Abstract
Mantle cell lymphoma (MCL) is an aggressive B-cell lymphoma resistant to conventional chemotherapy. The Bcl-2 pathway is deregulated in these tumors and may represent an interesting target for new therapeutic strategies. The new small-molecule pan–Bcl-2 inhibitor GX15-070 mimics BH3-only proteins by binding to multiple antiapoptotic Bcl-2 members. Here we show that GX15-070 induced apoptosis in vitro in MCL cell lines and primary cells from patients with MCL by releasing Bak from Mcl-1 and Bcl-XL at short incubation times and low micromolar doses. GX15-070 was effective in cells bearing defective DNA damage-sensor genes or cell-cycle regulators, inducing Bax and Bak conformational changes, mitochondrial depolarization, phosphatidylserine exposure, and caspase-3 activation. Furthermore, GX15-070 synergized with bortezomib, sensitizing MCL cells to low doses of this proteasome inhibitor, by neutralizing bortezomib-induced Mcl-1 accumulation and cooperating with Noxa to induce Bak displacement from this protein. These events led to an increased activation of the mitochondrial apoptotic pathway. Importantly, GX15-070 alone or in combination with bortezomib showed no significant cytotoxic effect in peripheral blood mononuclear cells from healthy donors. All these findings suggest that GX15-070 alone or in combination with bortezomib represents a new attractive therapeutic approach for MCL treatment.
Introduction
Mantle cell lymphoma (MCL) is a well-defined lymphoid neoplasm genetically characterized by the t(11;14)(q13;q32) translocation leading to a constitutive overexpression of cyclin D1.1 In addition to classical MCL, a blastoid variant of this disease has been described, characterized by high proliferation and associated with INK4a/ARF deletions, p53 mutations, and complex karyotypes.2–4 The clinical behavior is aggressive, and few patients reach long survival or can be considered cured with current therapies. Recent results from clinical trials using the proteasome inhibitor bortezomib have shown promising results in the management of patients with MCL.5–8
Bcl-2 family proteins are key regulators of apoptosis, determining cellular fate in response to numerous stress.9,10 In mammalian cells, the prosurvival members (Bcl-2, Bcl-XL, Mcl-1, Bcl-w, and A1) oppose 2 proapoptotic groups: the multidomain proteins (Bax, Bak, and Bok) and the BH-3–only proteins (Bim, Puma, Noxa, Bad, Bid, Bmf, Bik, and Hrk). A balance between prosurvival and prodeath Bcl-2 members dictates the outcome of many death-initiating signaling pathways. The BH3-only members function as sensors of cellular well-being, and when activated by cytotoxic signals, selectively engage the prosurvival members by inserting its BH3 domain into a hydrophobic groove on the antiapoptotic member surfaces.11 This event allows Bax and Bak displacement from antiapoptotic members, their oligomerization and permeabilization of the mitochondrion, provoking the release of proapoptotic factors, caspase activation and finally cell death.12,13
There is growing evidence that the Bcl-2 pathway is deregulated in most neoplasms. Several studies have described high levels of Bcl-2 in MCL cells.14,15 Bcl-XL overexpression has also been described in MCL cells, linked to constitutive activation of the NF-κB pathway.16 Moreover, Mcl-1 overexpression has been correlated with high-grade MCL.17 In addition, we have recently shown that bortezomib induces both the accumulation of the antiapoptotic protein Mcl-1 and the activation of the proapoptotic BH3-only protein Noxa, which counteracts, at least partially, the effect of its antiapoptotic partner.18 It is conceivable that Mcl-1 accumulation may delay bortezomib-induced apoptosis. In this context, the emergence of small-molecule inhibitors that modulate Bcl-2 pathway represents a rational approach for the treatment of this neoplasm and may synergize with bortezomib activity. GX15-070 (GeminX Biotechnologies, Montreal, QC, Canada) is a small-molecule pan–Bcl-2 inhibitor that belongs to the polypirrole class of molecules, which binds to Bcl-2, Bcl-w, Bcl-XL, and Mcl-1 with a Kd in the range of 0.5 μM.19 This compound has been designed to mimic proapoptotic BH3-only proteins in its binding to the antiapoptotic Bcl-2 family members, and appears to fall into the class of BH3 “sensitizers.”20 GX15-070 is currently in phase 1 clinical trials for the treatment of refractory solid tumors, and in phases 1 and 2a clinical trials for the treatment of refractory chronic lymphocytic leukemia (CLL) and myeloid malignancies.19
Our purpose was to assess the sensitivity of MCL primary tumor cells and cell lines to GX15-070–induced apoptosis and to analyze its effect in combination with bortezomib. These studies may help to establish the basis for a rational use of GX15-070 alone or in combination with bortezomib, hopefully improving the outcome of patients with MCL.
Patients, materials, and methods
Cell lines
The MCL cell lines Granta-519, Jeko, REC-1, UPN-1, and HBL-2, all of them bearing the t(11;14)(q13;q32) translocation, were used. Genetic alterations of these cell lines have been recently published.21 All cell lines (0.3-0.5 × 106 cells/mL) were cultured in RPMI 1640 culture medium (Gibco, Paisley, Scotland, United Kingdom), supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 50 μg/mL penicillin-streptomycin (Gibco), and 100 μg/mL normocin (Amaxa, Cologne, Germany). In Granta-519 cells, DMEM culture medium (Gibco) was used instead of RPMI 1640. All cultures were routinely tested for Mycoplasma contamination by polymerase chain reaction (PCR).
Patients
A total of 11 patients diagnosed with MCL according to the World Health Organization classification22 who had not received treatment for the previous 3 months were studied. Tumor cells were obtained from peripheral blood or the spleen. The percentage of malignant cells (CD19+, CD5+, and CD23− showing light-chain restriction) was analyzed by flow cytometry. Cyclin D1 overexpression was demonstrated in all cases by immunohistochemistry. The clinical characteristics of these patients are listed in Table 1The first 10 patients corresponded to those used in a previous report.18 Informed consent was obtained from each patient in accordance with the guidelines of the Ethical Committee of the Hospital Clinic (Barcelona, Spain) and the Declaration of Helsinki.
