Abstract
The tumor microenvironment plays an important role in the biologic behavior of follicular lymphoma (FL), but the specific cell subsets involved in this regulation are unknown. To determine the impact of FOXP3-positive regulatory T cells (Tregs) in the progression and outcome of FL patients, we examined samples from 97 patients at diagnosis and 37 at first relapse with an anti-FOXP3 monoclonal antibody. Tregs were quantified using computerized image analysis. The median overall survival (OS) of the series was 9.9 years, and the FL International Prognostic Index (FLIPI) was prognostically significant. The median Treg percentage at diagnosis was 10.5%. Overall, 49 patients had more than 10% Tregs, 30 between 5% to 10%, and 19 less than 5%, with a 5-year OS of 80%, 74%, and 50%, respectively (P = .001). Patients with very low numbers of Tregs (< 5%) presented more frequently with refractory disease (P = .007). The prognostic significance of Treg numbers was independent of the FLIPI. Seven transformed diffuse large B-cell lymphomas (DLBCLs) had lower Treg percentages (mean: 3.3%) than FL grades 1,2 (mean: 12.1%) or 3 (mean: 9%) (P < .02). In conclusion, high Treg numbers predict improved survival of FL patients, while a marked reduction in Tregs is observed on transformation to DLBCL.
Introduction
Follicular lymphoma (FL) is the second most common subtype of adult B-cell non-Hodgkin lymphoma (NHL) in Western countries and is characterized by the t(14;18) translocation. This abnormality promotes tumor cell survival through overexpression of the antiapoptotic Bcl-2 protein. Of interest, mice overexpressing Bcl-2 under the control of the immunoglobulin enhancer develop follicular hyperplasia rather than FL, indicating that pathogenetic mechanisms other than Bcl-2 overexpression exist in FL.1 The vast majority of FL patients has incurable disease with a generally indolent course with frequent relapses and the eventual development of resistance to chemotherapy or transformation to an aggressive diffuse large B-cell lymphoma (DLBCL) leading to death from disease. Despite a common underlying genetic abnormality, the clinical course of FL patients is heterogeneous. While the median survival of newly diagnosed FL patients is typically 8 to 10 years, there is a significant group of patients, 10% to 15%, having a more rapid disease course that proves fatal in 2 to 3 years. As yet, there is a lack of reliable prognostic markers that can be used at the time of diagnosis to identify the high-risk patients who will experience rapid disease progression. An international cooperative study has now proposed a simple and reproducible Follicular Lymphoma International Prognostic Index (FLIPI) based on easily available clinical data.2 The parameters used to define FLIPI represent surrogate markers of disease biology. The identification of prognostic models more closely related to the biologic mechanisms involved in the development and/or progression of the tumor may provide useful information, not only to predict survival but also to enable the design of more targeted therapeutic strategies.
In the last few years, microarray-based gene expression profiling has played an important role in the identification of clinically relevant subtypes within B-cell lymphoma entities. Intriguingly, survival signature analysis of FL patients at the time of diagnosis generated 2 immune response signatures. These were based on the molecular features of tumor-infiltrating cells and could be combined to effectively predict survival.3 Thus, in contrast to other B-cell lymphomas where gene expression in the malignant B-cell population determines survival, the composition of the lymphoma microenvironment at diagnosis seems to determine the survival of patients with FL. The immune response 1 (IR-1) signature conferred a favorable outcome and included T-cell markers together with genes highly expressed in macrophages. The immuneresponse signature 2 (IR-2) included genes preferentially expressed in macrophages and dendritic cells and conferred an unfavorable outcome. However, this signature was not determined solely by the total number of T cells since there was no correlation between a “pan-T-signature” and survival. A recent study has confirmed the clinical significance of macrophage numbers in FL showing that high numbers of these cells correlated with poor survival.4 On the contrary, further immunostaining studies in the same FL series using a range of commonly used T-cell markers failed to identify a significant association with overall survival.4
A subset of regulatory T cells (Tregs), with a CD4+CD25+ immunophenotype, has recently generated considerable interest through their critical role in regulating immunotolerance predominantly via the suppression of effector T-cell proliferation and cytokine production.5-7 Further studies have now convincingly identified a role for Tregs in enabling tumor cells to evade the host immune response.8-11 While a number of markers (including CTLA-4, GITR, and CD45RB) are expressed on Tregs, the FOXP3 transcription factor is currently accepted as the master regulator of this cell lineage and thus the most specific Treg marker. Our production and characterization of paraffin-reactive monoclonal antibodies to FOXP312 have recently provided essential reagents with which to investigate the clinical significance of FOXP3positive Tregs within the microenvironment of human tumors. To determine the role of Treg immunoregulatory cells in the progression and outcome of FL, we have examined a large series of patients at diagnosis and relapse. Our results indicate that the number of Tregs predicts survival in FL patients and that transformation to DLBCL is associated with a marked reduction in the number of these cells in the tumor microenvironment.
