with IL-2 into proteoliposomes. The vaccine was produced in 24 hours, compared with more labor-intensive and time-consuming hybridoma or recombinant DNA methods. The vaccine elicited T-cell immunity in vivo, as demonstrated by secretion of type 1 cytokines. It protected against tumor challenge at doses of tumor antigen 50 to 100 times lower than that previously observed using either liposomes formulated with IL-2 and secreted lymphoma immunoglobulin or a prototype vaccine consisting of lymphoma immunoglobulin conjugated to keyhole limpet hemocyanin. The increased potency justifies testing similar patient-specific human vaccines prepared using extracts from primary tumor samples.
Introduction
Immunization with tumor-specific idiotype (Id) protein conjugated to keyhole limpet hemocyanin (KLH) and administered with an adjuvant induces anti-tumor immunity in patients with follicular lymphoma, and is associated with prolonged disease-free survival.1–4 Previously we demonstrated that a liposomal vaccine, made by co-entrapping hybridoma-derived lymphoma idiotype protein and interleukin-2 (IL-2) in a multilamellar coalescence vesicle (MLCV),5 protected mice against a lethal challenge of lymphoma cells via a CD4+/CD8+ T-cell–dependent mechanism.6 IL-2 was chosen as a vaccine component due to its ability to expand activated T cells. Furthermore, we have previously demonstrated that IL-2 has a specific interaction with small unilamellar lipid vesicles, leading to the formation of MLCV used for vaccines.5 In a Phase I clinical trial, this liposomal vaccine formulation was found to be safe and immunogenic.7 Manufacture of individualized Id protein vaccines, however, using either KLH conjugation or liposome entrapment, required an expensive and time-consuming amplification of tumor antigen via hybridoma or recombinant DNA technology.8,9 The goal of the present study was therefore to streamline the production of patient-specific lymphoma vaccines. We developed a novel liposomal vaccine by replacing the hybridoma-secreted Id with natural tumor-derived cell membrane proteins and tested the formulation in a mouse lymphoma model. Our results indicate that the potency of the vaccine can be vastly increased by inserting in the MLCV liposomes either purified tumor-derived natural Id or cell membrane “patches” that may contain multiple tumor-associated antigens besides idiotype. Based on estimates of the amount of Id available from human tumor biopsies, this simple and rapid approach can be readily translated to clinical evaluation.
Materials and methods
Mice and tumor
Six- to 12-week-old C3H/HeN female mice were from the National Cancer Institute Frederick Cancer Research and Development Center (NCI-FCRDC), Frederick, MD. The carcinogen-induced C3H 38C13 B-cell lymphoma has been previously described.10
Tumor-derived IgM
Secreted 38C13 immunoglobulin M (IgM) Id and a control IgM4C5 were isolated for use in immunoassays and vaccines following somatic cell hyrbridization (R. Levy, Stanford University, CA) and purification from ascites.6 A control vaccine formulation was prepared by glutaraldehyde conjugation at a 1:1 ratio of 38C13 Id and KLH as described.11
Liposomal vaccine preparation
Cell membranes were prepared from 38C13 cells grown in tissue culture or as solid tumors in C3H mice. Membrane proteins were extracted with diheptanoylphosphatidylcholine (DHPC) detergent and then filtered through a 0.2 μ filter. Multilamellar ‘membrane-patched' proteoliposomes (MPL) were prepared by combining the membrane extract, IL-2 and small unilamellar vesicles of dimyristoylphosphatidylcholine (DMPC) and using an overnight coalescence process previously described for making multilamellar DMPC liposomes.5 A similar vaccine, “purified protein proteoliposomes” (PPL), was prepared using Id that had been extracted from cell membranes with DHPC and purified by immunoaffinity chromatography.
MPL and PPL vaccines were formulated with 80 mg/mL DMPC, 4 × 105 International Units/mL of IL-2 (determined by CTLL-2 bioassay6 ), and various amounts of 38C13 Id (determined by capture enzyme-linked immunosorbent assay (ELISA) following dissolution of lipid vesicles with detergent6 ). Incorporation of Id and IL-2 was generally 60-80% and 90-99%, respectively.