Isolation of MCL primary cells, PBMCs from healthy donors, and CD19+ cells
Mononuclear cells from peripheral blood samples (PBMCs) were isolated by Ficoll/Hypaque sedimentation (Seromed, Berlin, Germany). Tumor cells from spleen biopsies and cells from reactive tonsils were obtained after squirting with RPMI 1640 culture medium using a fine needle. Cells were either used directly or cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide and 20% heat-inactivated FCS. Manipulation due to freezing/thawing did not influence cell response. CD19+ cells from reactive tonsils were isolated by an immunomagnetic method (AutoMACS) using anti-CD19 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany).
Cell culture and treatments
Mononuclear cells from patients with MCL, PBMCs from healthy donors, and CD19+ from reactive tonsils (1-2 × 106 cells/mL) were cultured in X-VIVO 10 medium (Cambrers Bioscience, Verviers, Belgium). All MCL cell lines and primary cells were cultured at 37°C in a humidified atmosphere containing 5% carbon dioxide. Cells were incubated for 5 to 48 hours with GX15-070, either alone or in combination with the proteasome inhibitor bortezomib (Millenium Pharmaceuticals, Cambridge, MA). When specified, cells were preincubated for 1 hour with benzyloxy-carbonyl-Val-Ala-Asp-fluoro-methylketone (z-VAD.fmk; Bachem, Bubendorf, Switzerland) prior to GX15-070 addition.
Immunoblotting and immunoprecipitation
Cells were lysed in Triton buffer (1% Triton X-100, 50 mM Tris [tris-hydroxymethyl-aminomethane]–HCl [pH 7.6], 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 10 μg/mL leupeptin and benzamidine, and 1 mM PMSF [phenylmethanesulfonyl fluoride]). Solubilized proteins were analyzed in 12% to 15% polyacrylamide gels. Western blot analysis was performed as previously described.23 Equal protein loading was confirmed by analyzing α-tubulin expression. Chemiluminiscence was detected using an LAS3000 Fujifilm device, and relative protein quantification was done with Image Gauge software (Fuji, Tokyo, Japan).
The following monoclonal and polyclonal antibodies were used: anti–Mcl-1 (S-19), anti–Bcl-XL/S (2H12 and H62), and anti-Bak (N-20) (Santa Cruz Biotechnology, Santa Cruz, CA); anti–Mcl-1 (clone 22) (BD Pharmingen, San Diego, CA); anti-Noxa (clone 114C307) (Alexis Biochemicals, Lausen, Switzerland); anti–Bcl-2 (DAKO, Glostrup, Denmark); anti-Bak (NT) (Upstate, Lake Placid, NY); and anti–α-tubulin (Sigma, St Louis, MO).
For Mcl-1 immunoprecipitation, NP-40 buffer was used (0.5% NP-40 [vol/vol], 50 mM HEPES [pH 7.7], 150 mM NaCl, 0.1 mM EDTA, 1 μg/mL leupeptin and aprotinin, and 1 mM PMSF). Protein extracts were incubated for 3 hours at 4°C with anti–Mcl-1 antibody (S-19), then G-protein beads were added for 1 more hour. Supernatant (nonimmunoprecipitated fraction) was recovered by centrifugation, and G-protein beads (immunoprecipitated fraction) were washed three times with NP-40 buffer. Reducing 5 × sample buffer (400 mM Tris HCl [pH 6.8], 10% SDS, 50% glycerol, 500 mM DTT [DL-dithiothreitol], and 0.1% bromophenol blue) was added to both fractions, boiled, and analyzed in 12% polyacrylamide gels followed by Western blotting. Membranes were probed with the polyclonal anti-Bak (NT), monoclonal anti-Noxa, and monoclonal anti–Mcl-1 (clone 22) antibodies. Bcl-XL immunoprecipitation was performed similarly, except that CHAPS buffer (1% CHAPS [wt/vol], 20 mM Tris [pH 7.4], 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, 1 μg/mL leupeptin and aprotinin, and 1 mM PMSF) was used followed by incubation of protein extracts overnight at 4°C with anti–Bcl-XL antibody (2H12). Membranes were probed with polyclonal anti-Bak (NT) and anti–Bcl-XL (H62) antibodies.
Flow cytometry detection of apoptosis
Phosphatidylserine (PS) exposure was quantified by surface Annexin V–APC staining. Briefly, 0.2 to 0.5 × 106 cells were incubated in binding buffer (140 mM NaCl, 2.5 mM CaCl2, and 10 mM HEPES/NaOH [pH 7.4]) with 0.5 μL of Annexin V–APC (Bender Medsystems, Burlingame, CA) at room temperature for 15 minutes. A total of 10 000 cells per sample were acquired in a FACSCalibur flow cytometer using the Cell Quest software (Becton Dickinson, San Jose, CA). For the analysis of apoptosis in CD3+ and CD19+ subpopulations, PBMCs were labeled simultaneously, in Annexin-binding buffer, with anti-CD3–FITC (Immunotech, Marseille, France), anti-CD19–PE (Becton Dickinson), and Annexin V–APC at room temperature for 15 minutes. A total of 40 000 cells per sample were acquired in a FACSCalibur flow cytometer. Changes in mitochondrial transmembrane potential (ΔΨm) were evaluated by staining cells with 20 nM 3,3′-diexyloxacarbocyanine iodide (DiOC6[3]; Molecular Probes, Eugene, OR). A total of 10 000 cells per sample were acquired in a FACScan flow cytometer. Analysis of the labeled populations was assessed using Paint-a-Gate software (Becton Dickinson).
Detection of intracellular proteins by flow cytometry
Cells were fixed and permeabilized as previously described.24 Cells (0.5 × 106) were stained with 1 μg/mL of antibodies against the active form of caspase-3 (BD Pharmingen), Bax (clone 6A7; BD Pharmingen) and Bak (clone Ab-1; Oncogene Research, Boston, MA) for 30 minutes at room temperature, followed by goat anti–rabbit FITC (Sigma) or goat anti–mouse FITC (DAKO), and were analyzed in a FACScan flow cytometer.
