Abstract
The 2 subsets of chronic lymphocytic leukemia (CLL), of worse or better prognosis, likely derive from pre-GC unmutated B cells, or post-GC mutated B cells, respectively. Different clinical behavior could relate to the ability of tumor cells to respond to surface (sIg)–mediated signals. Unmutated cases (U-CLL) have an increased ability to phosphorylate p72Syk in response to sIgM ligation compared to mutated cases (M-CLL). We now confirm and further investigate this differential signaling in a large cohort by [Ca2+]i mobilization. Cases responding to sIgM ligation express higher levels of CD38, ZAP-70, and sIgM. However, CD38 does not influence signaling in vitro or associate with response in bimodal CD38-expressing cases. Similarly, ZAP-70 expression is not required for response in either U-CLL or M-CLL. Strikingly, partially or completely anergized sIgM responses from each subset can recover both sIgM expression and signal capacity spontaneously in vitro or following capping/endocytosis. This provides direct evidence for engagement of putative antigen in vivo. Signaling via sIgD differs markedly being almost universally positive in both U-CLL and M-CLL, with no association with CD38 or ZAP-70 expression. Downstream signaling pathways, therefore, appear intact in CLL, locating anergy to sIgM, mainly in M-CLL. Integration of differential isotype-specific effects mediated by (auto)antigen may determine tumor behavior.
Introduction
The division of chronic lymphocytic leukemia (CLL) into 2 major subsets, based on the presence or absence of a significant level of somatic mutations in the immunoglobulin variable (V) region genes has had a major effect on our understanding of this B-cell malignancy.1–3 The difference in clinical behavior, with unmutated CLL (U-CLL) showing a worse prognosis, is clearly relevant for clinical management, and has led to an intensive search for more easily measurable markers that parallel V-gene status. Meanwhile the subtle differences in the biology of the tumor cells that dictate the variable course are being sought. Gene expression analysis detected up-regulation of several cell cycle-associated genes in U-CLL.4 A powerful discriminator was identified as the protein tyrosine kinase ζ-associated protein 70 (ZAP-70), with a 4- to 5-fold increase in U-CLL.5 Expression at the protein level was also shown to be highly associated with U-CLL,6 and measurement by flow cytometry appears to provide a clinically relevant surrogate marker for prognosis.7 ZAP-70 is a member of the Syk family of tyrosine kinases and is known to be a critical component of the T-cell receptor signaling pathway.8 It was an unexpected component of a B-cell malignancy because it was thought to be confined to T cells and natural killer (NK) cells.9 However, ZAP-70 can be expressed in normal B cells,9 and the high level of expression in U-CLL, but not in mutated CLL (M-CLL), added to the interest in possible differences in signaling pathways between the subsets.
The B-cell receptor (BCR) is critical for survival of normal peripheral B cells.10 It is also likely to have a role in maintenance or growth of tumors derived from mature B cells, which are rarely sIg−. In CLL, levels are low compared to normal B lymphocytes and to other B-cell malignancies. Based on observations of self-reactive B cells in double transgenic mice continuously expressing antigen,11 it has been suggested that the low levels reflect an anergic state consequent on interaction with antigen in the absence of T-cell help.12 However, no direct evidence for this had been obtained.
The BCR includes sIg and the Ig-α/Ig-β heterodimer (CD79a and CD79b) associated noncovalently in a 1:1 ratio.13 Following engagement of antigen, receptors aggregate leading to phosphorylation of the Ig-α/Ig-β ITAM motifs by Src-family tyrosine kinases, including Lyn.14 Recruitment and phosphorylation of other kinases, with Syk being an important component, then activates intracellular signaling cascades. Phosphorylation of Syk therefore provides an indicator of membrane proximal events that depend on the structural integrity and oligomeric form of the BCR. It is this ability to signal via Ig-α/Ig-β that is critical for survival of mature normal B cells.15 We16 and others17 have reported that this early response to ligation of sIgM in CLL varies between the subsets, with an increased tendency for U-CLL to phosphorylate Syk. A parallel phosphorylation of ZAP-70 with recruitment to the BCR has also been observed.17 Our early data also indicated that 10 of 14 cases of M-CLL, which failed to signal via sIgM, were capable of signaling through sIgD.16
It is known that phosphorylated Syk itself phosphorylates several proteins including B-cell linker protein (BLNK), which then acts as a docking site for further proteins of the signaling cascade. One of these is phospholipase C γ (PLCγ), an enzyme that hydrolyzes polyphosphoinositide, producing inositol 1,4,5-triphosphate and diacylglycerol. The effect is to increase intracellular calcium [Ca2+]i with subsequent activation of protein kinase C and Ras. Because the rise in [Ca2+]i is a key event in the response to ligation of the BCR in CLL, and correlates with the induced phosphorylation of BLNK and PLCγ,18 we have used this as a more quantitative downstream measure to analyze events in single cells and to compare responses in the 2 subsets of CLL.
We find that the number of cells within a clone able to respond to sIgM ligation in vitro varies between cases but is generally higher in U-CLL. There is, therefore, an inevitable association with expression of CD38 and ZAP-70. However, we detected no direct influence of CD38 expression on signaling capacity. Also, although ZAP-70 remains a potential modulator of signaling, expression as currently measured is not a prerequisite, especially for M-CLL. A much stronger determinant of response in M-CLL is the level of expression of sIgM.
Strikingly, spontaneous recovery of signal capacity and of sIgM expression occurs in many cases on incubation in vitro. Recovery can also occur following removal of sIgM by anti-immunoglobulin–induced endocytosis. In both situations, reversal provides the first direct evidence for engagement of a BCR ligand, possibly an autoantigen, in vivo. The term “anergy” has been used widely to describe the tumor cell status in CLL.12 We are now better able to define this state as a reduced responsiveness to sIgM ligation, as compared to sIgD. Reversibility further refines the definition and points to antigen-induced modulation of sIgM, occurring in vivo. Differing levels of consequent anergy in U-CLL and M-CLL may influence malignant behavior.
Patients, materials, and methods
Patient material
Approval was obtained by the Southampton University Hospitals National Health Service (NHS) Trust from the Southampton and South West Hampshire Research Ethics Committee. Informed consent was provided in accordance with the Declaration of Helsinki. Blood was obtained from 112 patients with typical CLL who attended the hematology outpatient clinics at the Royal Bournemouth Hospital, Hammersmith Hospital, Leicester Royal Infirmary, Portsmouth Hospital, Southampton General Hospital, and the Royal Wolverhampton Hospitals NHS Trust. A total of 104 of the 112 were assigned to stage A, 5 to stage B, and 3 to stage C. All were untreated apart from 5 patients who had been treated previously but who had received no chemotherapy for more than 6 months. Peripheral blood mononuclear cells (PBMCs) were isolated by Lymphoprep gradient centrifugation (Axis-Shield Diagnostics, Dundee, United Kingdom) washed, and cryopreserved in RPMI 1640 medium supplemented with 10% DMSO and 15% fetal calf serum. CLL samples were thawed in complete RPMI 1640 medium containing 10% fetal calf serum, 2 mM glutamine, and 1% sodium pyruvate. Cells were pelleted by centrifugation, resuspended in complete medium, and counted. Cells were allowed to recover by incubation for 1 hour at 37°C. Cell viability was 90% to 100% and the proportion of contaminating normal B cells in each CLL sample as determined by fluorescence-activated cell sorting (FACS; CD19+CD5−) was less than 1.0%.