Patients and methods
Patients and samples
Ninety-seven patients consecutively diagnosed with FL in a single institution between 1988 and 2003 were included in the present study, with the only criterion for inclusion being the availability of histologic material. All of the cases were reviewed and reclassified according to the criteria of the World Health Organization (WHO) Classification.13 Approval for these studies was obtained from the institutional review board of Hospital Clinic, Barcelona. Informed consent was provided according to the Declaration of Helsinki. The median age of the patients was 55 years (range, 26-93 years), and the male-female distribution was 49/48. Advanced stage (Ann Arbor IV) was observed in 58 cases (60%), bulky disease in 14 (14%), and extranodal involvement in 63 (65%), including bone marrow infiltration in 64 cases (66%). Twenty (23%) of 87 patients with available data presented with high serum lactic acid dehydrogenase (LDH) levels, and 28 (38%) of 73 had high serum β2-microglobulin (β2m). The distribution of the series according to the FLIPI2 was the following: low risk, 32 cases (37%); intermediate risk, 23 cases (27%); and high risk, 31 cases (36%). In 11 cases, FLIPI could not be calculated.
Staging maneuvers included patient history and physical examination; blood cell counts and serum biochemistry, including LDH and β2m levels; computerized tomography scan of chest, abdomen, and pelvis; as well as unilateral bone marrow biopsy. Seventy-nine patients were treated with combination chemotherapy (9 with COP [cyclophosphamide, vincristine, and prednisone], 56 with CHOP-like [cyclophosphamide, adriamycin, vincristine, and prednisone] regimens, and 14 with fludarabine combinations), 7 with alkylating monotherapy, and 3 with radiotherapy, whereas in 8 cases a wait-and-see policy was established. Posttherapy restaging consisted of the repetition of the previously abnormal tests and/or biopsies. Response was assessed according to conventional criteria.14 Among the 89 patients with assessable response, 58% of the patients achieved a complete response (CR) and 33% a partial response, whereas 9% failed to respond to treatment. After a median follow-up of 5.6 years (range, 1.1-13.0 years) for surviving patients, 33 patients had died. The 5-year and 10-year overall survival [OS] was 72% (95% confidence interval [CI]: 62%-82%) and 52% (95% CI: 38%-66%), respectively.
In addition to the diagnostic tissue samples, sequential specimens at relapse were available for 18 patients. These were used to assess the numbers of FOXP3-positive Tregs over time. Finally, samples at relapse from another 19 patients from the same institution, for whom no additional material from the diagnostic biopsy was available, were also examined. All 37 samples were used to study the clinicopathological significance of FOXP3-positive Tregs at relapse in FL.
Finally, numbers of FOXP3-positive Tregs were analyzed in 6 reactive tonsils, in order to examine their frequency and distribution in normal lymphoid tissue.