RNAi assays
NOXA siRNA and nonsilencing siRNA (Qiagen, Hilden, Germany) were introduced in Jeko cells by electroporation using a Nucleofector system (Amaxa, Köln, Germany) as previously described.18 Briefly, 5 × 106 Jeko cells were resuspended in 100 μL of R-cell nucleofector solution containing 3 μg (2 μM) of double-stranded siRNAs and electroporated with the A23 Nucleofector program. Prewarmed cultured medium (500 μL) was added to each cuvette, and cells were transferred to culture plates and cultured at 3 × 106 cells/mL for 3 hours. Dead cells were removed by low-speed centrifugation, and viable cells were diluted at 1 × 106 cells/mL and incubated for 3 hours more before experiments were set up.
mRNA quantification by real-time RT-PCR
Total RNA was isolated using the guanidinium thiocyanate method (Trizol; Invitrogen). RNA (1 μg) was retrotranscribed to cDNA using the Taqman reverse-transcription (RT) reagents, and mRNA expression was analyzed using predesigned Assay-on-demand (Applied Biosystems, Foster City, CA). The comparative Ct method (ΔΔCt) for relative quantification of gene expression was used. β-glucoronidase (GUS) was used as an internal control, and mRNA expression levels were given as arbitrary units as previously described.25
Results
GX15-070 induces apoptosis in MCL cells while sparing normal PBMCs
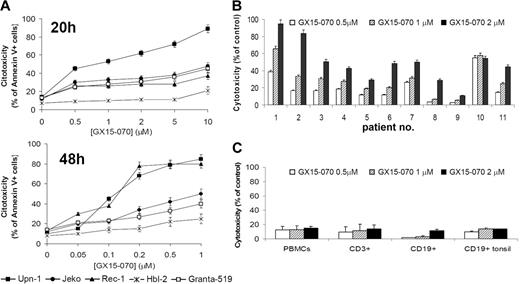
We assessed the effect of the BH3-mimetic GX15-070 in 5 MCL cell lines that differ in their p53-dependent pathway status, growth characteristics, and sensitivity to cytotoxic drugs.18,21,23 These MCL cell lines were treated with GX15-070 for 20 hours at doses ranging from 0.5 μM to 10 μM, and cytotoxicity was measured by Annexin V–labeling of externalized phosphatidylserine residues. Three different patterns of sensitivity to GX15-070 were observed among these cell lines (Figure 1A, left). UPN-1 was the most sensitive cell line, showing a significant cell death rate at 0.5 μM GX15-070. Jeko, Granta-519, and Rec-1 exhibited an intermediate sensitivity to GX15-070 at doses between 1 and 5 μM. HBL-2 was the least-sensitive cell line, reaching an LD50 around 5 μM at 72 hours (data not shown). Treatment of MCL cells with GX15-070 for 48 hours allowed a reduction on GX15-070 doses from 5- to 10-fold (range, 0.05-1 μM) to achieve the same cytotoxicity as that observed at 20 hours (Figure 1A, right). These results demonstrated that GX15-070 exerts a time- and dose-dependent cytotoxic effect in MCL cell lines.
Cytotoxic effect of GX15-070 apoptosis in MCL cells and nomal lymphocytes. (A) Dose response of GX15-070 in MCL cell lines at 20 hours (left panel) and 48 hours (right panel) analyzed by Annexin V–APC staining. (B) Cytofluorimetric analysis of Annexin V–positive cells after treatment of primary MCL cells with the indicated doses of GX15-070 for 20 hours. (C) PBMCs from healthy donors and CD19+ cells from reactive tonsils were incubated with the indicated doses of GX15-070 for 20 hours, and viability was analyzed with CD3-FITC/CD19-PE/Annexin V–APC. Results represent the mean ± SD of 3 independent experiments.
Cytotoxic effect of GX15-070 apoptosis in MCL cells and nomal lymphocytes. (A) Dose response of GX15-070 in MCL cell lines at 20 hours (left panel) and 48 hours (right panel) analyzed by Annexin V–APC staining. (B) Cytofluorimetric analysis of Annexin V–positive cells after treatment of primary MCL cells with the indicated doses of GX15-070 for 20 hours. (C) PBMCs from healthy donors and CD19+ cells from reactive tonsils were incubated with the indicated doses of GX15-070 for 20 hours, and viability was analyzed with CD3-FITC/CD19-PE/Annexin V–APC. Results represent the mean ± SD of 3 independent experiments.
Primary cells from 11 patients with MCL were also incubated for 20 hours with different doses of GX15-070 (range, 0.5-2 μM). The characteristics of these patients are summarized in Table 1. At 2 μM GX15-070, 3 profiles of response could be distinguished (Figure 1B): high response (patients no. 1 and no. 2), medium response (patients no. 3, no. 4, no. 6, no. 7, no. 10, and no. 11) and low response (patients no. 5, no. 8, and no. 9).
These results pointed out that GX15-070 was effective in cells bearing defective DNA damage-sensor genes such as p53 (Jeko, HBL-2, UPN-1, and patient no. 2) or ATM (Granta-519 and patients no. 1 and no. 3) and alterations in cell-cycle checkpoints (INK4a/ARF deletion in Rec, Granta-519, HBL-2, UPN-1, and patient no. 2).
Relationship between GX15-070 cytotoxicity and expression of antiapoptotic Bcl-2 members in MCL cell lines
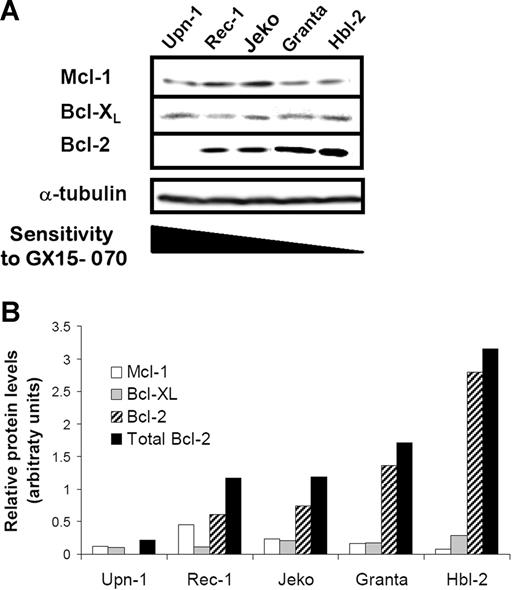
To evaluate whether the cytotoxicity of GX15-070 in MCL cell lines correlated with the expression levels of its targets, we analyzed the expression of the antiapoptotic proteins Mcl-1, Bcl-XL, and Bcl-2 by Western blot.