Immunoglobulin gene analysis
Flow cytometry
Thawed lymphocytes were stained for 30 minutes at 4°C with anti-CD5 PerCP-Cy5.5, anti-CD19 APC, and relevant FITC/PE-labeled antibodies. For CD38 analysis, anti-CD38 PE (clone HB7; BD Biosciences, Oxford, United Kingdom) was used, and cases with at least 30% expressing tumor cells were designated as positive.2 For sIg analysis, rabbit F(ab′)2 anti-IgM PE and rabbit F(ab′)2 anti-IgD FITC (Dako, Ely, United Kingdom) were used. Data were acquired on a BD FACSCalibur with CellQuest Pro software. Mean fluorescence intensity (MFI) and percent positive staining were measured relative to the appropriate isotype control for the CD5+CD19+ lymphocyte population. Determination of ZAP-70 status was carried out as described by Crespo et al.7 Cases where 20% or more of tumor cells expressed ZAP-70 were designated as ZAP-70+.7
Measurement of intracellular calcium
Calcium mobilization was measured using the fluorogenic probe, Fluo3-AM.21 Total lymphocytes were assayed to minimize cell handling, and complete RPMI 1640 medium was used to optimize viability and allow assessment of calcium mobilization from both intracellular and extracellular stores.22 PBMCs at 107 cells/mL were incubated with 4 μM Fluo3-AM (Invitrogen, Paisley, United Kingdom) and 0.02% (vol/vol) Pluronic F-127 (Sigma, Poole, United Kingdom) for 30 minutes at 37°C. Cells were then washed and resuspended at 5 × 106 cells/mL at room temperature. Cells (250 μL) were warmed to 37°C for 5 minutes prior to acquisition of background fluorescence (ie, of unstimulated cells), followed by addition of 20 μg/mL goat F(ab′)2 anti–human IgM or IgD (Southern Biotechnology, Cambridge, United Kingdom) and data acquisition for a further 5 minutes. Data were acquired on a BD FACScalibur and analyzed using FlowJo software (Tree Star, Ashland, OR). To confirm that absence of anti-IgM/IgD–induced [Ca2+]i fluxes observed in some samples was not due to technical artefacts, these samples were subsequently treated with 1 μM ionomycin (Sigma), which elicited robust calcium fluxes in all samples.
To calculate the percentage of cells that exhibited increased fluorescence following addition of anti-IgM/IgD we first established a background fluorescence threshold (T) for each sample at the fluorescence intensity of the 85th percentile of unstimulated cells (Figure 1); pilot experiments demonstrated that this level most clearly separated background and anti-immunoglobulin stimulated fluorescence. We then calculated the peak percentage of cells that exhibited an increase in fluorescence intensity above T following treatment with anti-IgM/IgD. The percentage of cells responding was corrected to take account of non-B cells (usually < 10%) in the live lymphocyte gate.
Variable levels of [Ca2+]i induced in cases of CLL by ligation of sIgM. Lymphocytes from 3 cases of CLL were labeled with the calcium-sensitive dye Fluo-3–AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Density plots illustrate (A) complete response, (B) no response, and (C) partial response. In panel D, responses were quantitated by determining the peak percentage of CLL cells with fluorescence intensity above the unstimulated threshold (T). T was set at the 85th percentile of the fluorescence intensity of unstimulated cells, as shown.
Variable levels of [Ca2+]i induced in cases of CLL by ligation of sIgM. Lymphocytes from 3 cases of CLL were labeled with the calcium-sensitive dye Fluo-3–AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Density plots illustrate (A) complete response, (B) no response, and (C) partial response. In panel D, responses were quantitated by determining the peak percentage of CLL cells with fluorescence intensity above the unstimulated threshold (T). T was set at the 85th percentile of the fluorescence intensity of unstimulated cells, as shown.
Anti-IgM–induced calcium mobilization was initially measured in a total of 112 samples, with reproducible results (mean SD ± 3.4%). Because some samples exhibited calcium fluxes in a relatively small percentage of cells (5%-15%), further assays were performed to determine the robustness of the measurements in this range. Reproducibility was critical for subsequent use of a cutoff value (5%) to assign cases as responders or nonresponders. Depending on availability of material, a minimum of 2 and up to 9 measurements were made for samples of nonresponders (n = 14) or modest responders (n = 14). The measurements were reproducible (mean SD = ± 2.6%). Of the 112 cases, 5 had a SD crossing the 5% cutoff, rendering it impossible to confidently assign a signaling status. These 5 samples were excluded from analyses requiring assignment of signaling status.
Endocytosis and re-expression of sIg
Cells were either untreated or treated with 20 μg/mL polyclonal goat anti–light chain F(ab′)2 (Southern Biotechnology) for 1 hour at 37°C. Both untreated and treated cells were washed twice in complete RPMI and distributed to 24-well plates. At the indicated time points, cells were removed to determine both sIgM and sIgD MFI and the ability of the cells to increase [Ca2+]i via ligation of each isotype.
Statistics
When comparing CLL subgroups, P values were calculated using the Mann-Whitney test (2-tailed, 95% CI). Statistical analyses were performed using GraphPad Prism software (San Diego, CA).
Results
Assessment of sIgM-mediated signaling capacity.
We measured signaling responses at the single-cell level by analyzing the increase in [Ca2+]i by flow cytometry. The calcium flux in response to cross-linking of sIgM varied between cases, ranging from a complete response, where all cells showed a significant increase in fluorescence (Figure 1A) to no response (Figure 1B). A number of cases gave a partial response, where only a proportion of the tumor cells showed an increase in fluorescence (Figure 1C). To demonstrate this, we calculated the percentage of cells showing an increased [Ca2+]i in each sample, rather than the median or mean increase in fluorescence. A cutoff of 5% was selected for 2 reasons: first, as the lowest level of a reproducible response, and second, as a distinguishing feature of a responding tumor cell population from that of nonresponders, which were totally and reproducibly unable to respond.
Because all our samples were frozen, we checked if there was any effect on signaling due to the freezing and thawing process. Four random samples with different levels of signal capacity were assessed for [Ca2+]i responses following ligation of sIgM or sIgD. Samples were assessed either fresh or after freezing/thawing. No significant difference in responses was detected, with remarkably consistent values of [Ca2+]i obtained (data not shown).
For 2 patients (232 and 201), samples were available at 2 separate time points, 1 year and 2 years apart, respectively. The sIgM-Ca2+ signaling status remained unchanged in both with the weak signaller remaining weak (7% versus 5%) and the strong signaller remaining strong (68% versus 54%). Overall, the ability to mobilize calcium in response to ligation of sIgM appeared to be reproducible in terms of the assay, and, in the limited available assessment, over time in individual patients.