Immunohistochemistry
Tissue samples were fixed in formalin and embedded in paraffin by routine methods. Immunostaining was performed in one laboratory (B.C.F. and A.H.B.) using undiluted hybridoma supernatant of the 236A/E7 anti-FOXP3 murine monoclonal antibody.12 Sections were dewaxed in Citro-clear (HD Supplies, Aylesbury, United Kingdom), and antigen retrieval was performed by microwave pressure cooking for 11 minutes in 50 mM Tris, 2 mM EDTA, pH 9. Serial sections were also stained for CD3 and CD4 (anti-CD3 monoclonal antibody, clone SP1, dilution 1:60; anti-CD4 monoclonal antibody, clone 4B12, dilution: 1:10; Novocastra, Newcastle upon Tyne, United Kingdom). Detection was performed using the EnVision-system (DakoCytomation, Glostrup, Denmark) according to the manufacturer's instructions. Tonsil sections were immunolabeled in parallel as a positive control together with the isotype-matched MR12 antibody as a negative control.
Quantitative assessment of FOXP3-positive Tregs, and CD3+ and CD4+ cells
The number of FOXP3-positive Tregs was quantified in whole-tissue sections of all samples using an automated scanning microscope and image analysis system (Ariol 2.1, SL-50; Applied Imaging, Melville, NY). Quantification of the FOXP3-positive cells was performed with the nuclear Kisight Assay provided by the manufacturer (Applied Imaging), using the following strategy. First, the tissue find phase was performed at 1× objective followed by an automated low-magnification scanning with the 5× objective. Subsequently, the optimal evaluation areas, which accounted for 60% to 100% of the whole section, were selected by the observer (J.C.) and scanned at high magnification with the 20× objective. In the next review phase, a nuclear high-resolution classifier was created and trained for the FOXP3 nuclear staining. The classifier consisted of a color class definition for the positive and negative nuclei and background, and a shape class definition for the positive and negative nuclei. The color class was defined by the hue, saturation, and intensity parameters, whereas the shape class was defined by the spot width, width, compactness, roundness, and axis ratio parameters. The FOXP3 high-resolution classifier was trained in several areas within a tissue sample in order to avoid miscalculation due to differences in staining and cell composition. Among tissue samples, the classifier was always trained and recalibrated under the visual supervision of the pathologist. FOXP3-positive and -negative cells were counted independently in the follicular and interfollicular compartments. The follicular compartment included the mantle zone when present. The total number of cells was recorded by adding the counts for the 2 compartments. To minimize the heterogeneous distribution of positive cells, a minimum number of 6 complete follicular and interfollicular areas was selected in tumors with a predominantly nodular pattern. In tumors with a predominantly diffuse pattern, a minimum of 6 high-power fields (HPFs) was evaluated. The selected areas had a similar number of total cells and were representative of the overall distribution of FOXP3-positive Tregs, and tangential sections of follicles were avoided. In areas in which the images of the nuclei were fused forming a net pattern, shape classes were established in clearly identifiable individual cells and the settings applied to the rest of the sample. In tumors with diffuse and nodular patterns, these areas were quantified and recorded separately. The mean number of total counted cells per case was 34 840 (range, 4754-169 330) corresponding to 21.2 HPFs (range, 3-103 HPFs; mean cell density, 1644 cells/HPF) (UplanFI 40×/0.75; Olympus). Once areas were selected by the observer, the software collected the data automatically. The observer (J.C.) was blinded to the clinical data of the patient.
The number of CD3+ and CD4+ cells was also quantified with the same methodology in whole tissue sections of all samples, using the same automated scanning microscope and image analysis system. Since the staining was cytoplasmic and membranous, we used the Gensight Multistain Assay also provided by the manufacturer (Applied Imaging) and only the color class parameters were trained.
Statistical analysis
The main initial and evolutive variables, including the histologic parameters, were recorded and analyzed for the prognostic significance. Categoric data were compared using Fisher exact test, 2-sided P value, whereas for ordinal data nonparametric tests were used. The multivariate analysis of the variables predicting response was performed by using a logistic regression. The actuarial survival analysis was performed according to the method described by Kaplan and Meier, and the curves were compared by the log-rank test. The independent prognostic value of FOXP3-positive Treg numbers and clinical data summarized in the FLIPI was tested by means of the stepwise proportional hazards model of Cox.