As shown in Figure 2A, a heterogenous expression of this family of proteins was observed. Indeed, Mcl-1 and Bcl-XL proteins were detected in all MCL cell lines, Rec-1 and Jeko being the cell lines with the highest protein levels. Granta-519 and HBL-2 cells showed high levels of Bcl-2 protein in accordance with the described amplification of the 18q21 region involving the BCL-2 gene.26,27 In contrast, Bcl-2 protein was not detected in UPN-1 cells that harbor a deletion at 18q12-22 (Silvia Bea, unpublished data, May 2006). Densitometric analysis of these proteins revealed an inverse correlation between the sensitivity to GX15-070 and the total amount of these antiapoptotic proteins, Bcl-2 being the protein that most contributed to the whole (Figure 2B).
Protein expression of Bcl-2 familly members in MCL cells. (A) Total protein extracts (50 μg) from 5 MCL cell lines were analyzed by Western blotting. Membranes were probed for Mcl-1, Bcl-XL, and Bcl-2 expression using suitable antibodies and α-tubulin was used to normalize protein loading. Western blot images are representative results from 3 independent experiments. (B) Relative protein quantification of Mcl-1, Bcl-XL, and Bcl-2 was performed using Image Gauge Fujifilm software.
Protein expression of Bcl-2 familly members in MCL cells. (A) Total protein extracts (50 μg) from 5 MCL cell lines were analyzed by Western blotting. Membranes were probed for Mcl-1, Bcl-XL, and Bcl-2 expression using suitable antibodies and α-tubulin was used to normalize protein loading. Western blot images are representative results from 3 independent experiments. (B) Relative protein quantification of Mcl-1, Bcl-XL, and Bcl-2 was performed using Image Gauge Fujifilm software.
GX15-070 activates the mitochondrial apoptotic pathway by inducing Bak displacement from Mcl-1 and Bcl-XL
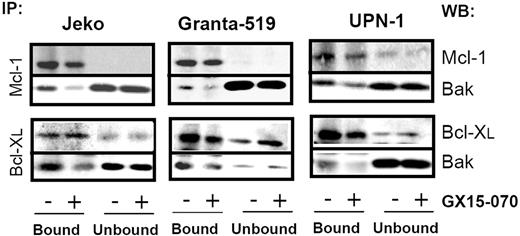
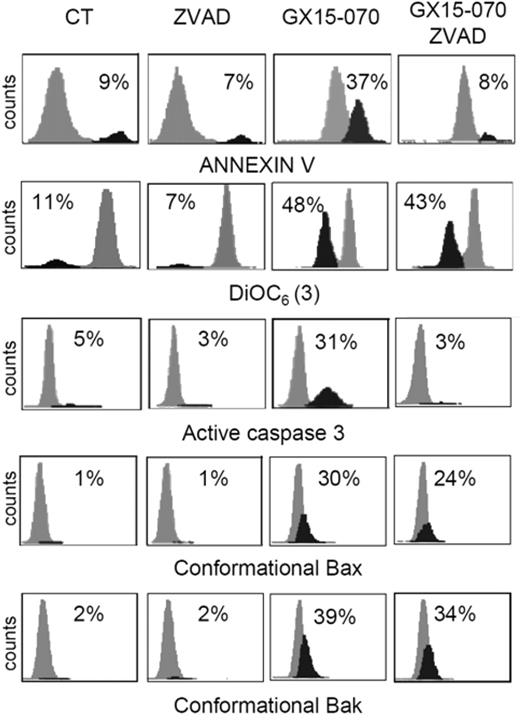
To characterize the mechanism of action of GX15-070 in MCL cells, we analyzed Bak/Mcl-1 and Bak/Bcl-XL interactions by coimmunoprecipitation experiments in MCL cell lines. It has been reported that Bak release from Mcl-1 and Bcl-XL is determinant for the onset of mitochondrial apoptotic pathway, and that the BH3-only proteins, such as Noxa, are known to be responsible for this release.13 Immunodetection of Bak in Mcl-1 and Bcl-XL precipitates, using an antibody against the Bak N-terminus, revealed that GX15-070 can bring about Bak release from Mcl-1 and Bcl-XL at 5 hours of incubation (Figure 3). This event initiated the typical mitochondrial apoptotic signaling that includes Bax and Bak conformational changes, loss of ΔΨm, phosphatydilserine exposure, and caspase-3 activation (Figure 4). As expected, Bax/Bak conformational changes and mitochondrial depolarization were independent of caspase activity, since these processes were not inhibited when UPN-1 cells were preincubated with the pancaspase inhibitor z-VAD.fmk (Figure 4). Similar results were obtained in the other MCL cell lines.
GX15-070 displaces Bak from Mcl-1 and Bcl-XL. MCL cell lines were treated for 5 hours with 5 μM (Jeko and Granta-519) or 0.5 μM (UPN-1) GX15-070. Mcl-1 and Bcl-XL immunoprecipitations were performed as described in “Patients, materials, and methods.” Immunoprecipitated (IP; bound) and nonimmunoprecipitated (unbound) fractions were analyzed by Western blotting for Bak, Mcl-1 and Bcl-XL proteins. Western blot (WB) images are representative results from 3 independent experiments.
GX15-070 displaces Bak from Mcl-1 and Bcl-XL. MCL cell lines were treated for 5 hours with 5 μM (Jeko and Granta-519) or 0.5 μM (UPN-1) GX15-070. Mcl-1 and Bcl-XL immunoprecipitations were performed as described in “Patients, materials, and methods.” Immunoprecipitated (IP; bound) and nonimmunoprecipitated (unbound) fractions were analyzed by Western blotting for Bak, Mcl-1 and Bcl-XL proteins. Western blot (WB) images are representative results from 3 independent experiments.
