Abstract
Platelet aggregation, the process by which platelets adhere to each other at sites of vascular injury, has long been recognized as critical for hemostatic plug formation and thrombosis. Until relatively recently, platelet aggregation was considered a straightforward process involving the noncovalent bridging of integrin αIIbβ3 receptors on the platelet surface by the dimeric adhesive protein fibrinogen. However, with recent technical advances enabling real-time analysis of platelet aggregation in vivo, it has become apparent that this process is much more complex and dynamic than previously anticipated. Over the last decade, it has become clear that platelet aggregation represents a multistep adhesion process involving distinct receptors and adhesive ligands, with the contribution of individual receptor-ligand interactions to the aggregation process dependent on the prevailing blood flow conditions. It now appears that at least 3 distinct mechanisms can initiate platelet aggregation, with each of these mechanisms operating over a specific shear range in vivo. The identification of shear-dependent mechanisms of platelet aggregation has raised the possibility that vascular-bed–specific inhibitors of platelet aggregation may be developed in the future that are safer and more effective than existing antiplatelet agents.
Introduction
The propensity of platelets to clump together at sites of vascular injury was first recognized more than 100 years ago.1–4 This phenomenon, most accurately described as platelet cohesion although more commonly referred to as platelet aggregation, was quickly identified as important for hemostatic plug formation.5 It was also recognized at the time that platelets played a key role in the development of thrombosis,6 but, it was not until almost a century later that it became widely accepted that platelets played a pivotal role in development of cardiovascular disease.7 As a consequence, inhibitors of platelet aggregation have become increasingly important parts of the armamentarium for the prevention and treatment of many atherothrombotic disorders.8,9
For more than 3 decades, the factors mediating platelet aggregation appeared conceptually straightforward, requiring a platelet stimulus (agonist), a soluble adhesive protein (fibrinogen), and a membrane-bound platelet receptor (integrin αIIbβ3 or GPIIb-IIIa), leading to a simple unified model of platelet aggregation (Figure 1). Although these core elements remain fundamental, recent technical advances allowing real-time analysis of platelet aggregation in vivo have demonstrated a much more complex and dynamic process than previously anticipated. It is now widely accepted that one of the key elements influencing the platelet aggregation process is blood flow, with evidence that distinct aggregation mechanisms operate under different shear conditions.10–13 This has raised the interesting possibility that vascular bed-specific inhibitors of platelet aggregation may be developed in the future and has stimulated a reevaluation of the temporal sequence of events underlying the aggregation process. In this review, I discuss recent developments in the study of platelet aggregation with particular focus on the mechanisms operating under rapid blood flow.
Traditional model of platelet aggregation. (A) Based on studies with the platelet aggregometer, 3 key elements have been identified as important for platelet aggregation: an activating stimulus (typically a soluble agonist), a plasma protein (predominantly fibrinogen), and a platelet surface receptor (integrin αIIbβ3 or GPIIb-IIIa). Agonist-induced activation of integrin αIIbβ3 is essential for the binding of fluid-phase fibrinogen, which as a consequence of its dimeric structure, can physically bridge 2 adjacent platelets. (B) The platelet aggregometer is a relatively simple technique that involves stirring a suspension of platelets in the presence of a platelet activating substance and by monitoring changes in light transmission, the device can accurately monitor platelet clumping (aggregation) in suspension.
Traditional model of platelet aggregation. (A) Based on studies with the platelet aggregometer, 3 key elements have been identified as important for platelet aggregation: an activating stimulus (typically a soluble agonist), a plasma protein (predominantly fibrinogen), and a platelet surface receptor (integrin αIIbβ3 or GPIIb-IIIa). Agonist-induced activation of integrin αIIbβ3 is essential for the binding of fluid-phase fibrinogen, which as a consequence of its dimeric structure, can physically bridge 2 adjacent platelets. (B) The platelet aggregometer is a relatively simple technique that involves stirring a suspension of platelets in the presence of a platelet activating substance and by monitoring changes in light transmission, the device can accurately monitor platelet clumping (aggregation) in suspension.
Historical aspects
Platelet aggregation was first recognized in the late 1800s after a series of pioneering studies by Zahn (1872), Hayem (1878), Bizzozero (1881 and 1882), Osler (1886), and Eberth and Schimmelbusch (1885-1888).1–4,6 Using intravital imaging techniques, Bizzozero clearly established that small cell elements (in which he originated the term “Blut Plattchen” or blood platelets) clumped together at sites of vessel damage,1,14 a process subsequently described as viscous metamorphosis.2 Through a series of insightful observations, Bizzozero described the changes platelets undergo after exposure to foreign surfaces, including the formation of aggregates and subsequent “white thrombi.” He also clearly described the plugging of small vascular punctures by platelet thrombi, heralded as a major discovery at the time.5 It was soon appreciated that quantitative or qualitative defects in platelets lead to a bleeding disorder; and in 1918, Glanzmann15 described a familial bleeding diathesis (“hereditary hemorrhagic thrombasthenia”) that was later shown to be a primary defect in platelet aggregation. Progress in the understanding of platelet aggregation was slow to develop until the advent of the platelet aggregometer in 1962 by Born16 and independently by O'Brien.17 This relatively simple technique involved stirring a suspension of platelets in the presence of a platelet activating substance and, by monitoring changes in light transmission, the device could accurately monitor platelet clumping (aggregation) in suspension (Figure 1). Aggregometry revolutionized the study of platelet function, providing fundamental insights into the molecular mechanisms underlying platelet-platelet adhesive interactions. The identification and characterization of all major physiological agonists, antagonists, surface receptors, and intracellular signaling pathways in platelets has involved platelet aggregation studies, and in many situations there is a good correlation between defects in aggregation and bleeding disorders in affected individuals. Similarly, antiplatelet drugs that increase bleeding risk in vivo can be detected using standard aggregation assays, further enhancing the clinical use of the assay. Thus, for many years, the aggregometer has provided an accurate readout of the fundamental processes regulating aggregation; as recognized in the early 1970s, however, this experimental system does not take into consideration the important influence of blood flow, a key variable increasing the complexity of the aggregation process.