Results
Clinical and histologic characteristics of patients at diagnosis
The main clinical and histologic features of the 97 patients at diagnosis are detailed in “Patients, materials, and methods” and summarized in Table 1. The histologic distribution according to the WHO classification was grade 1, 24 cases (25%); grade 2, 57 cases (58%); and grade 3, 16 cases (17%) (grade 3a, 12 cases [12%], grade 3b, 4 cases [4%]). The architectural pattern was predominantly follicular (follicular area > 75%) in 83 cases (86%), follicular and diffuse (follicular area 25%-75%) in 5 (5%), and predominantly diffuse (follicular area < 25%) in 9 (9%) cases. Two cases, one grade 1 and one grade 3 FL, had a small component of diffuse large B-cell lymphoma in the same lymph node biopsy at diagnosis.
FOXP3-positive Tregs in FL at diagnosis
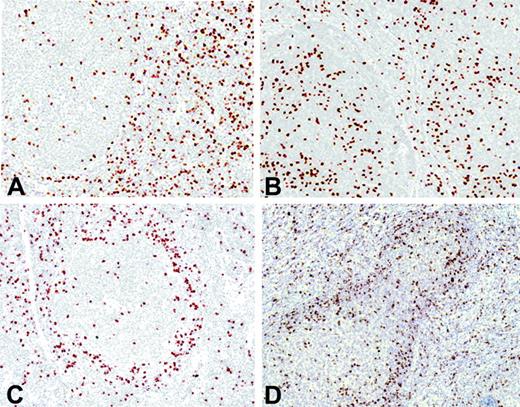
FOXP3-positive Tregs were observed in all cases, but the number and topographic distribution within the tumor varied from case to case. The distribution of the positive cells could be categorized into 4 major immunohistochemical patterns: interfollicular, follicular, perifollicular/mantle zone, and mixed perifollicular and interfollicular (Figure 1). In the interfollicular pattern, most of the Tregs were observed between the follicles (Figure 1A), whereas in the follicular pattern there were more Tregs within the follicles than in the interfollicular areas (Figure 1B). The perifollicular/mantle zone pattern was characterized by a prominent infiltration of Tregs surrounding the nodules (Figure 1C). Finally, some cases had a mixed pattern with strong infiltration in the perifollicular area and in the interfollicular spaces (Figure 1D). The distribution of the patients according to these patterns was as follows: follicular, 5 patients (5%), perifollicular/mantle zone, 13 (13%), interfollicular, 60 (62%), and mixed, 10 (10%). Nine patients had a predominantly diffuse architectural pattern, and in these cases Tregs were scattered throughout the tumor. These visual immunohistochemical patterns did not show any significant correlation with the clinical and histopathologic features of the patients.
To determine whether the total number of Tregs infiltrating the tumor could have a specific relationship with the clinicopathological characteristics of the patients, we quantified the number of FOXP3-positive Tregs using a computerized image analysis system with an automated scanning microscope. The cells were quantified separately in the interfollicular and in the follicular compartments. The total number of Tregs was calculated by adding these 2 counts. The mean number of total counted cells per case was 34 840 (range, 4754-169 330). The median percentage of Tregs in the 97 diagnostic biopsies was 10.5% (range, 0.9%-42.5%). Overall, 49 patients (50%) had a Treg count of more than 10%, 30 (31%) between 5% to 10%, and 18 (19%) less than 5%.
The relationship between the number of Tregs in samples at diagnosis and the pathological characteristics of the patients is shown in Table 2. The numbers of Tregs were lower in the follicular than in the interfollicular compartment (mean ± SD: 8.6% ± 5.2% vs 14.7% ± 9.7% for follicular and interfollicular areas, respectively; P < .001). Grade 3 FL had lower numbers of Tregs than grades 1 or 2 when the follicular compartment was assessed (6.7% vs 9%; P = .048) (Table 2).
Immunohistochemical FOXP3-positive Treg patterns. (A) Interfollicular pattern. (B) Follicular pattern. (C) Perifollicular pattern. (D) Mixed pattern.
Immunohistochemical FOXP3-positive Treg patterns. (A) Interfollicular pattern. (B) Follicular pattern. (C) Perifollicular pattern. (D) Mixed pattern.