GX15-070 activates the intrinsic apoptotic pathway in a caspase-independent manner. UPN-1 cells were treated with 0.5 μM GX15-070 for 16 hours in the presence or absence of z-VAD-fmk (50 μM). Bax/Bak conformational changes, caspase-3 activation, loss of ΔΨm, and PS exposure were analyzed as described in “Patients, materials, and methods.” The percentages inside each chart refer to the population in black. These experiments have been performed twice with similar results and therefore 1 representative experiment is shown.
GX15-070 activates the intrinsic apoptotic pathway in a caspase-independent manner. UPN-1 cells were treated with 0.5 μM GX15-070 for 16 hours in the presence or absence of z-VAD-fmk (50 μM). Bax/Bak conformational changes, caspase-3 activation, loss of ΔΨm, and PS exposure were analyzed as described in “Patients, materials, and methods.” The percentages inside each chart refer to the population in black. These experiments have been performed twice with similar results and therefore 1 representative experiment is shown.
GX15-070 interacts synergistically with the proteasome inhibitor bortezomib in MCL cell lines
Recent results from our laboratory reported that the proteasome inhibitor bortezomib induced undesirable accumulation of Mcl-1 due to the absence of its degradation by proteasome.18 Since Mcl-1 is 1 of the GX15-070 targets, we analyzed whether MCL sensitivity to bortezomib could be increased by cotreatment with this BH3-mimetic compound.
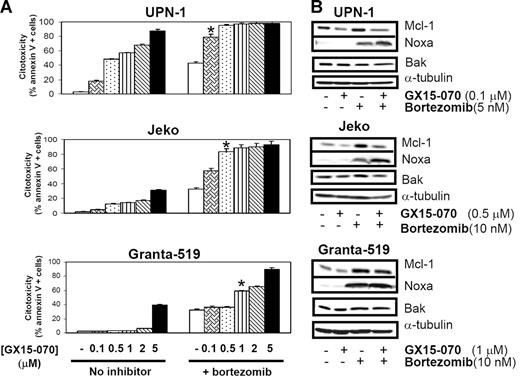
MCL cells were treated with 5 or 10 nM bortezomib in the presence or absence GX15-070 at doses ranging from 0.1 μM to 5 μM. In 3 representative MCL cell lines, a synergistic cytotoxic effect was observed (Chou and Talalay's combination indexes [CIs] lower than 0.7;28 Figure 5A). It is important to highlight that cotreatment with GX15-070 and bortezomib at the respective doses of 0.1 μM and 5 nM (UPN-1), 0.5 μM and 10 nM (Jeko), and 1 μM and 10 nM (Granta-519) induced similar cytotoxicity to that induced by bortezomib alone at the higher doses of 10 nM (UPN-1) and 50 nM (Jeko and Granta-519; data not shown). This synergistic interaction was not sequence dependent, for no significant differences were found when preincubating either of these compounds or when adding both simultaneously (data not shown).
Synergistic effect of GX15-070 and bortezomib in MCL cell lines. (A) Cells from 3 MCL cell lines were cotreated with 5 nM (UPN-1) or 10 nM (Jeko and Granta-519) bortezomib and increasing doses of GX15-070 (0.1-5 μM) for 18 hours. Cytotoxicity was evaluated by cytofluorimetric analysis of Annexin V–APC. *Doses needed to observe a synergistic effect between bortezomib and GX15-070. Results represent the mean ± SD of 3 independent experiments. (B) Mcl-1, Bak, and Noxa expression was analyzed by Western blot in 50 μg of total protein extracts from UPN-1, Jeko, and Granta-519 cells cotreated with GX15-070 and bortezomib for 18 hours. α-tubulin was also probed as an equal loading control. Western blot images are representative results from 3 independent experiments.
Synergistic effect of GX15-070 and bortezomib in MCL cell lines. (A) Cells from 3 MCL cell lines were cotreated with 5 nM (UPN-1) or 10 nM (Jeko and Granta-519) bortezomib and increasing doses of GX15-070 (0.1-5 μM) for 18 hours. Cytotoxicity was evaluated by cytofluorimetric analysis of Annexin V–APC. *Doses needed to observe a synergistic effect between bortezomib and GX15-070. Results represent the mean ± SD of 3 independent experiments. (B) Mcl-1, Bak, and Noxa expression was analyzed by Western blot in 50 μg of total protein extracts from UPN-1, Jeko, and Granta-519 cells cotreated with GX15-070 and bortezomib for 18 hours. α-tubulin was also probed as an equal loading control. Western blot images are representative results from 3 independent experiments.
In these conditions, Western blot analysis of Mcl-1, Bak, and Noxa showed that GX15-070 alone (0.1-1 μM) slightly reduced basal Mcl-1 levels without inducing significant cytotoxicity. This inhibitor was also able to overcome Mcl-1 accumulation induced by bortezomib, mainly in the cell lines (Jeko and UPN-1) where this combination exerted the highest cytotoxic effect (Figure 5B). The previously described bortezomib-induced Noxa up-regulation18 was increased after GX15-070 addition in UPN-1 and Jeko cells, where high apoptosis rates are achieved, while this Noxa up-regulation was not clearly observed in Granta-519 cells, where this combination is less effective. Total Bak levels remained constant independently of the drugs used in all MCL cell lines (Figure 5B).
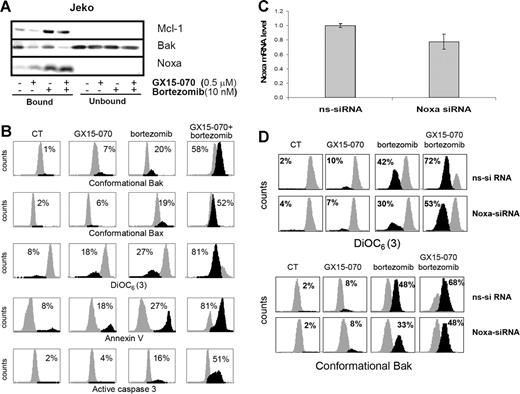
To confirm that GX15-070 facilitates bortezomib-induced apoptosis in MCL cells via Mcl-1 inhibition and Bak release, we performed Mcl-1 immunoprecipitation of Jeko cells treated with 10 nM bortezomib and/or 0.5 μM GX15-070 for 5 hours. As shown in Figure 6A, GX15-070 did not appear to modify the interaction between Noxa and Mcl-1. However, the release of Bak from Mcl-1 increased notably in the presence of GX15-070, suggesting that this BH3-mimetic compound could efficiently cooperate with Noxa to liberate Bak from its antiapoptotic counterpart (Figure 6A). As a consequence, while GX15-070 or bortezomib alone slightly activated the mitochondrial apoptotic pathway, the amplification of Bak-dependent signaling in cells treated with the combination led to enhanced Bak and Bax conformational activation, loss of ΔΨm, phosphatidylserine exposure, and caspase-3 activation (Figure 6B). Similar results were obtained in UPN-1 and Granta-519 cell lines (data not shown).