Importance of blood flow in the regulation of platelet aggregation
An important consideration for platelet aggregation, and for platelet adhesion mechanisms more generally, is the rheological (blood flow) conditions operating at sites of vascular injury. Platelets are exposed to a broad range of hemodynamic conditions in vivo, ranging from relatively low flow situations in venules and large veins (typical wall shear rates < 500 s−1) to small arterioles (shear rates up to 5000 s−1) to stenosed arteries with shear rates as high as 40 000 s−1.18 Platelets have the unique capacity to form stable adhesion contacts over all shear conditions operating in vivo and are indispensable for hemostatic plug formation and thrombosis at elevated shear rates.19 In an aggregometer, platelet clumping occurs under low, nonlaminar shear conditions, experimental conditions that do not adequately simulate the flow-dependent recruitment (cohesion) of platelets onto thrombogenic surfaces. Experimental systems designed to study platelet adhesion and aggregation on thrombogenic substrates were initially developed in the 1970s.20,21 Such systems enable assessment of platelet aggregation in flowing whole blood (with or without anticoagulant) providing insight into the role of thrombin generation in regulating platelet aggregation at different flow rates. These assays have been instrumental in defining the adhesive steps mediating platelet adhesion to extracellular matrices, including a key role for the von Willebrand factor (VWF)–GPIb interaction in promoting initial platelet tethering to the vessel wall22,23 and a major role for collagen through engagement of GPVI and integrin α2β1 in promoting platelet arrest.21,24–26 These experimental systems have also confirmed an essential role for integrin αIIbβ3 (GPIIb-IIIa) in mediating platelet aggregation over the full range of shear conditions operating in vivo. Thus, flow-based studies were pivotal in the development of the concept that platelet adhesion (mediated by VWF and collagen) is distinct from platelet aggregation (mediated by fibrinogen).
Conceptual advances in the understanding of platelet aggregation
A number of studies in the 1980s suggested that the processes underlying platelet aggregation under flow conditions may be more complicated than those operating in the aggregometer. Thus, at high shear rates, VWF27,28 and fibronectin29 were demonstrated to not only promote primary adhesion, but also subsequent platelet aggregation. However, widespread recognition of the growing complexity of the platelet aggregation process was slow to develop. A number of technical advances in the late 1990s, including the development of genetically engineered mouse models and improved live cell imaging techniques enabling high-resolution visualization of the platelet aggregation process in vitro30 and in vivo,31,32 have profoundly impacted the study of platelet aggregation, heralding a renaissance in the field. In combination, these techniques have been instrumental in clarifying many aspects of the platelet aggregation process, including (a) the demonstration of a role for multiple adhesive ligands and receptors in the aggregation process; (b) identifying the importance of membrane tethers in initiating platelet aggregation; (c) improved insight into the role of individual adhesion and soluble agonist receptors in regulating the dynamics of platelet aggregation; and (d) the identification of surface ligands and receptors involved in stabilizing and sustaining formed aggregates. In the remainder of this review, I focus on recent developments in each of these areas.
Involvement of multiple adhesion receptors and ligands in platelet aggregation
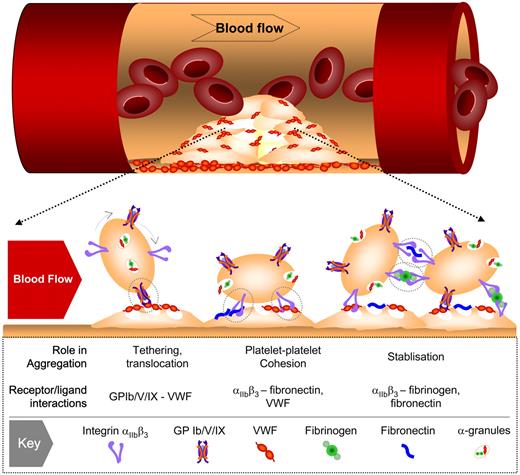
One of the most dramatic conceptual changes in the understanding of platelet aggregation has been the demonstration that multiple adhesive ligands, such as VWF, fibrinogen, and fibronectin, regulate platelet-platelet interactions, with each ligand having distinct roles in the thrombotic process (Figure 2). In vitro perfusion studies on blood obtained from individuals with afibrinogenemia or type III von Willebrand's disease,12,13,27,28,33,34 as well as in vivo studies on mice with a targeted deletion of VWF or fibrinogen,31,35 have demonstrated that VWF plays a major role in initiating aggregation under high shear with fibrinogen (and fibrin) playing a secondary role in stabilizing formed aggregates. Strikingly, mice lacking both VWF and fibrinogen still retained the capacity to form aggregates in vivo35 through an adhesion process dependent on plasma fibronectin36 and possibly other ligands. Why multiple integrin αIIbβ3 ligands are required for platelet aggregation and thrombus growth remains unclear.