Tumors with a predominantly diffuse pattern showed a lower number of Tregs (9.3%) than cases with a predominantly follicular pattern (11.8%), although this difference was not statistically significant. Of interest, in 5 FLs with mixed follicular and diffuse pattern at diagnosis, the number of Tregs in the diffuse component was significantly lower than in the follicular areas (8.03% vs 13.1%; P = .043) (Table 3). Similarly, in 2 patients that presented at diagnosis with a DLBCL component, the number of Tregs in these DLBCL areas (1.02%) was lower than in the FL areas (6.9%) of the same biopsy (Table 4).
Finally, FOXP3-positive Tregs were also evaluated in 6 reactive tonsils. The percentage of total Tregs in these samples was 9.5% ± 5.9%, whereas the FL samples contained 11.6% ± 7.5% (P > .1). The number of Tregs in the follicular and interfollicular compartments was also counted separately. Of interest, the number of Tregs in the follicular compartment of FL was higher than in reactive tonsils (8.6% ± 5.6% vs 3.7% ± 1.9%; P = .012). Conversely, no differences between the FL and reactive tonsils were found when analyzing the interfollicular compartment (13.3% ± 10.2% vs 12.6% ± 7.7%).
Relationship between FOXP3-positive Treg numbers, clinical features, and outcome
Treg numbers were compared with the main clinical features of the patients at diagnosis (Table 5). No significant correlation was observed between the Treg number and the main initial characteristics, including Ann Arbor stage, nodal and extranodal involvement, serum LDH, or serum β2-microglobulin. However, patients with high-risk FLIPI more frequently exhibited a Treg count of less than 10% than did those with low- or intermediate-risk FLIPI (74% vs 44%, respectively; P = .007).
There were no differences in the treatment given to the patients on the basis of the number of Tregs. Regarding the response to therapy, those patients with a very low number of Tregs (< 5%) presented more frequently with refractory disease (failure to first-line treatment) than the others (5 [30%] of 15 vs 4 [6%] of 70, respectively; P = .007). No significant differences were found in terms of event-free survival according to the Treg number.