GX15-070 and bortezomib combination enhances Bak-dependent apoptotic signaling in MCL cell lines. (A) Jeko cells were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 5 hours. Mcl-1 immunoprecipitation was performed as described in “Patients, materials, and methods,” analyzing Mcl-1–bound and –unbound fractions by Western blotting for Mcl-1, Bak, and Noxa proteins. Western blot images are representative results from 3 independent experiments. (B) Jeko cells were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 18 hours. Bax/Bak conformational changes, caspase-3 activation, loss of ΔΨm, and PS exposure were analyzed as described in “Patients, materials, and methods.” The percentage inside each chart refers to the population in black. These experiments have been performed twice with similar results, and therefore 1 representative experiment is shown. (C) NOXA siRNA and nonsilencing siRNA were introduced in Jeko cells by electroporation as described in “Patients, materials, and methods.” Total RNA was isolated 6 hours after transfection. NOXA mRNA levels were determined by quantitative RT-PCR (Taqman technology) using GUS as a housekeeping gene. The results showed are the mean ± SD of 2 different experiments. (D) Jeko cells transfected with nonsilencing siRNA (ns-siRNA) and with NOXA siRNA (NOXA-siRNA) were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 18 hours. Loss of ΔΨm and Bak conformational change were analyzed as described in “Patients, materials, and methods.” The percentage inside each chart refers to the population in black.
GX15-070 and bortezomib combination enhances Bak-dependent apoptotic signaling in MCL cell lines. (A) Jeko cells were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 5 hours. Mcl-1 immunoprecipitation was performed as described in “Patients, materials, and methods,” analyzing Mcl-1–bound and –unbound fractions by Western blotting for Mcl-1, Bak, and Noxa proteins. Western blot images are representative results from 3 independent experiments. (B) Jeko cells were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 18 hours. Bax/Bak conformational changes, caspase-3 activation, loss of ΔΨm, and PS exposure were analyzed as described in “Patients, materials, and methods.” The percentage inside each chart refers to the population in black. These experiments have been performed twice with similar results, and therefore 1 representative experiment is shown. (C) NOXA siRNA and nonsilencing siRNA were introduced in Jeko cells by electroporation as described in “Patients, materials, and methods.” Total RNA was isolated 6 hours after transfection. NOXA mRNA levels were determined by quantitative RT-PCR (Taqman technology) using GUS as a housekeeping gene. The results showed are the mean ± SD of 2 different experiments. (D) Jeko cells transfected with nonsilencing siRNA (ns-siRNA) and with NOXA siRNA (NOXA-siRNA) were treated with 0.5 μM GX15-070 and/or 10 nM bortezomib for 18 hours. Loss of ΔΨm and Bak conformational change were analyzed as described in “Patients, materials, and methods.” The percentage inside each chart refers to the population in black.
To validate the suggested cooperation of Noxa with GX15-070 in bortezomib-induced apoptosis in MCL cells, we performed RNAi experiments to knock down the expression of this protein. NOXA and nonsilencing siRNA oligonucleotides were introduced in Jeko cells by electroporation using Nucleofector technology as described in “Patients, materials, and methods.” As we have previously reported, MCL cell lines are difficult to transfect;18 as a consequence, our transfection conditions only allowed us to reduce around 25% of NOXA mRNA expression (Figure 6C). The effect of Noxa down-regulation was analyzed in Jeko cells after treatment with 10 nM bortezomib and/or 0.5 μM GX15-070 by flow cytometry activated cell sorting (FACS). Interestingly, the 25% knockdown in NOXA mRNA levels reduced in a similar proportion the mitochondrial depolarization and Bak conformational change induced by bortezomib alone, as described previously.18 More important, Noxa expression knockdown also diminished the synergistic combination between GX15-070 and bortezomib (Figure 6D).
GX15-070 sensitizes primary MCL cells to bortezomib
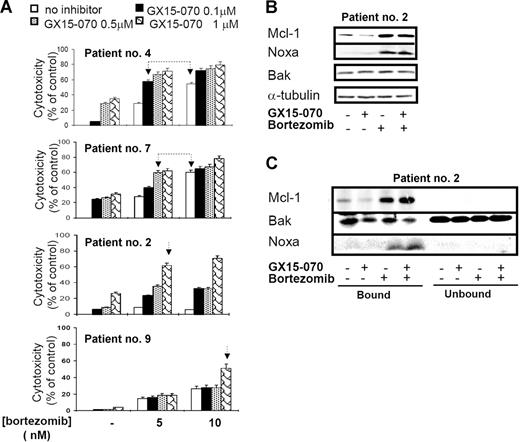
To verify these results, we tested the cytotoxic effect of GX15-070 combined with bortezomib in primary cells from 11 patients with MCL. In all patients, a synergistic effect between the 2 compounds was observed, although the doses needed to obtain this effect varied among individuals. Figure 7A shows the results obtained in cells from 4 representative patients with MCL treated with 5 or 10 nM bortezomib and/or GX15-070 (0.1-1 μM). For instance, in cells from patient no. 4, the combination of 0.1 μM GX15-070 with 5 nM bortezomib exerted a similar cytotoxicity to that observed with bortezomib used alone at 10 nM. Similarly, in cells from patient no. 7, the same cytotoxic pattern was achieved with 0.5 μM GX15-070 and 5 nM bortezomib. Most important, 1 μM GX15-070 was able to sensitize bortezomib-resistant cells from patients no. 2 and no. 9 to low doses of the proteasome inhibitor (5 and 10 nM, respectively). In these 2 patients, 200 nM bortezomib (the maximum tolerated dose) was needed to obtain a similar cytotoxic effect. In summary, GX15-070 sensitized MCL cells to low doses of bortezomib and overcame MCL resistance to this proteasome inhibitor. Furthermore, this synergistic effect was specific to neoplasic cells, since this combination therapy showed no cytotoxic effect in PBMCs from healthy donors treated in vitro with doses of 2 μM GX15-070 plus 10 nM bortezomib (data not shown).