Involvement of multiple adhesion receptor-ligand interactions in platelet aggregation under high shear flow. Under conditions of rapid blood flow (typically wall shear rates > 1000 s−1), the initial tethering of platelets to the surface of immobilized platelets involves VWF-GPIb interaction. This adhesive interaction is rapidly reversible and at shear rates up to 10 000 s−1 does not readily support stable platelet-platelet adhesion, resulting in platelet movement (translocation) across the thrombus surface. Platelet stimulation by one or more soluble agonists during translocation promotes the binding of VWF and fibronectin to integrin αIIbβ3, leading to sustained platelet-platelet adhesion. At elevated shear rates, the principal role of the fibrin(ogen)-integrin αIIbβ3 interaction is to stabilize formed aggregates.
Involvement of multiple adhesion receptor-ligand interactions in platelet aggregation under high shear flow. Under conditions of rapid blood flow (typically wall shear rates > 1000 s−1), the initial tethering of platelets to the surface of immobilized platelets involves VWF-GPIb interaction. This adhesive interaction is rapidly reversible and at shear rates up to 10 000 s−1 does not readily support stable platelet-platelet adhesion, resulting in platelet movement (translocation) across the thrombus surface. Platelet stimulation by one or more soluble agonists during translocation promotes the binding of VWF and fibronectin to integrin αIIbβ3, leading to sustained platelet-platelet adhesion. At elevated shear rates, the principal role of the fibrin(ogen)-integrin αIIbβ3 interaction is to stabilize formed aggregates.
Fibrinogen
The importance of the fibrinogen-integrin αIIbβ3 interaction in supporting platelet aggregation has been reviewed in detail elsewhere.37 Fibrinogen, VWF, and fibronectin all have similar binding affinities to the activated form of integrin αIIbβ3; under low shear conditions, however, fibrinogen is thought to be the dominant ligand supporting platelet aggregation, principally attributable to its high molar ratio in plasma relative to other integrin αIIbβ3 ligands.38 Furthermore, with increasing shear (> 1000 s−1), the initiation of aggregation becomes progressively more dependent on VWF and fibronectin, with in vivo studies of fibrinogen-deficient mice demonstrating normal or even increased rates of initial thrombus growth.35 These thrombi are invariably unstable, and studies on mice expressing mutants forms of fibrinogen that have a selective defect in their ability to engage integrin αIIbβ3 have confirmed that part of the thrombus-stabilizing effects of fibrinogen are linked to its capacity to bridge integrin αIIbβ3 receptors on the surface of adjacent platelets,39 whereas fibrin formation anchors the thrombus to the vessel wall.35
von Willebrand factor
The importance of VWF in initiating platelet-platelet adhesion contacts under conditions of rapid blood flow is well established,40 with evidence that it participates over a much broader (and lower) shear range than previously anticipated.12,28,33,41 There are a range of factors that lead to the preferential binding of platelets to VWF under flow, including (a) VWF's very large multimeric structure, which provides an array of binding sites for platelet receptors; (b) VWF's efficiency at capturing platelets from bulk flow (through engagement of GPIb), thereby increasing its spatial proximity to integrins30 ; (c) potential shear-dependent changes in VWF's globular conformation that increases the availability of receptor binding sites; and (d) the ability of the VWF-GPIb interaction to transmit signals that initiate integrin αIIbβ3 activation, thereby increasing the affinity of integrin αIIbβ3-VWF bonds.42,43 These factors, combined with the fact that VWF-GPIb bonds are positively modulated by shear, provides a mechanistic explanation for the dominant role of VWF in promoting platelet aggregation with progressive increases in blood flow. The importance of VWF multimer size in regulating platelet thrombus formation has recently been highlighted through the study of mice lacking the VWF cleaving metalloproteinase ADAMTS13 whose deficiency predisposes to thrombotic thrombocytopenic purpura.44 Mice lacking ADAMTS13 have a much faster rate of thrombus formation leading to premature vasculature occlusion. Furthermore, infusion of recombinant ADAMTS13 promotes thrombus dissolution in injured mouse arterioles, raising the possibility that cleavage of VWF multimers may represent an effective antithrombotic approach.
Fibronectin
For many years, fibronectin was thought to play a minor role in platelet aggregation. For example, deficiency of plasma fibronectin in mice is not associated with a defect in platelet aggregation using standard aggregometer assays, nor is it associated with a prolonged bleeding time.45 The demonstration more than 20 years ago that depleting fibronectin from plasma reduced thrombus formation under flow raised the possibility for its involvement in shear-dependent platelet aggregation.29 Recent in vivo studies have supported these findings,35,36 with Wagner et al demonstrating persistent thrombus formation in arterioles of mice lacking VWF and fibrinogen, suggesting the involvement of a third adhesive ligand in platelet aggregation.35 Follow-up studies on mice expressing low levels of plasma and platelet fibronectin have confirmed an important role for fibronectin in promoting platelet aggregation and thrombus growth.36,46 Interestingly, all stages of thrombus development appear to be affected by fibronectin deficiency, including thrombus initiation, growth, and stability. Fibronectin influences both platelet adhesion and aggregation as crosslinking fibronectin with polymerized fibrin in combination with added plasma fibronectin, synergistically enhanced platelet adhesion, aggregation, and thrombus growth.47 Fibronectin's role in shear-dependent platelet aggregation is incompletely understood and is likely to involve multiple factors. Fibronectin is known to bind other proteins involved in aggregation, such as fibrinogen and thrombospondin, a process that may increase the stability of platelet-platelet interactions.48 As with VWF, fibronectin has been demonstrated to undergo conformational changes in response to mechanical stress,49 a finding that may partially explain the preferential role for fibronectin in promoting thrombus formation under high shear. Fibronectin has also been noted to exhibit a distinct pattern of matrix assembly on the surface of platelets50 and have distinct binding characteristics from fibrinogen to the activated form of integrin αIIbβ3.50,51 How these distinct binding interactions with platelets facilitate platelet aggregation remains unknown.