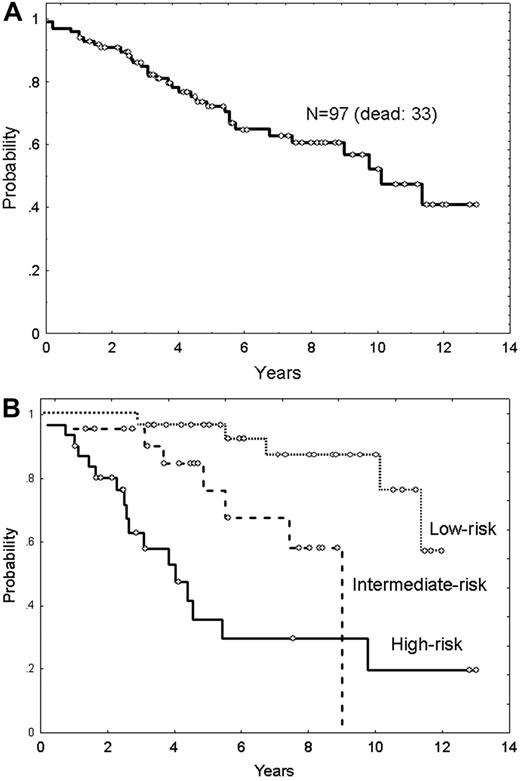
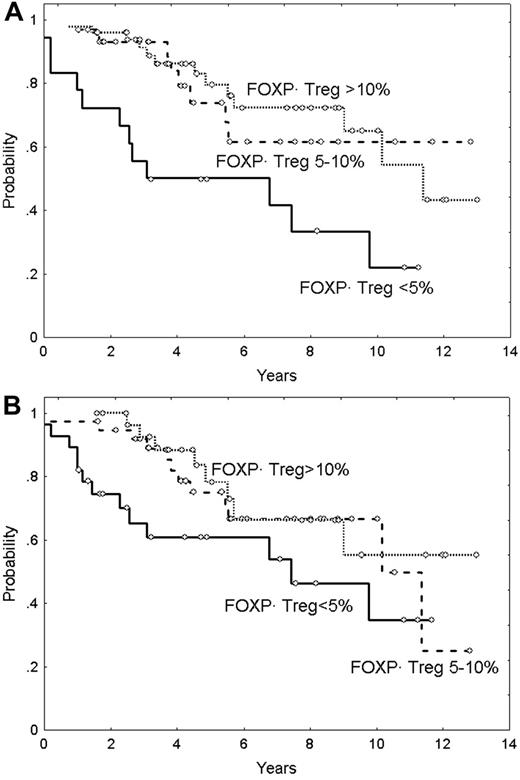
Median overall survival (OS) of the series was 9.9 years, and the OS curve is plotted in Figure 3A. The main clinicobiologic variables with favorable impact on OS were the following: age younger than 60 years; ambulatory performance status (ECOG < 2); absence of B symptoms; number of nodal sites less than 4; and normal hemoglobin level, serum LDH, β2m, and histologic grade (grade 1 and 2 vs 3). FLIPI score divided the series into 3 groups of low, intermediate, and high risk with median OS of 3.9 years, 9.3 years, and not reached, respectively (Figure 3B). OS according to the Treg number is plotted in Figure 4. Five-year OS of patients with Tregs less than 5%, 5% to 10%, and more than 10% was of 50% (95% CI: 26%-74%), 74% (95% CI: 54%-94%), and 80% (95% CI: 68%-92%), respectively (P = .001) (Figure 4A). Moreover, the quantity of Tregs in the follicular compartment also showed prognostic value for OS, with a 5-year OS of 79%, 76%, and 61% for Tregs more than 10%, 5% to 10%, and less than 5%, respectively (P = .02) (Figure 4B). In contrast, interfollicular Treg numbers were not predictive of patient outcome.
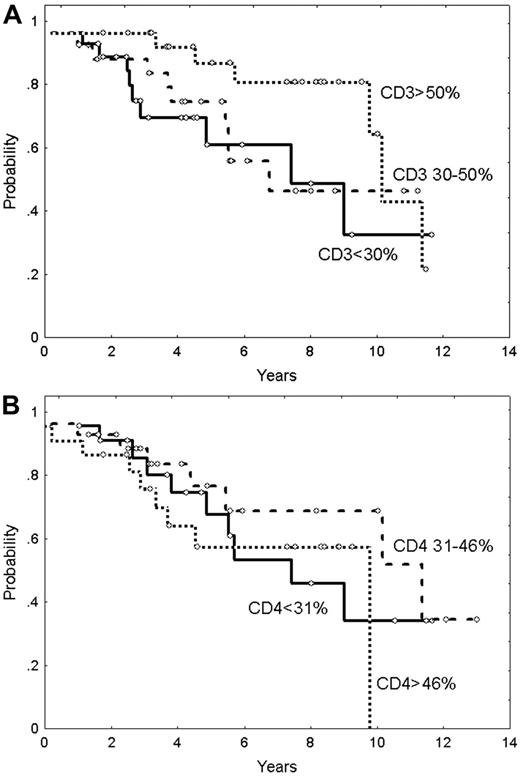
To determine whether the prognostic significance of FOXP3-positive Tregs was only a surrogate marker of the number of CD3+ T cells, 80 FL samples at diagnosis were stained and quantified for CD3. Although the number of Tregs showed a correlation with the number of CD3+ cells when analyzed as a categoric variable (CD3 percentage median: 30% vs 49% for cases with CD3+ cells below and above the Treg median, respectively; P = .001), the ratio was not significant in a linear regression test (R square = 0.092). The number of CD3+ cells counted in the whole section separated in 3 groups did not show predictive significance for OS. Five-year OS of patients with CD3 less than 30%, 30% to 50%, and more than 50% was 72% (95% CI: 54%-90%), 63% (95% CI: 39%-87%), and 87% (95% CI: 73%-100%), respectively (P = .15) (Figure 5A). The same study carried out in the follicular and interfollicular compartments did not show predictive value for OS.