We compared signaling responses as measured by [Ca2+]i and by immunoprecipitation of phosphorylated Syk16 in 27 samples. Overall, the calcium signaling response to anti-IgM correlated well with the fold increase in Syk phosphorylation (Spearman r = 0.8056, P < .001, data not shown). However, of 13 samples classified as nonsignallers by analysis of Syk phosphorylation, 6 showed increases in [Ca2+]i in more than 5% of cells. By contrast, of 14 samples classified as signallers by analysis of Syk phosphorylation, 13 also exhibited more than 5% [Ca2+]i increase in anti-IgM–treated cells. Thus, analysis of Syk phosphorylation by immunoprecipitation, which measures the average increase in a cell population, is therefore a less sensitive method compared to analysis of [Ca2+]i fluxes, which can detect significant responses in relatively small subpopulations of cells.
Comparison of subsets of CLL
The percentage of cells responding to sIgM ligation by calcium flux was highly variable between cases but, using the cutoff point of 5% of responding cells, 59 of 112 (53%) cases were designated as “responders.” U-CLL had significantly more responders than M-CLL (Figure 2A) with a majority of cases (30 of 41; 73%) showing a [Ca2+]i flux as compared to 29 of 71 (41%) of M-CLL (P = .013). Cases of U-CLL appeared variable in terms of signal capacity (Figure 2A), but heterogeneity in M-CLL was even more striking. Levels of sIgM-[Ca2+]i signal were also increased in the CD38+ group (P = .001) (Figure 2B) and in the ZAP-70+ group (P = .031; Figure 2C). Analysis of this large cohort confirms that sIgM-mediated signaling is more commonly detected in U-CLL and correlates with the other markers of U-CLL, CD38 and ZAP-70. We next sought to determine factors that might influence signaling in each subset.
sIgM-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgM cross-linking was determined for 112 patients with CLL. The percentage of cells responding in subgroups defined by (A) somatic mutational status, (B) CD38 expression, (C) ZAP-70 expression status, and (D) sIgM MFI was assessed. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative. For sIgM, the median MFI of all the samples in the cohort was determined to be 50. sIgMhi samples were those with an MFI greater than 50, whereas sIgMlo samples had an MFI below 50. Horizontal bars indicate median values.
sIgM-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgM cross-linking was determined for 112 patients with CLL. The percentage of cells responding in subgroups defined by (A) somatic mutational status, (B) CD38 expression, (C) ZAP-70 expression status, and (D) sIgM MFI was assessed. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative. For sIgM, the median MFI of all the samples in the cohort was determined to be 50. sIgMhi samples were those with an MFI greater than 50, whereas sIgMlo samples had an MFI below 50. Horizontal bars indicate median values.
Influence of known prognostic factors
CD38.
To assess the influence of the level of CD38 expression, we used the cutoff of 5% to define sIgM-mediated calcium signaling capacity (sIgM-Ca2+) as positive or negative. As anticipated (Figure 2B), CD38 expression was higher in the sIgM-Ca2+ responders (Figure 3A). Superimposition of mutational status reveals that responders in U-CLL are usually CD38+, whereas the small number of nonresponders tend to be CD38− (Figure 3B). Although expression was heterogeneous in the nonresponders, this difference was significant. Figure 3B also confirms previous data16 showing that there are more CD38+ cases (21 of 38) in U-CLL than in M-CLL (9 of 69). In M-CLL, where the majority are CD38−, the 4 cases expressing the highest levels of CD38 (77%-97% positive) were responders, but 4 further cases expressing significant levels (41%-66%) were not. In M-CLL therefore, CD38 did not have statistically significant impact on signaling (Figure 3B). The overall association of response with CD38 (Figure 2A) is therefore determined mainly by the fact that U-CLL tends to be CD38+.
Association of sIgM-mediated signal responses with CD38, ZAP-70, and sIgM expression in subsets of U-CLL and M-CLL. Cases (107) with signaling responses above (S) or below (N) the cutoff of 5% response were analyzed for expression of (A) CD38, (C) ZAP-70, or (E) sIgM by flow cytometry. Superimposition of mutational status (U or M) was then used to assess differential expression of (B) CD38, (D) ZAP-70, or (F) sIgM in responders or nonresponders within U-CLL or M-CLL. Horizontal bars indicate median values. Statistical analysis was done using the Mann-Whitney test (2-tailed, 95%CI). The dotted lines show the cutoff values, 30% and 20%, respectively, used to define (A-B) CD38 and (C-D) ZAP-70 status.
Association of sIgM-mediated signal responses with CD38, ZAP-70, and sIgM expression in subsets of U-CLL and M-CLL. Cases (107) with signaling responses above (S) or below (N) the cutoff of 5% response were analyzed for expression of (A) CD38, (C) ZAP-70, or (E) sIgM by flow cytometry. Superimposition of mutational status (U or M) was then used to assess differential expression of (B) CD38, (D) ZAP-70, or (F) sIgM in responders or nonresponders within U-CLL or M-CLL. Horizontal bars indicate median values. Statistical analysis was done using the Mann-Whitney test (2-tailed, 95%CI). The dotted lines show the cutoff values, 30% and 20%, respectively, used to define (A-B) CD38 and (C-D) ZAP-70 status.
Identification of “bimodal” cases in which the tumor clone can be divided into discrete CD38+ and CD38− populations23 allowed assessment of the influence of expressed CD38 on sIgM-mediated signaling. As a prerequisite, we showed in 4 of 4 cases (3 U-CLL and 1 M-CLL), of which 3 of 4 were sIgM-Ca2+ positive, that the binding of the detecting HB7 anti-CD38 monoclonal antibody (mAb) per se did not induce any detectable [Ca2+]i responses (data not shown). We also showed in 5 cases (3 U-CLL and 2 M-CLL), of which 4 were sIgM-Ca2+ positive, that addition of anti-CD38 mAb prior to measuring sIgM-mediated signal had no effect (data not shown). Seven cases with bimodal expression of CD38 were then investigated for the ability of the 2 populations to respond to sIgM engagement. An example is illustrated in Figure 4A. Both the percentage of responding cells (Figure 4B) and the fold-increase in [Ca2+]i in those cells (data not shown) was essentially identical in the CD38+ and CD38− populations. Similar results were obtained for all 7 cases, with a slight reduction in response in CD38− cells in only one case, and are summarized (Figure 4C). Overall, expressed CD38, therefore, does not directly influence responsiveness to sIgM cross-linking but appears to be an independent feature more frequently found in U-CLL.
sIgM-mediated signal responses in CD38+ or CD38− subpopulations of cases with bimodal expression. (A) Bimodal expression of CD38 in a representative case. (B) sIgM-mediated [Ca2+]i responses, as percent of responding cells in CD38+ and CD38− subpopulations. (C) Cumulative responses in 7 bimodal cases.
sIgM-mediated signal responses in CD38+ or CD38− subpopulations of cases with bimodal expression. (A) Bimodal expression of CD38 in a representative case. (B) sIgM-mediated [Ca2+]i responses, as percent of responding cells in CD38+ and CD38− subpopulations. (C) Cumulative responses in 7 bimodal cases.
ZAP-70.