Assays and measurements
Results and discussion
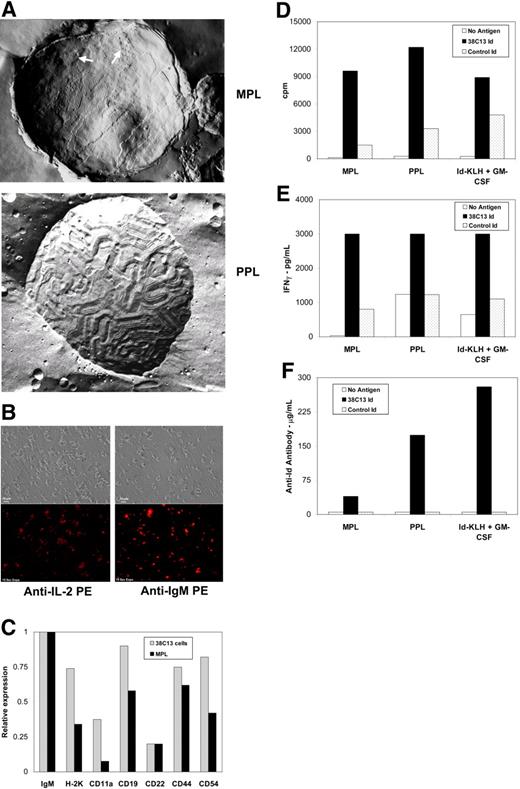
Electron microscopic analysis of the MPL liposomes, mean diameter 2 to 3 μm, revealed patches with a bumpy texture surrounded by a ripple phase characteristic of DMPC bilayers (Figure 1A). The PPL liposomes, mean diameter 2 to 3 μm, in contrast had a predominantly ripple phase texture with bulges possibly caused by protein and associated water pockets trapped between the lamellae. Surface immunofluorescent staining of the MPL vesicles showed the presence of IL-2 and Id (Figure 1B) and other proteins found on 38C13 cells, such as H-2K, CD19, CD54 and CD44 (Figure 1C), while similar staining of the PPL vesicles showed only surface IL-2 and Id (data not shown).
Characteristics of MPL and PPL lymphoma vaccines and immune responses induced following vaccination. (A) Freeze fracture electron microscopic image of membrane proteoliposome (MPL) demonstrates a “bumpy” texture (arrows) resembling that of tumor cell membranes and sections with a ripple texture characteristic of DMPC bilayers. The purified protein proteoliposome (PPL) shows a predominant DMPC ripple texture with bulges that may be caused by protein and associated water pockets trapped between the lamellae. Samples without cryoprotectant were placed between copper specimen carriers and rapidly plunged into liquid propane. Fracturing and replicating was performed at −115°C in a Balzers BAF 400T Freeze-Etch unit (Liechtenstein). Cleaned replicas were viewed on a Philips EM 300 transmission electron microscope (Netherlands) at a magnification of approximately 55 000 fold and photographed using built-in plates. (B) Immunofluorescent staining of membrane proteoliposomes (MPL) with anti-IL-2 PE and anti-mouse IgM PE antibodies demonstrated the presence of IL-2 (bottom left panel) and tumor-derived idiotype (bottom right panel) on the surface of the liposomes. Phase contrast images are also shown for comparison (top panels). Samples consisting of wet mounts in phosphate buffered saline were viewed using a Nikon Optiphot II Epifluorescence microscrope (Melville, NY) at 500 fold magnification with a 40×/0.75 Plan Fluor Ph2 DLL objective (Nikon, Melville, NY). Images were collected using a Spot RT Color Digital camera (Diagnostic Instrumentations, Inc., Sterling Heights, MI) and Image-Pro-Plus #4 acquisition software (Media Cybernetics, Bethesda, MD). (C) Relative expression levels of membrane proteins were measured on 38C13 murine lymphoma cells and on MPL proteoliposomes prepared from a membrane extract of the same cells. Cells and MPL proteoliposomes were incubated with biotin-labeled monoclonal antibodies to the indicated surface proteins, washed, and then incubated with europium (Eu)-labeled streptavidin. Following washing, the amount of bound Eu was measured on a Delfia 1232 time-resolved fluorometer (Wallac, Gaithersburg, MD). The relative expression level of each membrane protein was determined by dividing its Eu value by that of the 38C13 IgM measured in the same preparation (38C13 cells or MPL proteoliposomes; ie the IgM was assigned a relative expression value of 1.0). (D-F) Mice were inoculated once intraperitoneally with MPL vaccine, PPL vaccine, or Id-KLH + GM-CSF and tested for immune responses after 14 days. Draining lymph node cells were cultured in the presence or absence of tumor-specific 38C13 idiotype or control idiotype and proliferation assay (d) and cytokine induction assay for IFN-γ production (E) were performed as previously described.11 Plasma antibody levels specific for 38C13 idiotype were determined by ELISA (F).11 Data in panels d-f are representative of those obtained in 3 independent experiments each.