Synergistic interaction between GX15-070 and bortezomib in MCL primary cells. (A) Primary cells from 4 representative patients with MCL were treated with 5 or 10 nM bortezomib and increasing doses of GX15-070 (0.1-1 μM) for 18 hours. Cytotoxicity was evaluated by cytofluorimetric analysis of Annexin V–APC. Linked arrows in patients no. 4 and no. 7 indicate equivalent cytotoxicities. Single arrows in patients no. 2 and no. 9 indicate the sensitizing effect of GX15-070 in these bortezomib-resistant patients. Results represent the mean ± SD of 3 independent experiments (B) Mcl-1, Bak, and Noxa expressions were analyzed by Western blot in 50 μg of total protein extracts from cells of patient no. 2 cotreated with 1 μM GX15-070 and/or 5 nM bortezomib for 18 hours. α-tubulin was also probed as an equal loading control. (C) Cells from patient no. 2 were treated with 1 μM GX15-070 and/or 5 nM bortezomib for 5 hours. Mcl-1, Bak, and Noxa proteins were analyzed in Mcl-1–immunoprecipitated and nonimmunoprecipitated fractions as described in “Patients, materials, and methods.” Western blot images are representative results from 3 independent experiments.
Synergistic interaction between GX15-070 and bortezomib in MCL primary cells. (A) Primary cells from 4 representative patients with MCL were treated with 5 or 10 nM bortezomib and increasing doses of GX15-070 (0.1-1 μM) for 18 hours. Cytotoxicity was evaluated by cytofluorimetric analysis of Annexin V–APC. Linked arrows in patients no. 4 and no. 7 indicate equivalent cytotoxicities. Single arrows in patients no. 2 and no. 9 indicate the sensitizing effect of GX15-070 in these bortezomib-resistant patients. Results represent the mean ± SD of 3 independent experiments (B) Mcl-1, Bak, and Noxa expressions were analyzed by Western blot in 50 μg of total protein extracts from cells of patient no. 2 cotreated with 1 μM GX15-070 and/or 5 nM bortezomib for 18 hours. α-tubulin was also probed as an equal loading control. (C) Cells from patient no. 2 were treated with 1 μM GX15-070 and/or 5 nM bortezomib for 5 hours. Mcl-1, Bak, and Noxa proteins were analyzed in Mcl-1–immunoprecipitated and nonimmunoprecipitated fractions as described in “Patients, materials, and methods.” Western blot images are representative results from 3 independent experiments.
In primary MCL cells, GX15-070 alone slightly reduced basal Mcl-1 levels. After a bortezomib combination, a modest decrease of Mcl-1 was detected in accordance with the degree of apoptosis. As described here in MCL cell lines, bortezomib-induced Noxa up-regulation was moderately increased by GX15-070 and total Bak levels did not vary with any treatment (Figure 7B). Importantly, Mcl-1 immunoprecipitation of primary cells showed that GX15-070 and bortezomib cotreatment enhanced Bak release from Mcl-1 when compared with that observed using either compound individually (Figure 7C). All these results agreed with those described in MCL cell lines and supported the cooperation between Noxa and GX15-070.
Discussion
Bcl-2 family proteins are critical regulators of cell life and death. In mammalian cells, the prosurvival members oppose 2 proapoptotic groups: the Bax group (Bax, Bak, and Bok) and the BH3-only proteins. The life-death switch is flipped by the BH3-only proteins.11,29 High levels of Bcl-2, Bcl-XL, and Mcl-1 have been previously described in MCL cells and in a wide variety of human cancers.14–17,30 High expression of the antiapoptotic Bcl-2 proteins mediates the resistance of cancers to multiple cellular stress by blocking the cell-death signals they triggered.31 This fact has led to the development of novel agents targeting Bcl-2 antiapoptotic proteins. Several approaches have been described, including BCL-2 antisense oligonucleotides that shut down Bcl-2 expression.32 In this sense, it has been reported that the combination of bortezomib and the BCL-2 antisense molecule oblimersen sensitizes MCL cells to cyclophosphamide.33 Currently, a favored strategy for Bcl-2 antagonism is based on small molecules that target Bcl-2 antiapoptotic proteins by mimicking a BH3 domain. Hence, several compounds have been isolated or chemically synthesized, showing different binding specificity and affinity for these proteins and promoting apoptosis.34
Among them, GX15-070 is a polypirrole small-molecule pan–Bcl-2 inhibitor that fits into the groove of prosurvival Bcl-2 members mimicking a BH3-only protein. GX15-070 has been found to bind to the antiapoptotic members Mcl-1, Bcl-2, Bcl-XL, and Bcl-w with high affinity.19 The therapeutic effect of GX15-070 has been described in a variety of hematologic malignancies, including CLL and myeloid malignancies.35–37
In this report, we demonstrate that GX15-070 in vitro exhibits activity in MCL cell lines and primary MCL specimens when used as a single agent at low micromolar concentrations. GX15-070 cytotoxic activity is time and dose dependent, and shows 2 important characteristics. First, it is effective in cells bearing defective DNA damage-sensor genes and alterations in cell-cycle checkpoints. This is particularly important in MCL cells, where these control points are often impaired and are related to aggressive forms of the tumor.2,4 Furthermore, we have also demonstrated that GX15-070 is also active in samples from relapsed patients (patients no. 2 and no. 8). The second significant feature of GX15-070 is that it exerts no significant cytotoxicity in CD3+ and CD19+ lymphocytes from PBMCs of healthy donors or CD19+ cells from reactive tonsils, suggesting its potential benefit in clinical studies.