A similar analysis of ZAP-70 expression, which showed a higher expression overall in sIgM-Ca2+ responders (Figures 2C and 3C), was carried out. Superimposition of mutational status revealed no apparent influence of ZAP-70 expression on sIgM-signaling response in either U-CLL or M-CLL (Figure 3D). In U-CLL, most cases expressed ZAP-70 (27 of 37) and were responders, but a few ZAP-70− cases were evident in both responders and nonresponders (Figure 3D). For M-CLL, known to be largely ZAP-70−,6,24 11 of 67 cases were positive in our study. These few cases included responders (6) and nonresponders (5), and the majority of responders expressed little or no ZAP-70 (Figure 3D). It appears, therefore, that the association of ZAP-70 expression with signaling ability is again due mainly to the fact that this group is largely within U-CLL. It appears not to be a signaling discriminator within each subset.
Influence of sIgM expression
The level of sIgM expression is clearly a determinant of sIgM-Ca2+ signaling in the whole cohort of cases (Figures 2D and 3E). Dissection into U-CLL and M-CLL revealed no significant association with signal capacity in U-CLL (Figure 3F). This suggests that, in addition to sIgM levels, there may be other factors influencing signal capacity. In M-CLL, however, there was a clear division into responders with sIgM levels comparable to U-CLL and nonresponders with reduced sIgM expression (Figure 3F). Expression of sIgM is, therefore, a determinant of sIgM-Ca2+ response in M-CLL, and these cases account for the overall association of nonsignallers with reduced sIgM levels. However, even within M-CLL, there were overlapping cases with similar sIgM expression but different signal capacity (Figure 3F). We identified 6 cases with identical sIgM levels (MFI in the 30-35 range) but with sIgM-Ca2+ responses of 11%, 9%, and 30% of cells responding (positive responses) and 0%, 0%, and 2% of cells responding (negative responses). These results are consistent with other factors influencing signal capacity.
In M-CLL, there was an exceptional cohort of 7 cases that expressed high levels of sIgM (MFI > 200), with positive sIgM-Ca2+ responses (Figure 3F). We investigated these for expression of other prognostic markers. We found that 4 of 7 expressed CD38 and 1 of 6 evaluable cases expressed ZAP-70. It appears therefore that there is no obvious correlation with these markers.
Surface IgD-mediated signaling capacity (sIgD-Ca2+) in defined CLL subsets
To assess at which level signal competence was compromised in cases failing to respond to ligation of sIgM, we investigated [Ca2+]i flux following ligation of sIgD. The majority of cases of both U-CLL and M-CLL were able to respond to engagement of sIgD (cutoff of ≥ 5%) and there was no apparent difference between the 2 subsets (Figure 5). Among U-CLL, 37 of 38 cases responded beyond the cutoff point with levels mainly (33 of 38) at 15% to 100%, and only 3 of 38 at 5% to 15% and 1 of 38 at 0%. Among M-CLL, 64 of 68 cases responded, with 60 of 68 at 15% to 100% and 4 of 68 at 5% to 15%. There was, therefore, no significant difference between U-CLL and M-CLL in either the level or the frequency of cases responding to sIgD ligation. Because almost all cases were sIgD-Ca2+ positive, there was no influence of the expression of CD38 (P = .876) or ZAP-70 (P = .335). However, the few signal-negative cases (1 U-CLL and 4 M-CLL) were all CD38− and three quarters of the M-CLL (but not the single U-CLL) cases were ZAP70−. These results indicate that the downstream signaling machinery is intact in 95% of CLL cases overall. Anergy therefore is specific to each sIg isotype, is more common in sIgM, and occurs at the membrane proximal level
sIgD-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgD cross-linking was determined for 111 patients with CLL. The percentage of cells responding in U-CLL or M-CLL was assessed. Horizontal bars indicate median values. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative.
sIgD-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgD cross-linking was determined for 111 patients with CLL. The percentage of cells responding in U-CLL or M-CLL was assessed. Horizontal bars indicate median values. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative.
One explanation for the differential effects of ligating sIgM or sIgD could be a lower ability of the exogenous anti-μ antibody to bind to and cross-link the target sIgM. Although both antibodies were and used at saturation, it was necessary to check this point. To maximize overall avidity, we added a secondary antibody (polyclonal rabbit anti–goat IgG F(ab′)2) to bound (Fab′)2 anti-μ antibodies. In 2 cases that had failed to signal, this did not induce a signal. Similarly, in 2 cases that were signal responsive, the level of response did not change (data not shown).
Cross-talk between sIgM and sIgD
In cases that were sIgM signal-incompetent and sIgD signal-competent, the effect of simultaneous ligation of sIgM on the signal mediated via sIgD was assessed. In 3 of 3 cases, selected for significant expression of sIgM (MFI = 51, 43, 67) coaddition of anti-IgM did not affect the signal via sIgD (data not shown). This indicates that there is no detectable negative influence from the anergized sIgM-mediated pathway on the nonanergized sIgD-mediated pathway.
Spontaneous recovery of sIgM-Ca2+ signal capacity by incubation in vitro
Initial sIgM-Ca2+ signal responses were highly variable between patients, especially within the M-CLL subset, where the majority appeared to be low (Figure 2A). Because the apparent anergy may be mediated by engagement of antigen in vivo, we explored the possibility of reversing this state by incubating tumor cells in vitro. We assessed the effect of incubation on the level of response both in cases already showing some degree of response “responders” (7 cases), and in those with less than 5% initial response “nonresponders” (7 cases). Recovery was considered positive if the level increased by more than 6% at 2 time points or, if 2 time points were not available, more than 10%.
Although the majority of cases of U-CLL are responders, we also included nonresponders in the analysis. Similarly for M-CLL, we selected both responders and nonresponders.
In a total of 10 of 14 cases (4 of 4 U-CLL, 6 of 10 M-CLL), there was clear recovery of sIgM-Ca2+ signal capacity generally evident by 24 hours and usually reaching a plateau at 48 hours (Figure 6A-B). The responder group showed recovery in 4 of 7 cases (2 U-CLL, 2 M-CLL; 255, 231, 191, and 239) and the nonresponder group in 6 of 7 (2 U-CLL, 4 M-CLL; 159, 201, 169, 153, 261, and 299; Figure 6B). The remaining 4 cases showed no change.