Although considerable progress has been made in recent years in defining the relative roles of VWF, fibrin(ogen), and fibronectin in promoting shear-dependent platelet aggregation, many important issues remain, not least, the mechanisms controlling deposition of these proteins on the platelet surface. It also remains to be determined what the relative contributions of platelet-derived and plasma VWF, fibrinogen, and fibronectin are in platelet aggregation. Confocal imaging of fibrinogen and VWF distribution during thrombus development reveal distinct spatial localization of the different protein pools with evidence that platelet stores of fibrinogen are important for consolidating the thrombus core.52 It is also unclear why a combination of ligands (including VWF/fibrinogen or fibronectin/fibrin) are far more reactive to platelets than in isolation, a finding that may have direct clinical relevance because elevated plasma levels of VWF, fibrinogen, and possibly fibronectin independently increase the risk of atherothrombotic complications.
Unraveling the complex dynamics of platelet aggregation
One of the most striking findings from intravital studies of thrombosis is the dynamic nature of platelet aggregation in vivo. A characteristic feature of initial thrombus formation is the high proportion of platelets that translocate (movement from the point of initial attachment) on the injured vessel wall and on the surface of thrombi before forming firm adhesion contacts.12,39 This adhesive behavior is similar to the rolling interactions of leukocytes with postcapillary endothelial cells at sites of inflammation, where initial tethering and rolling is mediated by the P-selectin-PSGL-1 interaction and firm adhesion is mediated by leukocyte β2-integrins.53 The initial tethering of platelets is principally mediated by the VWF-GPIb interaction, whereas firm adhesion depends on ligand engagement of β1 and β3-integrins.19 Platelet translocation is typically stop-start in nature and, depending on the type and extent of vascular injury, a considerable proportion of tethering platelets will only interact transiently with the thrombus surface.12 Defining the factors regulating the stop-start nature of platelet translocation is a critical issue, because it is one of the key variables regulating the rate and extent of thrombus growth.
Platelet shape and translocation dynamics
An important factor influencing cell rolling behavior is morphology.53 In the case of leukocytes, their round morphology is well suited to rotational motion (rolling); the flat discoid morphology of platelets, however, is less ideal for smooth rolling interactions. Platelets have been demonstrated to assume different morphologies in vitro after prolonged exposure to adhesive surfaces such as VWF,54–56 raising the possibility that signals generated during surface translocation may induce morphological changes that facilitate a rolling phenotype. It has also been demonstrated that platelets can translocate as flat discs through a rotational side-to-side flipping mechanism57 or through a sliding, rotational movement of the cell body.58 Recent high-resolution imaging of platelets in vivo have revealed that the majority of platelets translocate on the vessel wall and on the surface of thrombi in vivo as flat, sliding discs.59 Most platelets slide in a stop-start manner, although rotational flipping of discoid platelets can also be observed. Relative to rotating discs or rolling spheres, sliding platelets experience reduced drag force and have much lower tensile stresses on adhesive bonds. A flat disc also enables maximal surface contact area with the adhesive surface, increasing the potential for multivalent adhesive interactions. Thus, by adopting a sliding, adhesive behavior, platelets appear to have evolved a unique translocation mechanism that minimizes drag forces on adhesive bonds. Combined with their small dimensions, this translocation mechanism provides an efficient means of limiting the detaching effect of blood flow on translocating platelets. As discussed subsequently, this sliding mechanism appears to be greatly facilitated by the development of small membrane protrusions (tethers), which further reduce tensile stress on adhesive bonds.
Importance of membrane tethers in the initiation of platelet aggregation
A significant recent development in the study of platelet adhesive interactions under flow has been the finding that membrane tethers play an important role in the initiation of platelet-matrix and platelet-platelet adhesive interactions56,58–60 (Figure 3A). Membrane tethers are smooth cylinders of lipid bilayer that are pulled from the surface of platelets under the influence of hemodynamic drag force. These structures are dynamic and extend from localized adhesion contacts and appear to play a role in regulating platelet translocation behavior.60 Similar structures have been demonstrated in a variety of cell types, including red blood cells,61 neutrophils,62 neurons,63 fibroblasts,64 and endothelial cells,65 and similar to platelets, membrane tethers are thought to play an important role in regulating leukocyte rolling behavior.62
Membrane tethers regulate platelet-matrix and platelet-platelet adhesive interactions. (A) (Left) Scanning electron microscopy of a single discoid platelet forming membrane tethers during adhesion to immobilized VWF (1800 s−1). Note that tethers can be readily distinguished from filopodia in that the latter is inhibited by pretreating platelets with the actin polymerization inhibitor, cytochalasin D. (Right) Shear-dependent formation of adhesion contacts between discoid platelets involves the development of membrane tethers (arrows). (B) (Left) Reversible aggregation of discoid platelets. Note that in this image, all platelets cluster around a central activated platelet through the development of membrane tethers. (Right) The development of stable platelet aggregates is associated with classic platelet shape change characterized by sphering of the platelet body and the extension of multiple filopodial projections. (Modified from Brass et al68 ; used with permission.) (This research was originally published in Maxwell et al.59 and was adapted with permission. Platelets were imaged on a Hitachi S570 scanning electron microscope [Tokyo, Japan] at 20 kV accelerating voltage, 3 mm working distance.)