Characteristics of MPL and PPL lymphoma vaccines and immune responses induced following vaccination. (A) Freeze fracture electron microscopic image of membrane proteoliposome (MPL) demonstrates a “bumpy” texture (arrows) resembling that of tumor cell membranes and sections with a ripple texture characteristic of DMPC bilayers. The purified protein proteoliposome (PPL) shows a predominant DMPC ripple texture with bulges that may be caused by protein and associated water pockets trapped between the lamellae. Samples without cryoprotectant were placed between copper specimen carriers and rapidly plunged into liquid propane. Fracturing and replicating was performed at −115°C in a Balzers BAF 400T Freeze-Etch unit (Liechtenstein). Cleaned replicas were viewed on a Philips EM 300 transmission electron microscope (Netherlands) at a magnification of approximately 55 000 fold and photographed using built-in plates. (B) Immunofluorescent staining of membrane proteoliposomes (MPL) with anti-IL-2 PE and anti-mouse IgM PE antibodies demonstrated the presence of IL-2 (bottom left panel) and tumor-derived idiotype (bottom right panel) on the surface of the liposomes. Phase contrast images are also shown for comparison (top panels). Samples consisting of wet mounts in phosphate buffered saline were viewed using a Nikon Optiphot II Epifluorescence microscrope (Melville, NY) at 500 fold magnification with a 40×/0.75 Plan Fluor Ph2 DLL objective (Nikon, Melville, NY). Images were collected using a Spot RT Color Digital camera (Diagnostic Instrumentations, Inc., Sterling Heights, MI) and Image-Pro-Plus #4 acquisition software (Media Cybernetics, Bethesda, MD). (C) Relative expression levels of membrane proteins were measured on 38C13 murine lymphoma cells and on MPL proteoliposomes prepared from a membrane extract of the same cells. Cells and MPL proteoliposomes were incubated with biotin-labeled monoclonal antibodies to the indicated surface proteins, washed, and then incubated with europium (Eu)-labeled streptavidin. Following washing, the amount of bound Eu was measured on a Delfia 1232 time-resolved fluorometer (Wallac, Gaithersburg, MD). The relative expression level of each membrane protein was determined by dividing its Eu value by that of the 38C13 IgM measured in the same preparation (38C13 cells or MPL proteoliposomes; ie the IgM was assigned a relative expression value of 1.0). (D-F) Mice were inoculated once intraperitoneally with MPL vaccine, PPL vaccine, or Id-KLH + GM-CSF and tested for immune responses after 14 days. Draining lymph node cells were cultured in the presence or absence of tumor-specific 38C13 idiotype or control idiotype and proliferation assay (d) and cytokine induction assay for IFN-γ production (E) were performed as previously described.11 Plasma antibody levels specific for 38C13 idiotype were determined by ELISA (F).11 Data in panels d-f are representative of those obtained in 3 independent experiments each.
Specific cellular immune responses to free 38C13 Id, but not to control IgM, were observed in the draining lymph node cells from mice inoculated once with either the MPL or the PPL vaccine. The magnitudes of the cellular responses assessed by proliferation and cytokine induction assays (release of IFN-γ and GM-CSF) were comparable to those observed in mice vaccinated with 38C13 Id-KLH + GM-CSF despite the fact that the MPL and PPL vaccines contained only 1.25 μg of 38C13 cell-membrane–derived Id while the KLH vaccine contained 50 μg secreted hybridoma-derived Id (Figures 1D,E and data not shown). In contrast, the anti-Id antibody titer was lower in MPL and PPL vaccinated mice compared with Id-KLH + GM-CSF vaccinated mice (Figure 1F).
Protection experiments demonstrated that survival following tumor challenge was highly dependent upon having the membrane proteins and IL-2 in the same MPL vesicle (Figure 2A). Liposomes containing only the 38C13 membrane extract or only IL-2 were ineffective (P < .001) and administering these formulations as a mixture resulted in partial protection (P= .004). These results were consistent with a previously published report that demonstrated optimal protective immunity in mice required the incorporation of purified hybridoma-derived idiotype protein and IL-2 in the same liposomal vesicle.6 The MPL vaccine was not significantly superior to control Id-KLH vaccine containing 50 μg secreted hybridoma-derived Id (P= .26) although the trend was in favor of the MPL vaccine (Figure 2A). The mice killed at day 50 were necropsied and were found to be tumor free by visual examination.