Interestingly, we have observed that GX15-070 cytotoxicity inversely correlates with the levels of the targeted antiapoptotic Bcl-2 proteins. Since GX15-070 functions as an antagonist of prosurvival Bcl-2 proteins, it is conceivable that higher drug doses are needed to inhibit elevated levels of these proteins. In the same context, it has been recently described that the BH3-mimetic ABT-737, which exhibits low affinity for Mcl-1, is more efficient in those tumors expressing low levels of this protein.38,39
It has recently been proposed that only certain Bcl-2 protein pairs associate inside cells.12 Indeed, Bim and Puma engage all prosurvival Bcl-2 proteins, while Bad couples preferentially to Bcl-2, Bcl-XL, and Bcl-w, and Noxa binds only to Mcl-1 and A1. Moreover, it is now known that proapoptotic Bak is sequestered by the antiapoptotic Mcl-1 and Bcl-XL, its displacement by BH3-only proteins being required for apoptosis onset.13 Evidence of Mcl-1/Bak complex disruption has been reported in CLL cells incubated with GX15-070 ex vivo.19 Our immunoprecipitation experiments demonstrate that GX15-070 exerts an inhibitory effect on Mcl-1/Bak and Bcl-XL/Bak interactions in MCL cells. This event allows Bak displacement from its antiapoptotic counterparts to occur. The mitochondrial apoptotic pathway is then directly activated, detecting Bax and Bak conformational changes, loss of ΔΨm, phosphatydilserine exposure, and caspase-3 activation. Our observation that the pancaspase inhibitor z-VAD-fmk is unable to block Bax/Bak conformational changes and mitochondrial depolarization confirms that GX15-070 acts upstream on the mitochondria, and that caspases participate in a postmitochondrial phase.
We have previously described that bortezomib induces a marked accumulation of Mcl-1 in MCL, as it prevents its degradation by proteasome.18 Although this event does not inhibit the bortezomib cytotoxic effect, it may well delay the apoptosis induction.40 Thus, to increase the effectiveness of bortezomib, it is attractive to consider the idea of adding a second agent that can interfere with Mcl-1 accumulation. In this context, we have investigated the potentially beneficial effect of combining GX15-070 with bortezomib in MCL. We have found that low doses of GX15-070 reduce Mcl-1 protein levels that could be linked to the BH3-mimetic nature of this compound. We therefore hypothesize that GX15-070, by binding to Mcl-1, might target this protein for proteasomal degradation similarly to the mechanism described for Noxa.13 However, the reduction of Mcl-1 protein levels observed in cells cotreated with bortezomib and GX15-070, compared with those observed in cells treated with bortezomib alone, must be dependent on caspases since the 26-kDa Mcl-1 cleaved form41 was detected (data not shown). As previously described, bortezomib induces Noxa up-regulation, and this BH3-only protein couples to Mcl-1, inducing Bak release.18 In the present work, the analysis of Mcl-1/Bak interactions and Noxa knock-down experiments in MCL cells support the hypothesis that GX15-070, due to its BH3-mimetic nature, could efficiently cooperate with Noxa in the activation and displacement of Bak from Mcl-1 and the subsequent mitochondrial injury and apoptosis. As a consequence, GX15-070 sensitizes MCL cells to low doses of bortezomib and, most important, this compound is able to overcome the in vitro resistance of primary MCL cells to bortezomib. These results are of special interest since, although bortezomib as a single agent provides significant clinical activity, an important group of patients remain resistant.5–8 For this reason, GX15-070 and bortezomib combination therapy may represent a good treatment option in patients with MCL. The efficacy of combining bortezomib with a Bcl-2 inhibitor has also been described in multiple myeloma using HA14-142 and the BH3-mimetic ABT-737.43 However, GX15-070 appears to be a more suitable option for this combination since HA14-1 is only capable of inhibiting Bcl-2,44 and ABT-737 uncovers Mcl-1 inhibition.39
In conclusion, this is 1 of the first studies providing evidence that Bcl-2 family proteins are suitable targets for the treatment of MCL. This new strategy that combines GX15-070 with bortezomib demonstrates for the first time that GX15-070 synergizes with bortezomib in vitro and sensitizes MCL cells to low doses of this proteasome inhibitor. We proposed a mechanism of action in which GX15-070, by neutralizing bortezomib-induced Mcl-1 accumulation, cooperates with Noxa to induce Bak displacement from its antiapoptotic counterpart. This drug combination circumvents 1 of the drawbacks of proteasome inhibition-based therapies, validating this approach as a rational drug combination therapy. Finally, our current results support further in vivo studies that may well provide substantial clinical benefit in the treatment of MCL patients.
Authorship
Contribution: P.P.-G. contributed to the design and conception of the study, performed the research, analyzed and interpreted the data, and participated in writing the paper; G.R. performed research, analysis, and interpretation of data and contributed critically to the revision of the article; N.V. contributed to the analysis and interpretation of data and the revision of the article; E.C. contributed to the design of the study and to the revision of the manuscript critically, and provided supervision of the study; and D.C. was the principal investigator and contributed to the design of the study, interpretation of data, and the writing of the paper. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dolors Colomer, Hematopathology Unit, Hospital Clinic, Villarroel 170, 08036 Barcelona, Spain; e-mail: dcolomer@clinic.ub.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge Dr Gordon C. Shore (Geminx Biotechnologies) for kindly providing the compound GX15-070 and for valuable discussion of results and revision of this manuscript. We also appreciate technical advice on immunoprecipitation experiments from Dr Mark Watson (Geminx Biotechnologies). Finally, we thank N. Peiró and S. Cabezas for their technical assistance.
This work was supported by Fondo Investigaciones Sanitarias (FIS 03/0398) and Ministerio de Educación y Ciencia (SAF 06/8850) (D.C.), European Commission contracts SLMM-CT-2004-503351, the Lymphoma Research Foundation, and Redes temáticas de Investigación Cooperativa en Salud RDOC/0020/0014, Instituto de Salud Carlos III. P.P.-G. holds a postdoctoral contract from the Juan de la Cierva program (Ministerio de Educación y Ciencia [MEC]). G.R. holds a postdoctoral contract from the Contratació i reincorporació de doctors estrangers, Generalitat de Catalunya (c-RED) program.