Membrane tethers regulate platelet-matrix and platelet-platelet adhesive interactions. (A) (Left) Scanning electron microscopy of a single discoid platelet forming membrane tethers during adhesion to immobilized VWF (1800 s−1). Note that tethers can be readily distinguished from filopodia in that the latter is inhibited by pretreating platelets with the actin polymerization inhibitor, cytochalasin D. (Right) Shear-dependent formation of adhesion contacts between discoid platelets involves the development of membrane tethers (arrows). (B) (Left) Reversible aggregation of discoid platelets. Note that in this image, all platelets cluster around a central activated platelet through the development of membrane tethers. (Right) The development of stable platelet aggregates is associated with classic platelet shape change characterized by sphering of the platelet body and the extension of multiple filopodial projections. (Modified from Brass et al68 ; used with permission.) (This research was originally published in Maxwell et al.59 and was adapted with permission. Platelets were imaged on a Hitachi S570 scanning electron microscope [Tokyo, Japan] at 20 kV accelerating voltage, 3 mm working distance.)
One of the key features of membrane tethers is their ability to reduce the pulling forces imposed on adhesive bonds66 and, as a consequence, increase the probability of sustaining adhesion in a shear field. Strikingly, tethers of up to 30 μm have been observed extending from the surface of platelets,58,60 a massive extension of membrane from a cell that is only 1 to 3 μm in diameter. Because surface membranes can only be normally stretched by approximately 4% before causing cell lysis,67 the creation of such tethers must involve utilization of membrane reserves from the surface-connecting canalicular system. These membrane reserves can also buffer changes in surface membrane tension, helping to dampen the platelet activating effects of rapid fluctuations in blood flow.64
Role of membrane tethers in a 2-stage platelet aggregation process
What is the role of membrane tethers in promoting the formation of stable platelet aggregates? Recent high-resolution imaging of platelets during thrombus development has provided new insights into this process59 (Figure 3B). These studies have revealed the existence of a 2-phase platelet aggregation process, an initial reversible phase of aggregation occurring between discoid platelets and a subsequent stable aggregation phase associated with platelet shape change and granule release. The formation of discoid platelet aggregates is shear-dependent and primarily mediated through the development of membrane tethers. This initial phase of aggregation involves platelet activation and the adhesive function of both GPIb and integrin αIIbβ3; conspicuously, however, it does not require soluble agonists such as adenosine diphosphate (ADP), TXA2, or thrombin. Nonetheless, these aggregates are always reversible with conversion to stable aggregates dependent on soluble agonist generation, most notably ADP. Thus, tethers appear to play an important role in maintaining close physical proximity between platelets, which may provide a mechanism of facilitating autocrine/paracrine stimulation by locally generated agonists. Such a mechanism may help limit the “washout” effects of blood flow on released soluble agonists, providing a means of maintaining a high local concentration of activating signals within the confines of a developing aggregate.68 One of the key features of membrane tethers is that they provide a mechanism of sustaining platelet interactions with the thrombus surface without the requirement for global platelet activation.
Role of adhesion receptor signals and soluble agonists in coordinately regulating platelet aggregation dynamics under flow
One of the more vexing issues with respect to platelet aggregation is the significance of GPIb and integrin αIIbβ3-derived activating signals. In general, there is limited insight into the spatiotemporalsignaling relationship operating between adhesion receptors and soluble agonists in regulating platelet or leukocyte activation under flow. For example, leukocyte rolling velocity is critically influenced by the level of cell stimulation by specific chemokines69 ; these input signals, however, do not occur in isolation and are likely to be modified (and amplified) by signals emanating from the adhesion receptors themselves.70 Based on perfusion studies on immobilized VWF, GPIb and integrin αIIbβ3 appear to elicit distinct, cooperative activating signals71,72 that can regulate platelet translocation dynamics partially through the reversible activation of integrin αIIbβ3.71 In general, GPIb and integrin αIIbβ3 signals are inefficient at inducing global platelet activation in the absence of a costimulus such as ADP, thrombin, TXA2, or collagen.73 Most current models of thrombus development propose a key role for collagen (and possibly vessel wall-derived thrombin) in initiating platelet activation in primary adherent platelets, whereas subsequent propagation of thrombi (platelet aggregation) is primarily driven by agonists released or generated from the platelet surface, including ADP, TXA2, and thrombin. Soluble agonists do not recruit platelets into forming thrombi, however, adhesion receptors do, which begs the question whether activating signals downstream of GPIb and integrin αIIbβ3 are important to promote initial platelet activation and thrombus development. The answer to this, at least in the context of initial thrombus growth, remains uncertain and controversial.73–76 There is currently limited evidence that signals downstream of GPIb are essential for the initiation of hemostatic plug formation or thrombus development. Similarly, the role of integrin αIIbβ3-derived signals in initiating platelet aggregation and thrombus development remains unclear. It is possible that activating signals downstream of GPIb play an important role in initiating localized integrin αIIbβ3 activation necessary for membrane tether formation and reversible aggregation. Similarly, there is evidence that integrin αIIbβ3-derived signals, in combination with soluble agonists, synergistically enhance platelet activation under flow.73,77 In isolation, GPIb and integrin αIIbβ3 signals are weak; nonetheless, it is conceivable that localized signals from these receptors may play an important role in facilitating the initial formation of reversible aggregates and, in combination with soluble agonists, enhance the incorporation of these aggregates into stable thrombi. Definitive resolution of this issue will require the development of mouse models expressing mutant forms of GPIb and integrin αIIbβ3 that have preserved adhesive function but a selective signaling defect.