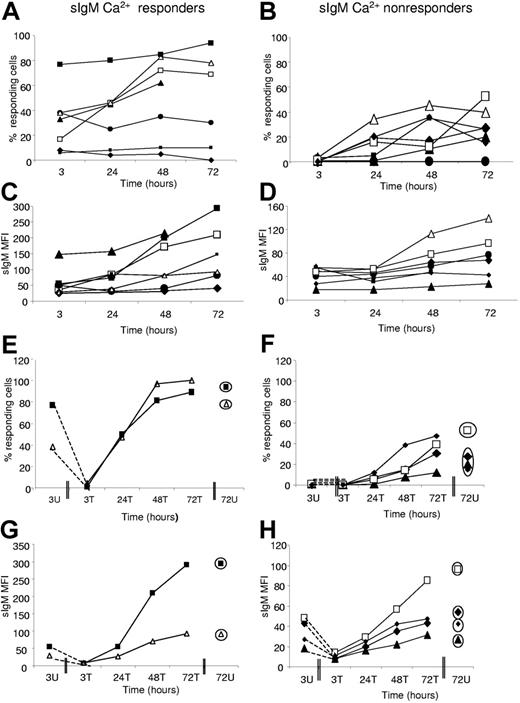
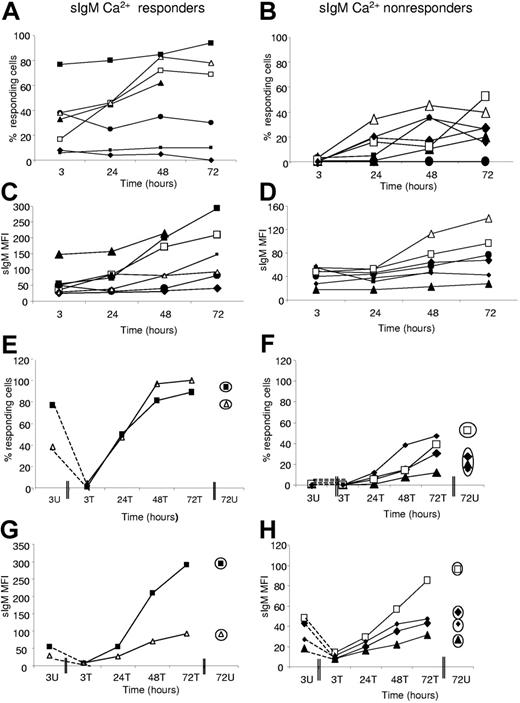
Effect of incubation or endocytosis/re-expression on sIgM signaling. Cells were either untreated (A-D) or treated (E-H) with polyclonal goat anti–L chain F(ab′)2. The change in the ability of untreated cell populations to respond to ligation of sIgM was monitored over time in cases previously designated as (A,C,E,G) “responders” or (B,D,F,H) “nonresponders.” In parallel, (C-D) the level of expression of sIgM (MFI) was measured. Antibody-treated cell populations from “responders” or “nonresponders” underwent endocytosis of sIgM. (E-F) The ability of cell populations to reacquire signaling capacity and (G-H) sIgM expression was monitored. Unmutated cases are represented by open symbols; mutated cases, by filled symbols. Circled symbols in panels E-H indicate values at 72 hours for untreated cultures. Case numbers were as follows: “Responders” (A,C,E,G): 255 (□), 191 (▴), 269 (•), 273 (▪), 189 (♦), 231 (△), and 239 (▪). “Nonresponders” (B,D,F,H): 236 (•), 169 (large ♦), 159 (△), 153 (▪), 261 (▴), 201 (□), and 299 (small ♦).
Effect of incubation or endocytosis/re-expression on sIgM signaling. Cells were either untreated (A-D) or treated (E-H) with polyclonal goat anti–L chain F(ab′)2. The change in the ability of untreated cell populations to respond to ligation of sIgM was monitored over time in cases previously designated as (A,C,E,G) “responders” or (B,D,F,H) “nonresponders.” In parallel, (C-D) the level of expression of sIgM (MFI) was measured. Antibody-treated cell populations from “responders” or “nonresponders” underwent endocytosis of sIgM. (E-F) The ability of cell populations to reacquire signaling capacity and (G-H) sIgM expression was monitored. Unmutated cases are represented by open symbols; mutated cases, by filled symbols. Circled symbols in panels E-H indicate values at 72 hours for untreated cultures. Case numbers were as follows: “Responders” (A,C,E,G): 255 (□), 191 (▴), 269 (•), 273 (▪), 189 (♦), 231 (△), and 239 (▪). “Nonresponders” (B,D,F,H): 236 (•), 169 (large ♦), 159 (△), 153 (▪), 261 (▴), 201 (□), and 299 (small ♦).
The 4 responders that acquired further signaling capacity also showed an increasing expression of sIgM (Figure 6C). However, 2 cases (269 and 273) showed an increase in sIgM expression but failed to recover signaling capacity. One case (189) failed both to recover signal capacity and to increase sIgM expression significantly. Among the 6 initial nonresponders that showed recovery of signal capacity in vitro, the 5 evaluable at 72 hours all increased expression of sIgM, although one (261) was low (Figure 6D). The single nonresponder (236) that failed to recover signal capacity did increase sIgM expression. In summary, therefore, correlation between recovery of signal capacity and sIgM expression was strong, but not all cases that re-express sIgM were able to recover signal capacity.
Viability was falling in all cases over the 72-hour period, but there were no overall differences between the responders and nonresponders, or between cases showing recovery of sIgM signal capacity or not (data not shown). One exception was case 189, a responder, which failed to recover and showed a relatively rapid loss of viability.
Recovery of sIgM-Ca2+ signal capacity following endocytosis and re-expression
Because bound antigen could potentially be removed by an active process of endocytosis and re-expression of sIg, we investigated the effect of this on signaling capacity. We selected 2 sIgM-Ca2+ responders (1 U-CLL [231] and 1 M-CLL [239]), and 4 nonresponders (1 U-CLL [201], 3 M-CLL [261, 169, and 299]). Cells from each case were incubated either alone or with F(ab′)2 anti–light (L) chain, the latter to remove sIg without interfering with the ability to monitor expression of the individual isotypes.
In all cases of both responders and nonresponders, the effect of the F(ab′)2 anti-L was to remove all signal capacity and sIgM expression (Figure 6E-H). Subsequent incubation then restored both signaling (Figure 6E,F) and sIgM expression (Figure 6G,H), remarkably reaching almost exactly the levels achieved by incubation alone at 72 hours (circled symbols). In all 6 cases, recovered signal capacity and sIgM expression were greater than in the original tumor cells. These data confirm the link between sIgM expression and signaling and indicate again the ability of CLL cells to reverse a weakened signal capacity in vitro either spontaneously or by active endocytic removal/re-expression.
The [Ca2+]i response to engagement of sIgD was positive (> 5%) in all 14 cases of either sIgM-Ca2+ responders or nonresponders, and did not change significantly over the 72-hour incubation period (Figure 7A-B). Only one case (255) showed some increase in the sIgD response (Figure 7A), and this was a sIgM responder. Others were stable in signal level out to72 hours. In no case was there clear evidence of an increase in expression of sIgD (Figure 7C-D), but rather a tendency to decrease over time.
Effect of incubation or endocytosis/re-expression on sIgD signaling. Cells were either untreated (A-D) or treated (E-H) treated with polyclonal goat anti–L chain F(ab′)2. The change in the ability of untreated cell populations to respond to ligation of sIgD was monitored over time in cases previously designated as (A,C,E,G) “responders” or (B,D,F,H) “nonresponders” to sIgM ligation. In parallel, (C-D) the level of expression of sIgD (MFI) was measured. Antibody-treated cell populations from “responders” or “nonresponders” underwent endocytosis of sIgD. (E-F) The ability of cell populations to reacquire signaling capacity or (G-H) sIgD expression was monitored. Unmutated cases have open symbols and mutated cases have filled symbols. Circled symbols in panels E-H indicate values at 72 hours for untreated cultures. Case numbers were as follows: “Responders” (A,C,E,G): 255 (□), 191 (▴), 269 (•), 273 (▪), 189 (♦), 231 (△), and 239 (▪). “Nonresponders” (B,D,F,H): 236 (•), 169 (large ♦), 159 (△), 153 (▪), 261 (▴), 201 (□), and 299 (small ♦).