Protection against tumor challenge required both membrane extract and IL-2 in the same MPL vesicle. (A) Mice (10 per group) were inoculated once i.p. with the indicated preparations and challenged 14 days later with 2 × 103 38C13 lymphoma cells. Survival was assessed 50 days after challenge. Vaccines tested included the standard MPL formulation with membrane extract (1.25 μg Id/dose) and 4 × 105 IU/dose IL-2 (MPL); an MPL formulation containing membrane extract but no IL-2; an MPL formulation without IL-2 that was mixed with multilamellar liposomes containing 4 × 105 IU/dose IL-2; multilamellar liposomes containing 4 × 105 IU/dose IL-2 (L[IL-2]); and secreted hybridoma-derived Id conjugated to KLH (Id-KLH; 50 μg Id/dose). All p values shown are with respect to the standard MPL vaccine (closed circle). (B) Vaccine formulations protected against tumor challenge at very low antigen doses. MPL and PPL vaccine formulations were prepared with a constant amount of IL-2 (4 × 105 IU/dose) and different concentrations of 38C13 cell membrane Id. Mice (10 per group) received a single immunization intraperitoneally and were challenged with 2 × 103 38C13 lymphoma cells 14 days later. Survival was assessed at 50 days following tumor challenge. No mice survived in the saline control group (data not shown).
Protection against tumor challenge required both membrane extract and IL-2 in the same MPL vesicle. (A) Mice (10 per group) were inoculated once i.p. with the indicated preparations and challenged 14 days later with 2 × 103 38C13 lymphoma cells. Survival was assessed 50 days after challenge. Vaccines tested included the standard MPL formulation with membrane extract (1.25 μg Id/dose) and 4 × 105 IU/dose IL-2 (MPL); an MPL formulation containing membrane extract but no IL-2; an MPL formulation without IL-2 that was mixed with multilamellar liposomes containing 4 × 105 IU/dose IL-2; multilamellar liposomes containing 4 × 105 IU/dose IL-2 (L[IL-2]); and secreted hybridoma-derived Id conjugated to KLH (Id-KLH; 50 μg Id/dose). All p values shown are with respect to the standard MPL vaccine (closed circle). (B) Vaccine formulations protected against tumor challenge at very low antigen doses. MPL and PPL vaccine formulations were prepared with a constant amount of IL-2 (4 × 105 IU/dose) and different concentrations of 38C13 cell membrane Id. Mice (10 per group) received a single immunization intraperitoneally and were challenged with 2 × 103 38C13 lymphoma cells 14 days later. Survival was assessed at 50 days following tumor challenge. No mice survived in the saline control group (data not shown).
To determine the dose dependence of vaccine protection, groups of 10 mice were vaccinated once with either MPL or PPL vaccine containing various amounts of 38C13 cell membrane-derived Id and then challenged with a lethal dose of lymphoma cells (Figure 2B). A high level of survival was consistently observed in this and similar experiments at low Id doses of 0.5 to 1 μg. In contrast, the same level of protection required at least 50 μg Id for the vaccine consisting of hybridoma Id-coupled KLH, with or without added GM-CSF (Figure 2A and data not shown), and at least 40 μg Id for a vaccine consisting of liposomes containing hybridoma Id and IL-2.6
To see whether an effective amount of antigen could be obtained directly from human follicular lymphoma patient samples, we extracted Id protein from lymph node biopsies. Using a capture ELISA assay for quantitation, the mean, range, and n of Id (in μg) per 2 × 109 tumor cells for each group of samples was as follows: IgM-positive tumors= 131, 50.4 to 374, n= 12; IgG-positive tumors= 9.8, 3.6 to 18.8, n= 7; and IgA-positive tumors= 144, 22.8 to 212, n = 3. These amounts are sufficient to prepare multiple doses of vaccine at the Id concentrations (0.5 to 1 μg/dose) found to be effective for the MPL and PPL vaccines in mice.
The substantial potency of the MPL and PPL vaccines at low antigen doses may have arisen from long-lasting in vivo loading of antigen-presenting dendritic cells (DC) associated with liposome vesicles.12–14 Norbury et al and Wolkers et al further demonstrated that in vivo cross-priming is critically dependent on metabolically-intact, stable antigenic structures such as the cell membrane-derived Id used in the MPL and PPL vaccines, rather than peptides or chaperone-bound peptides.15,16 That the vaccines appeared to be 50 to 100 fold more potent than vaccines (KLH-conjugated or liposome entrapped) containing secreted hybridoma-produced antigen6 (Figure 2A) could be due to the membrane insertional domain present on cell-derived Id (but not on secreted hybridoma-derived Id) that would serve to anchor and orient the metabolically-intact proteins in the liposome bilayers, potentially leading to increased immune stimulation. That the MPL vaccine in general resulted in slightly higher protection than the PPL vaccine (Figure 2B), suggests that there may be a contribution from unidentified tumor antigens in the MPL and/or that other membrane proteins, such as co-stimulatory and MHC molecules in the MPL, facilitated Id loading and/or enhanced activation of the immune system. Alternatively, the higher protection observed with the MPL vaccine could have been due to inclusion of retroviral antigens that could be present in the 38C13 lymphoma cell line. However, we believe that this is unlikely due to the induction of specific immune responses against the tumor-derived idiotype protein by the MPL vaccine, which was comparable to the prototype Id-KLH + GM-CSF vaccine that induced equivalent protection (Figures 1D,E and 2A).