Sustaining platelet aggregation and thrombus stability
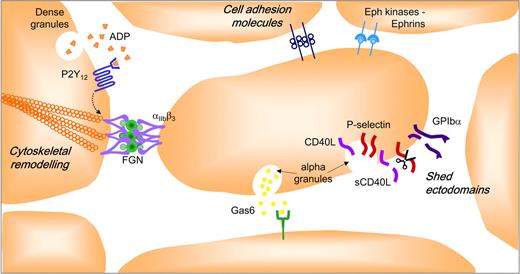
Platelets must not only be able to develop adhesive contacts under rapid blood flow, but must also sustain them to prevent thrombus detachment (embolization) from the site of vascular injury. Recent studies have revealed that a complex repertoire of adhesion and signaling receptors are present on the platelet surface that may serve to regulate the stability of forming aggregates (Figure 4). The details of this system have recently been reviewed68 and only the core elements are briefly presented here. Central to thrombus stability is the maintenance of high-affinity/avidity integrin αIIbβ3 adhesion bonds. Initiation of integrin αIIbβ3 activation is controlled by well-characterized signaling events operating downstream of soluble agonist (Gq and G12/13) and adhesion (nonreceptor tyrosine kinase) coupled receptors.9 Sustaining integrin αIIbβ3 activation is critically dependent on signals operating downstream of Gi-coupled receptors, principally the purinergic P2Y12 receptor.78 Thus, ADP plays a key role in both initiating (through the Gq-linked P2Y1 receptor) and sustaining integrin αIIbβ3 activation necessary for the development of stable platelet-platelet adhesion contacts. There is growing evidence that the P2Y12 signals do not operate in isolation. For example, once engaged by ligand, integrin αIIbβ3 initiates the formation of membrane-proximal signaling complexes that not only serve to sustain platelet activation, but also induce cytoskeletal changes that promote integrin αIIbβ3 clustering and increased receptor avidity.79 Recent evidence suggests that members of the tetraspanin family, CD15169 and TSSC6,80 play an important role in regulating integrin αIIbβ3 outside-in signaling, with a deficiency in TSSC6 leading to a defect in thrombus stability.80 The development of close platelet-platelet contacts also enables the juxtaposition of ligands on one platelet with receptors on adjacent platelets. Examples of this include various members of the immunoglobulin superfamily (PECAM-1,81 JAM-A,82 JAM-C,83 ESAM, 84 and CD22685 ), Eph kinases/ephrins,86 and Gas6 and its receptors, Axl-Tyro3-Mer.87 The role of these individual components in regulating the stability of platelet aggregates is only beginning to be addressed, although there is evidence that Eph kinases/ephrins88 and Gas687 and its receptors play an important role in this process. The exodomains of various platelet surface proteins, including P-selectin,89 CD40L,90 GPIb,91 GPV,92 GPVI,93 and Sema4D,68 are also shed from the surface of platelets with evidence that the soluble form of CD40L promotes thrombus stability by engaging integrin αIIbβ3.94
Factors stabilizing formed platelet aggregates. The intercellular space between aggregating platelets facilitates interaction between integrins and their ligands and other adhesion molecules, and promotes activation of Eph receptor kinases by cell surface ephrins. This narrow space also provides a protective environment for the accumulation of soluble agonists (ADP, thrombin, and TXA2), Gas-6, and the proteolytically shed exodomains of platelet surface proteins (GPIb, P-selectin, sCD40L).
Factors stabilizing formed platelet aggregates. The intercellular space between aggregating platelets facilitates interaction between integrins and their ligands and other adhesion molecules, and promotes activation of Eph receptor kinases by cell surface ephrins. This narrow space also provides a protective environment for the accumulation of soluble agonists (ADP, thrombin, and TXA2), Gas-6, and the proteolytically shed exodomains of platelet surface proteins (GPIb, P-selectin, sCD40L).