Effect of incubation or endocytosis/re-expression on sIgD signaling. Cells were either untreated (A-D) or treated (E-H) treated with polyclonal goat anti–L chain F(ab′)2. The change in the ability of untreated cell populations to respond to ligation of sIgD was monitored over time in cases previously designated as (A,C,E,G) “responders” or (B,D,F,H) “nonresponders” to sIgM ligation. In parallel, (C-D) the level of expression of sIgD (MFI) was measured. Antibody-treated cell populations from “responders” or “nonresponders” underwent endocytosis of sIgD. (E-F) The ability of cell populations to reacquire signaling capacity or (G-H) sIgD expression was monitored. Unmutated cases have open symbols and mutated cases have filled symbols. Circled symbols in panels E-H indicate values at 72 hours for untreated cultures. Case numbers were as follows: “Responders” (A,C,E,G): 255 (□), 191 (▴), 269 (•), 273 (▪), 189 (♦), 231 (△), and 239 (▪). “Nonresponders” (B,D,F,H): 236 (•), 169 (large ♦), 159 (△), 153 (▪), 261 (▴), 201 (□), and 299 (small ♦).
Following treatment with F(ab′)2 anti-L, both sIgD expression and signal capacity were lost or significantly reduced (Figure 7E-H). Recovery occurred in both sIgM-Ca2+ responders and nonresponders over time, generally reaching the levels achieved by spontaneous recovery in 72 hours. For sIgD, therefore, there is less evidence for anergy in vivo, but the recovery following endocytosis is similar to that for sIgM.
In summary, the increase in signal capacity and sIgM expression indicates loss of an anergizing BCR ligand following incubation in vitro. Increases appear possible in both M-CLL and U-CLL. The lack of effect on sIgD is consistent with the finding that sIgD is apparently not anergized to the same extent in vivo by BCR engagement.
Discussion
Although the clinical course of CLL appears highly dependent on whether the cell of origin has acquired somatic mutations in the immunoglobulin V genes, only minimal differences in phenotypic or gene expression profiles between U-CLL and M-CLL have been detected so far.4,5 One reason for this may be that critical activation and proliferative events occur in the tissues, and cells obtained from blood have had time to lose the features associated with these events.25 This must also apply to signaling events mediated via the BCR and might account for the detection of trends rather than of absolute differences between the subsets.
In measuring the ability of blood cells to respond to cross-linking of sIg, we are assessing the signaling capacity remaining after stimulation that might be occurring in vivo. This is a challenging setting especially because there is little information from normal B cells on how BCR activation occurs in vivo and for how long the intracellular pathways maintain the activated state. We have used mobilization of [Ca2+]i as an indicator known to correlate with phosphorylation of Syk, BLNK, and PLCγ.18 We have distinguished responders from nonresponders by the ability of 5% or more of the clone to increase [Ca2+]i on stimulation in vitro. Single-cell analysis has allowed reproducible detection of these small responsive populations within the clone. The clear distinction from nonresponders suggests a functional dichotomy between completely unresponsive cases and those showing residual ability to respond, a distinction that might be more evident in tissue sites.
The 3 current indicators of poorer prognosis in CLL are: V-gene mutational status, expression of CD38, and expression of ZAP-70. Signaling via sIgM in vitro correlates with all 3, but that does not prove functional linkage. Although CD38 can apparently act as a signaling molecule in CLL and, in the presence of IL-2, influence BCR signaling,26 our studies failed to show any difference in signal capacity in CD38+ or CD38− subpopulations of the same clone. Our results differ from another study that reported a higher level of global phosphorylation in sorted CD38+ subpopulations.27 In fact, we checked global phosphorylation in our cases and could not confirm this difference (data not shown). One possibility is that the long-term engagement by anti-CD38 during sorting affected outcome. It remains possible that CD38 could modulate tumor behavior at tissue sites, or, alternatively, it could be simply a marker of activation.
ZAP-70 is a tyrosine kinase and is likely to influence BCR signaling. It is a strong prognostic factor6,7 and when transfected into ZAP-70− CLL cells, enhances signal competence.18 A recent study failed to detect direct phosphorylation of ZAP-70 following BCR ligation and suggested instead that signal enhancement by ZAP-70 might result from prolongation of phosphorylation of Syk.28 We find that signal competence as measured by increased [Ca2+]i does correlate with ZAP-70 expression. However, we could not detect a specific association of expression with signal competence within the 2 subsets. The apparent irrelevance of ZAP-70 expression for signaling, especially in M-CLL, could indicate that ZAP-70 has different roles in U-CLL and M-CLL.
An investigation of CLL using apoptotic indicators as a measure of response to cross-linkage of sIgM by immobilized antibody also revealed independence from ZAP-70. Responding cases generally correlated with poor prognosis but a group of responsive ZAP-70− M-CLL cases was identified, which had evidence of disease progression.29 This proposes signaling as a stronger determinant of clinical behavior than mutational status or expression of ZAP-70, a conclusion that requires further investigation. We are currently accumulating more outliers (U-CLL, sIgM-Ca2+ nonresponders/M-CLL, responders/ZAP-70+, nonresponders/ZAP-70−, responders) to address this point, but larger numbers and longer follow-up will be required to provide a clear answer.
The main determinant of sIgM-mediated signaling is the level of sIgM expression. In general, U-CLL expresses more sIgM and M-CLL has down-modulated expression to the point of signal anergy. However, differences in responsiveness among cases with similar sIgM expression levels suggest that additional factors could be contributing to the failure to respond. Prior to molecular definition, we are using the term “anergy” to describe the overall failure to respond. In contrast to sIgM, as suggested previously in a small cohort,16 sIgD remains signal competent in almost all cases. Differential responsiveness of sIgM and sIgD to antigen engagement has been observed in mouse B cells, and appears due to a weaker association of the transmembrane regions with Ig-α/Ig-β.30
The key question for CLL is whether relative or complete anergy derives from engagement of antigen in vivo, and what effect this has on proliferation or apoptosis. Indications of a role for (super)antigen in the pathogenesis of CLL include asymmetry of V-gene usage in both subsets, and conservation of certain CDR3 sequences, especially evident in U-CLL.1,3,31–33 Expression of activation markers is also consistent with a continuing role for antigen after transformation.34 Direct evidence for antigen engagement in vivo is now provided by the finding that, in the majority of cases from both subsets, sIgM-mediated signal competence can be reversed by incubation in vitro or by endocytosis/re-expression. Although alternative events occurring in vitro, such as a spontaneous reprogramming of sIgM signaling pathways, cannot be discounted, removal of antigen seems the likely explanation in most cases. Removal from an anergy-inducing signal operating in vivo apparently allows sIgM to recover, and this reversibility argues against an intrinsic defect in the biosynthesis of BCR components.35 It is remarkable given that tumor cells are losing metabolic vigor in vitro and need to reacquire both sIgM expression and to reverse anergic status. These demands could explain why all cases do not show this phenomenon, but the alternative explanation may be that some putative autoantigens are expressed within the tumor cell population.36 Also there may be additional anergy-inducing pressures operating in vivo, which are irreversible in vitro. In line with the nonanergized status of sIgD, incubation or endocytosis/re-expression had little or no effect on sIgD signaling or expression.