The major advantage of the MPL and PPL vaccines are their ability to induce effective anti-tumor protection with small amounts of antigen. Preparing a human MPL or PPL lymphoma vaccine using the antigenic material directly extracted from a lymph node biopsy would circumvent the expensive and time-consuming steps (2 to 6 months) of preparing Id protein vaccines by hybridoma or recombinant DNA technologies.8,9 Manufacturing such a vaccine would require only 24 hours and the vaccine would be ready for administration immediately following standard release and sterility testing. This accelerated manufacturing process opens the possibility for innovative and potentially more effective vaccination regimens. In an accompanying article by Neelapu et al17 , page 5160, we present the results of a phase 1 trial testing an MPL-type vaccine in patients with follicular lymphoma.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC ection 1734.
Acknowledgments
A research grant and financial support was provided by the Cooperative Research and Development agreement between Biomira USA and the National Cancer Institute.
National Institutes of Health
Authorship
Contribution: M.C.P., R.J.R., and L.W.K. designed the research; R.J.R., M.M.B., L.T.B., M.E.N., and R.W.P. performed the research; R.J.R., M.M.B., L.T.B., M.E.N., and S.S.N. analyzed the data, M.C.P., R.J.R., S.S.N., and L.W.K. wrote the paper.
Conflict of interest disclosure: M.C.P., R.J.R., and L.W.K. have declared a financial interest in XEME Biopharma, Inc., whose potential products were studied in the present work.
Correspondence: Mircea C. Popescu, XEME Biopharma, Inc., 5 Parkway Ave., Plainsboro, NJ 08536; Email: mpopescu@xemebiopharma.com.

![Figure 2. Protection against tumor challenge required both membrane extract and IL-2 in the same MPL vesicle. (A) Mice (10 per group) were inoculated once i.p. with the indicated preparations and challenged 14 days later with 2 × 103 38C13 lymphoma cells. Survival was assessed 50 days after challenge. Vaccines tested included the standard MPL formulation with membrane extract (1.25 μg Id/dose) and 4 × 105 IU/dose IL-2 (MPL); an MPL formulation containing membrane extract but no IL-2; an MPL formulation without IL-2 that was mixed with multilamellar liposomes containing 4 × 105 IU/dose IL-2; multilamellar liposomes containing 4 × 105 IU/dose IL-2 (L[IL-2]); and secreted hybridoma-derived Id conjugated to KLH (Id-KLH; 50 μg Id/dose). All p values shown are with respect to the standard MPL vaccine (closed circle). (B) Vaccine formulations protected against tumor challenge at very low antigen doses. MPL and PPL vaccine formulations were prepared with a constant amount of IL-2 (4 × 105 IU/dose) and different concentrations of 38C13 cell membrane Id. Mice (10 per group) received a single immunization intraperitoneally and were challenged with 2 × 103 38C13 lymphoma cells 14 days later. Survival was assessed at 50 days following tumor challenge. No mice survived in the saline control group (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-08-039446/4/m_zh80120702070002.jpeg?Expires=1765150161&Signature=oJRDWE42L2OJVG8qmUoPqM3IX1Zd9xAWrIMUvXcj-tn295sRvJHYhGLzdxzXOs6fZxKY6CSppLNFLnjjtb5V7XyV2EozfZwoVPbzu-hsmSBbSIgtauOCfYSDLi7s3urKTS6SFsjvMToXRHXc0TjDoYWEjQrLgM3yIZF1REC8ZNNwg6RLjt9ma~ymiZuy7E8m2jhS3T4WsFFN-8bMjetv-GbsV3zEoGmFhriTTAlP-fiO9yH7JflCCgYLJft6O5vbvf99jz4ZfXZS5vdpbc~ZalgkaZMyp1AfI4PN17~svIkwEynJgh3WhuNdG713BTMks1C4L8CBp~j0xlzFEsc1ow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