Multiple adhesion mechanisms initiating platelet aggregation
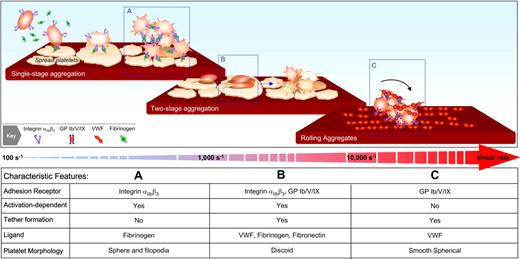
Based on findings from in vitro perfusion systems12,28,33,34,95 and in vivo thrombosis models,35,36,39,59 at least 3 distinct mechanisms of platelet aggregation can be identified with the relative contribution of each mechanism dependent on the prevailing shear conditions. As demonstrated in Figure 5, the key components of the traditional model of platelet aggregation, in which aggregation is mediated exclusively by fibrinogen and integrin αIIbβ3, is likely to be the predominant mechanism operating under relatively low shear conditions (< 1000 s−1). Under such conditions, integrin αIIbβ3 on the surface of free-flowing platelets can engage fibrinogen adsorbed onto the surface of thrombi. Note that this adhesive interaction can occur independent of VWF-GPIb, although even at these shear rates, the latter adhesive interaction may have a role.41 Subsequent stimulation of platelets by locally generated soluble agonists induces platelet shape change and an increase in integrin αIIbβ3 affinity, which helps stabilize/sustain integrin αIIbβ3-fibrinogen bonds. At shear rates between 1000 and 10 000 s−1, the processes initiating aggregation are quite distinct involving additional receptors, ligands, and membrane tethers. At these shear rates, platelet-platelet interactions become progressively more VWF-dependent with an important role for both GPIb and integrin αIIbβ3 in promoting the initial formation of discoid platelet aggregates. Whether fibronectin is also involved in these earliest stages remains to be seen; nonetheless, at these shear rates, there are essential contributions of VWF, fibrin(ogen), and fibronectin to the formation of stable platelet aggregates. Recently, a third mechanism initiating platelet aggregation has been identified that is operative at very high shear rates (> 10 000 s−1).95 Strikingly, this aggregation mechanism does not require platelet activation or the adhesive function of integrin αIIbβ3 and is exclusively mediated by VWF-GPIb adhesive bonds. Aggregate formation is dependent on both immobilized and soluble forms of VWF, and at such high shear, marked deformation to the membrane of nucleating platelets (the nidus for aggregate formation) is a hallmark feature of the aggregation process. How important these membrane changes are to the formation/maintenance of adhesive bonds remains to be established; nonetheless, the findings that nonactivated platelets can form large aggregates under very high shear have potentially important implications for the mechanisms promoting occlusive thrombus formation in stenosed arteries. It is important to recognize that none of these models are identical to the traditional model of aggregation developed from studies in the aggregometer. In the latter situation, regardless of the physiological agonist, the platelet response is essentially the same; platelets undergo shape change, become “sticky,” form aggregates, and release granule contents, a temporal sequence of events distinct from those occurring under flow.
Distinct mechanisms initiating platelet aggregation at various shear rates. (A) Platelet aggregation at shear rates < 1000 s−1 is predominantly mediated by the interaction of fibrinogen with integrin αIIbβ3. Under these conditions, stable aggregation typically occurs between shape-changed platelets. (B) At shear rates between 1000 and 10 000 s−1, a distinct 2-stage aggregation process can be identified. The initial formation of aggregates occurs between discoid platelets, is mediated by membrane tethers, and is dependent on the adhesive function of both GPIb and integrin αIIbβ3. Conversion of reversible aggregates to stable aggregates is associated with platelet shape change and is dependent on the generation of soluble agonists, particularly ADP. (C) At shear rates > 10 000 s−1, platelet aggregation can be initiated independent of integrin αIIbβ3 or platelet activation and is exclusively mediated by the VWF-GPIb interaction. Although the initial aggregates form between discoid platelets, at such high shears, the platelets adopt a smooth spherical morphology and roll (translocate in a rotational manner) across the VWF surface (unpublished observations, Erik Westein and Shaun P. Jackson). (Figure was adapted with permission from Maxwell et al.59 )
Distinct mechanisms initiating platelet aggregation at various shear rates. (A) Platelet aggregation at shear rates < 1000 s−1 is predominantly mediated by the interaction of fibrinogen with integrin αIIbβ3. Under these conditions, stable aggregation typically occurs between shape-changed platelets. (B) At shear rates between 1000 and 10 000 s−1, a distinct 2-stage aggregation process can be identified. The initial formation of aggregates occurs between discoid platelets, is mediated by membrane tethers, and is dependent on the adhesive function of both GPIb and integrin αIIbβ3. Conversion of reversible aggregates to stable aggregates is associated with platelet shape change and is dependent on the generation of soluble agonists, particularly ADP. (C) At shear rates > 10 000 s−1, platelet aggregation can be initiated independent of integrin αIIbβ3 or platelet activation and is exclusively mediated by the VWF-GPIb interaction. Although the initial aggregates form between discoid platelets, at such high shears, the platelets adopt a smooth spherical morphology and roll (translocate in a rotational manner) across the VWF surface (unpublished observations, Erik Westein and Shaun P. Jackson). (Figure was adapted with permission from Maxwell et al.59 )
Translating basic insights into clinical benefit
The identification of discrete shear-specific mechanisms initiating platelet aggregation, coupled with a clearer understanding of the key elements propagating and sustaining thrombi, raises the possibility that antiplatelet therapies may be developed that preferentially operate in certain vascular territories and at distinct stages of the thrombotic process. Evidence supporting such a possibility has been derived from in vivo studies targeting specific platelet adhesion events. For example, deficiency of plasma fibronectin produces a platelet aggregation defect that is distinct from that observed with VWF or fibrinogen deficiency35,36 without producing a marked prolongation in bleeding time. Given the preferential role for fibronectin in promoting thrombus growth and stability under elevated shear,46 selective blockade of the fibronectin-integrin αIIbβ3 interaction may be an attractive approach to prevent thrombotic vascular occlusion while maintaining mural thrombus formation. Similarly, blockade of the integrin αIIbβ3-binding sequence of fibrinogen (C-terminal γ-chain dodecapeptide sequence) also produces a marked defect in thrombus formation,39,96 raising the possibility that selective inhibition of specific integrin αIIbβ3 ligands, rather than global inhibition of integrin αIIbβ3 itself, may represent a more targeted antithrombotic approach. Furthermore, given the well-defined role for the GPIb-VWF interaction in supporting platelet adhesive interactions under high shear, inhibition of this adhesive event has been considered an attractive approach to limit thrombus formation in high shear flow situations. Inhibitors of the VWF-GPIb interaction are highly effective in preventing thrombotic vascular occlusion,97 although it remains to be established whether such approaches will ultimately have an improved therapeutic window relative to conventional integrin αIIbβ3 antagonists.8
Similar concepts are beginning to emerge in the context of soluble agonists. For example, there is a considerable body of evidence supporting a major role for ADP in not only potentiating platelet aggregation, but also in sustaining formed aggregates necessary for the development of stable thrombi. Genetic deletion or pharmacological blockade of the P2Y12 receptor leads to a major defect in thrombus growth and stability regardless of the primary thrombogenic stimulus.98 These amplifying effects of ADP are likely to be important for thrombus development/stability in most areas of the circulation, producing a significant hemostatic defect at high levels of receptor blockade. Several signaling components acting downstream of the P2Y12 receptor appear to also play an important role in thrombus development/stability but are less critical for hemostasis. These include 2 members of the PI 3-kinase family, p110β and p110γ, which play an important role in sustaining integrin αIIbβ3 activation and stable platelet aggregation.99,100 Other platelet components that have been demonstrated to be important for thrombus stability, but less important for initial thrombus growth, include sCD40L94 and Gas687 and its receptors. Whether other agonist receptors and their downstream signaling components regulate platelet aggregation under specific rheological conditions and at discrete stages in the thrombotic process remains to be seen.