If antigen bound in vivo mimics the effects of BCR engagement in vitro, the outcome for the cell will depend on the integration of signals via sigM and sIgD. Anergized normal B cells have a reduced life span in mice,37 but anergy appears isotype dependent in CLL. Evidence for isotype-specific effects of signaling is available from a mouse cell line where apoptosis was induced by cross-linkage of sIgM but not sIgD.38 For a single case of CLL, it was observed that anti-IgM antibodies suppressed subsequent cytokine-induced proliferation, suggestive of apoptosis, whereas anti-IgD did not.39 More recently, a study of CD38+ CLL reported a similar isotype-specific effect on apoptosis.40 Another group obtained opposing results,41 but neither study defined the cases by mutational status.
Collectively these observations point toward differing outcome of signaling via sIgM and sIgD. The effects of antigen binding to both isotypes in vivo cannot yet be determined. Cross-talk between the isotype-mediated effects may occur, but, in contrast to mouse studies of transfected cells, there appears to be no effect of ligating anergized sIgM on nonanergized sIgD. The effects of coligation using anti–L chain antibodies are currently being investigated. So far, our investigation has focused on membrane proximal events. The pathways between those and apoptosis need to be explored for isotype-specific effects. We plan to dissect the downstream intracellular pathways activated by either sIgM or sIgD in individual CLL clones that respond to both, using single-cell phosphorylation analysis of key signaling intermediates.42 This strategy should also allow investigation of intrinsic levels of phosphorylation in freshly isolated cells, thereby providing insight into events occurring in vivo.
Although dynamic, sIgM-mediated signal competence in vitro appears to unite the 3 important prognostic markers, VH-gene mutational status, CD38, and ZAP-70, pointing to relevance for tumor behavior. The route to anergy seems easier for M-CLL and continuing antigen-induced anergy in vivo may control tumor expansion. The role of ZAP-70 remains enigmatic but could differ between U-CLL and M-CLL. It is highly likely that ZAP-70 is involved in signaling in expressors18 and ZAP-70–mediated modulation of downstream events could influence final outcome for the cell thereby providing the key to malignant behavior.
Authorship
Contribution: C.I.M., K.N.P. G.P., and F.K.S. participated in designing the research; C.I.M., K.N.P., I.W., and L.A.N. performed research; C.I.M., K.N.P., and F.K.S. collected data; C.I.M., K.N.P., G.P., and F.K.S. analyzed and interpreted data; C.I.M. performed statistical analysis; C.I.M., K.N.P., G.P., and F.K.S. drafted the manuscript.
Conflict-of-interest disclosure: The authors declare there is no financial conflict of interest.
C.I.M. and K.N.P. contributed equally to this study; G.P. and F.K.S. contributed equally to this study.
Correspondence: Freda K Stevenson, Molecular Immunology Group, Tenovus Laboratory, Southampton University Hospitals Trust, Southampton SO16 6YD, United Kingdom; e-mail: fs@soton.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by Tenovus, the Leukaemia Research Fund, Cancer Research United Kingdom, the National Translational Cancer Research Network (NTRAC) United Kingdom, and the CLL Global Research Foundation.
We thank Drs A. S. Duncombe, D. S. Richardson, A. Jacob, H. McCarthy, B. Kennedy, S. Wagner, and Professor T. J. Hamblin for providing clinical material and obtaining LREC approvals, and Dr C. Ottensmeier and Julie Gwilt for obtaining M-REC approval.

![Figure 1. Variable levels of [Ca2+]i induced in cases of CLL by ligation of sIgM. Lymphocytes from 3 cases of CLL were labeled with the calcium-sensitive dye Fluo-3–AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Density plots illustrate (A) complete response, (B) no response, and (C) partial response. In panel D, responses were quantitated by determining the peak percentage of CLL cells with fluorescence intensity above the unstimulated threshold (T). T was set at the 85th percentile of the fluorescence intensity of unstimulated cells, as shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020001.jpeg?Expires=1766347163&Signature=uQ3CxmlGu8atIoUxgweyQ5lNP0Ho1Nf4Q4kmXdDH52Z1iZvH~8MYyxMOAp8qxgjiO46k8Er90kHnU6VjN~p7Hs~5762h0-YdHEHBlhNk6b89U0IxBeEJIg5ek-ahrHFe32uN7RAw5iE~N~EKoXAeRqeDtTvKpwuCxuG34KTr9bl31zO~qgxFNJRttNlwCvxQ8tsdIMc5niIkDIBrLH5UXQ50Zm569alydnUEpfDOSlHJXftmaJYyA-838tsXGFmjdhPAOlISUM9fU-ctuD98o7vYYZU2I6awlz44jr6zxI4oDN1WGRiD0uGn6~ZxyLi1gPQA26W4ZQXCHiHtOfFJAw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. sIgM-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgM cross-linking was determined for 112 patients with CLL. The percentage of cells responding in subgroups defined by (A) somatic mutational status, (B) CD38 expression, (C) ZAP-70 expression status, and (D) sIgM MFI was assessed. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative. For sIgM, the median MFI of all the samples in the cohort was determined to be 50. sIgMhi samples were those with an MFI greater than 50, whereas sIgMlo samples had an MFI below 50. Horizontal bars indicate median values.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020002.jpeg?Expires=1766347163&Signature=R94kAJ8jeGa3mHTcjHaC9qqJac7WhZm1DcORky~z1ZIoZ13HcaBpNKzSTLsBsNJo6Zr95g9nHE2ltRr94zt2RtHt-6sp-b-bTdhvoKqG6CUtMFyzWtHWthNAJ8Wn0AhRpPhJwdpYZ2AZCQSDiiW~WmDiIGE7mAi3NzJOP7nCVKQs2LvNJw-sLjlJjj1wt3UkaBGEDzw~JI6arC0o-mHRNeJ9cBv8AExNupth0IA5c8BngoMC5~csChC8v-30ktJ4733pbO5agdAe9tg3OjdGmMXL3uqO7PcSrL7nN2Hc1SILQ7XChX6zN6xfBK2MlNElsR4scfyCghAmcu-YWSEjlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. sIgM-mediated signal responses in CD38+ or CD38− subpopulations of cases with bimodal expression. (A) Bimodal expression of CD38 in a representative case. (B) sIgM-mediated [Ca2+]i responses, as percent of responding cells in CD38+ and CD38− subpopulations. (C) Cumulative responses in 7 bimodal cases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020004.jpeg?Expires=1766347163&Signature=fJ1YAx9NcT22O8z5iGI5-7v-vVITqlIH-jT5j7FX-J24mpyTpQ3Lxq6blLTUR8kSnd1qcn79Y~B0fYmCugdbsbtlDCrGvbAdSCUREa9flQhLYBl4z6IqK6r6JsVHrODSKUAbmegJFn-qKvEhTN4LtdndOcI7IXN3vQH2OunuY6SRhv3jtxVV0lufSPgvwjz-q0yAF7pTAuUrBIpzY3tLAkyvQGASg2CrbM9mIJRHpQDPVjkgu2ofuWHHn-D7O24XJz2juCjWy7zLfHdsBwvZ8HW8WfefPg1-Od9ygG2ZJ3Z9fzVEDuNZ1OOMz1RKXQAFNLKLLA9TXCygwaNDJU7Bbw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. sIgD-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgD cross-linking was determined for 111 patients with CLL. The percentage of cells responding in U-CLL or M-CLL was assessed. Horizontal bars indicate median values. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020005.jpeg?Expires=1766347163&Signature=uhNel7y-WExFO1AzCgaLtYdWgWPZavY3Gi7-nbIA6MeAtjEAHgVoyEXbQWBoQm7hm39pwJ05Rv8yqGrhek4TufTYHtNJy2390o7ZPJOU2oDcKOb25aEHrvv-1VGBQ6b6OasupxmUD9FSFhZkEiMEqosSc5XBDipvpmq6CcYrXzm5x7MOsNENcaJsT6ZJIA7XixlpGu-HHudZ2rg4V2XPD9mIyWjKRNdxftZqOzswVYtkv61ReqQ3sDvzQMNKhp8Mo0XXWWysucqDTZHj0VvfUOky1JqZeXtc1-jchG5g2OmHUZsCw3iL6rZ~Kq2DLrJVhGOQYl00ISL8gCQIOle2ag__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Variable levels of [Ca2+]i induced in cases of CLL by ligation of sIgM. Lymphocytes from 3 cases of CLL were labeled with the calcium-sensitive dye Fluo-3–AM and analyzed by flow cytometry before and after addition of F(ab′)2 anti-IgM. Density plots illustrate (A) complete response, (B) no response, and (C) partial response. In panel D, responses were quantitated by determining the peak percentage of CLL cells with fluorescence intensity above the unstimulated threshold (T). T was set at the 85th percentile of the fluorescence intensity of unstimulated cells, as shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020001.jpeg?Expires=1766347164&Signature=znSC6UgGWHYHqDJq~9~LFHQc3hccDax68lOSszrzUz9UtfvlKVy3u364DvMV-b4z5T-Spj75ZxO36gvDe-jSZtaNxfNfqotKCA9QiRvz73mc5Jkv7Eua0a99vqftO6rKf9KocYCL-IYt2PKnNpubctSFdvL8XCapoOE8XE8gZkSoRUFQuDQl-XBTYw-8F2dlrZuGlnW1mPk81rG66SaXjzW3Gmddz8ozaokxdhU4cToQlt0ese-LyjiS3SFsLzKTykaEK5AGvAHQ5WC5LR5VZVgdkSwjWAip9QuNU-KEtiwP8MfaznQnwrDDecZnaSC4w6uhnBKXcDrxG-Z1MUFMWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. sIgM-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgM cross-linking was determined for 112 patients with CLL. The percentage of cells responding in subgroups defined by (A) somatic mutational status, (B) CD38 expression, (C) ZAP-70 expression status, and (D) sIgM MFI was assessed. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative. For sIgM, the median MFI of all the samples in the cohort was determined to be 50. sIgMhi samples were those with an MFI greater than 50, whereas sIgMlo samples had an MFI below 50. Horizontal bars indicate median values.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020002.jpeg?Expires=1766347164&Signature=Mp-xAvgz2-b2QgHN87a0WoVRcXuEizIEBf6lWLBoXLYIoRvYNu2CpdC-YSS5P-9So4b0bwcmZ26pis~mDQBJBK2pfZQNs5aoQydcXQ8I7eeFDsOTROTYh9AoBHLyFlwDReCE3lxNFb0zzQpHX9Zl2NDDibNmtW6XZebcwQ84ht8vM4b3vFkAyPLtFIMmz4ugVaBQ3YJ4OPwukPjDpU3qrZQi0UXl8~4lrQJj6CR7RdL6T0XtkIMgkkHKn8Bmhd1y~eCA6nPnrt5QKmHRR8kVYAQ1EZZ65pAfCcIy3GQ3eQeDwCjz~tVJMH27sb3GEVjCX5e3jKEvJZy5HLtpFUJK3A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 4. sIgM-mediated signal responses in CD38+ or CD38− subpopulations of cases with bimodal expression. (A) Bimodal expression of CD38 in a representative case. (B) sIgM-mediated [Ca2+]i responses, as percent of responding cells in CD38+ and CD38− subpopulations. (C) Cumulative responses in 7 bimodal cases.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020004.jpeg?Expires=1766347164&Signature=tLYul07Q5yXJ9v8Xtg4fs-a3NDHNe~TFwysI61lc8sdOQG~2hetRLHunTdox06AYuMAwJFe3J7m9ZhjQuoooI0MO2s6g6djq3mj64g8-M8E0XCH3Jvrtb2WFAYKIw8DhiQGr~pOSLImDNBAWzFpeuCh4XO1Q0gVlr9w3eltw7Cwd4JwM9ZewafKDISDcr9qAmuNrSgKQk9x~fiHH96R0JhOO-~qzGEiXQuPzw~uKUvPll3tHOV9BcwvVOEiN8mvsGRuAHJNQBnCE5kN7-I4EVpZG49XwMkzKcHLIIeCULyEQf1XSA91xUBnlEsQD3vv77Jd2lF88FXN-m-hWzOOgkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. sIgD-mediated signal responses in CLL subgroups. The [Ca2+]i response to sIgD cross-linking was determined for 111 patients with CLL. The percentage of cells responding in U-CLL or M-CLL was assessed. Horizontal bars indicate median values. Significance of differences was derived from the Mann-Whitney test (2-tailed, 95% CI). The dotted line at 5% shows the cutoff used to define signaling status as positive or negative.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-11-056648/4/m_zh80100701020005.jpeg?Expires=1766347164&Signature=oiJFxe7gYmCE7Mo0bkbw9vsJ~17z13YsiRWOuAvgKBhXQJyn-N3NbbfkO-2shNLDPJGww2UAy19mnPNh-zNysMWefwfLfop3jvF9eRn8mOXC6Oy0DO1m7mhT~4JXFbvWLUKwVly4EIriEn75bBXg1dKf9r6jTfUpq3Bif5I4PMnnV595hF9sjGXYHPxyq038DWyoMw8NhZRfcwLliynGGBPT56~jt8yapXcWzM3HcwmyZwdHgVaGH5EAgn3WwSV7mTnEQaVzm4xX~huGAFSGAT8Uskci2aU0PdMJ2NhdftXV-ld-X4Mvl6PqgBY4p0C8FGw8pBh44oJFkmEWvTEtGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)