It should be acknowledged that a great deal of the recent progress in the understanding of platelet aggregation has been based on experimental animal models, particularly the mouse, which inevitably raises issues of relevance to humans. In this context, none of the commonly used in vivo thrombosis models (involving ferric chloride, photochemical or laser injury of healthy vessels) accurately reproduce the conditions of arterial thrombosis in humans, in which thrombi typically form on ruptured or eroded atherosclerotic plaques. Moreover, the rheological conditions in the mouse microcirculation used for intravital studies are likely to be significantly different from those operating in stenosed human arteries. It also remains unclear whether observations from in vivo thrombosis models can be extrapolated to platelet aggregation processes relevant to hemostatic platelet plug formation. However, as with any experimental model, the biological plausibility of findings can only be established when carefully analyzed in the context of other experimental data and clinical observations. Thus, in the same way that findings in the aggregometer helped validate the importance of various adhesive proteins, soluble agonists/receptors, and signaling cascades in hemostasis, insights from ex vivo perfusion studies on human platelets, combined with data from carefully performed clinical studies, will be required to more precisely validate the role of the growing list of “new players” in the platelet aggregation/thrombosis process.
Conclusions
Great progress has been made over the last decade in unraveling the complex and dynamic processes regulating platelet aggregation, particularly in the context of arterial thrombosis. Although most of the key elements have probably been identified, some unexpected findings continue to surface that challenge many long-held beliefs in the field, not least the recent demonstration that under certain experimental conditions, platelet aggregation can occur independent of platelet activation and integrin αIIbβ3 engagement.95 These new insights, although currently of primary interest to the academic community, are likely to be rapidly translated into new experimental approaches that may ultimately hold great promise in the clinic. What was once considered a straightforward phenomenon, platelet aggregation has evolved into a complex field of investigation with many of its most jealously guarded secrets only now beginning to emerge.
Acknowledgments
I thank Drs Denisa Wagner and Zaverio Ruggeri, members of my laboratory and the Department of Haematology, Alfred Hospital, for constructive advice on the review. The assistance with preparation of figures by Dr. Simone Schoenwaelder is also gratefully acknowledged.
This work was supported by grants from the National Health and Medical Research Council of Australia, the Australian Research Council, and the National Heart Foundation of Australia.
Authorship
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Shaun P. Jackson, Australian Centre for Blood Diseases, Monash University, 6th Floor, Burnet Tower, 89 Commercial Rd., Melbourne, Australia, 3004; e-mail: shaun.jackson@med.monash.edu.au.


![Figure 3. Membrane tethers regulate platelet-matrix and platelet-platelet adhesive interactions. (A) (Left) Scanning electron microscopy of a single discoid platelet forming membrane tethers during adhesion to immobilized VWF (1800 s−1). Note that tethers can be readily distinguished from filopodia in that the latter is inhibited by pretreating platelets with the actin polymerization inhibitor, cytochalasin D. (Right) Shear-dependent formation of adhesion contacts between discoid platelets involves the development of membrane tethers (arrows). (B) (Left) Reversible aggregation of discoid platelets. Note that in this image, all platelets cluster around a central activated platelet through the development of membrane tethers. (Right) The development of stable platelet aggregates is associated with classic platelet shape change characterized by sphering of the platelet body and the extension of multiple filopodial projections. (Modified from Brass et al68; used with permission.) (This research was originally published in Maxwell et al.59 and was adapted with permission. Platelets were imaged on a Hitachi S570 scanning electron microscope [Tokyo, Japan] at 20 kV accelerating voltage, 3 mm working distance.)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-12-027698/4/m_zh80120701420003.jpeg?Expires=1769295146&Signature=3ZiaZ-xWkP6UyO3WBF0E1WxBtN03VVee2tw59xihnn8KNKrlWx0iDu7D418-1sG7l7LKW2y6BJdyOljjcfNkTBMbeumrFhkly1vIs7QQfd3x1Umt8-tCRO0BEjlkS50huiHi7uyWvKYGNS-ilFyucCrSO5XGkaUIYDto3cSEHK94vSwg~SXkW6~NUxRbm3EOi7hkYLIjgIIrbRYJfdwUugQMMnuAXg~chnwnTTBtuu2KGMrIkR0a8Xzp0bzZRc3LLMXw1jgGRrQWKew2zmjYQfmz6PU6MipRnXEiDIOyfKqTbCOf7-I6mNn3CP85TKO5~w6LMUE-cLT4jDHDCjZFtw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)