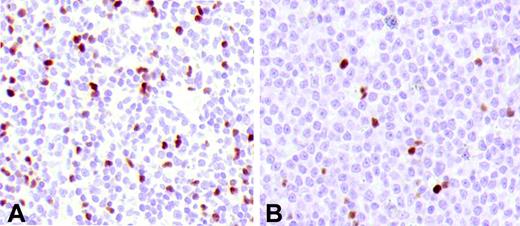
Tregs in FL and DLBCL. (A) FL at diagnosis. (B) The lymph node biopsy of the same patient at relapse shows a transformed DLBCL with a lower number of Tregs.
Tregs in FL and DLBCL. (A) FL at diagnosis. (B) The lymph node biopsy of the same patient at relapse shows a transformed DLBCL with a lower number of Tregs.
Moreover, to determine whether the prognostic significance of FOXP3-positive Tregs was only a surrogate of the number of CD4+ T cells, 71 FL samples at diagnosis were stained and quantified for CD4 as well. The total number of CD4+ cells did not show a correlation with the number of Tregs either when analyzed as a categoric variable (median CD4: 41 vs 40 for cases below and above Treg median, respectively; P = .8) or in the linear regression test (R square: 0.007). In addition, CD4+ cells did not have prognostic value for OS (5-year OS of patients with CD4 < 31%, 31%-46%, and > 46% was 69% [95% CI: 48%-90%], 74% [95% CI: 54%-94%], and 58% [95% CI: 35%-81%], respectively; P = .7) (Figure 5B). The same negative result was observed when the analysis was performed in the follicular and the interfollicular compartments. Of note, in these sets of patients, the number of FOXP3-positive Tregs maintained its predictive value for OS.
To further analyze the prognostic importance of the number of Tregs, a Cox analysis was performed, including Treg number (≤ 10% vs > 10%) and FLIPI score (low, intermediate, and high). In the final model, with 86 assessable cases, Treg number maintained its predictive value (relative risk: 0.59 [95% CI: 0.34-0.84]; P = .04) along with the FLIPI (relative risk: 2.7 [95% CI: 2.47-2.94]; P < .001). However, when the histologic grade (grade 1,2 vs grade 3) was also included in the analysis, only FLIPI and histologic grade showed prognostic significance, whereas the Treg number had only marginal value.
Overall survival of the study population. (A) Overall survival of 97 patients with FL. (B) Overall survival according to the FLIPI score (P = .003).
Overall survival of the study population. (A) Overall survival of 97 patients with FL. (B) Overall survival according to the FLIPI score (P = .003).
Overall survival in patients with FL in relation to FOXP3-positive Tregs. (A) Overall survival according to FOXP3-positive Tregs (P < .05). (B) Overall survival according to follicular FOXP3-positive Tregs (P < .05).
Overall survival in patients with FL in relation to FOXP3-positive Tregs. (A) Overall survival according to FOXP3-positive Tregs (P < .05). (B) Overall survival according to follicular FOXP3-positive Tregs (P < .05).
FOXP3-positive Tregs in FL at first relapse
A sample obtained at the first relapse after the diagnosis of FL was available from 37 patients. The histologic distribution of these biopsies at relapse was FL grade 1, 5 cases (14%); grade 2, 20 cases (54%); grade 3, 7 cases (18%); and transformation to a DLBCL, 5 (14%) cases. After excluding the DLBCL cases, 24 FL cases (75%) had a predominantly nodular pattern and 8 cases (25%) a predominantly diffuse pattern.
Overall survival in patients with FL in relation to CD3+ and CD4+ cells. (A) Overall survival according to CD3+ cells (P = NS). (B) Overall survival according to CD4+ cells (P = NS).
Overall survival in patients with FL in relation to CD3+ and CD4+ cells. (A) Overall survival according to CD3+ cells (P = NS). (B) Overall survival according to CD4+ cells (P = NS).
The mean of Tregs in the relapsed samples was 8.7%. Similar to samples at diagnosis, the number of Tregs was lower in the follicular than in the interfollicular compartment (8.4% vs 14.3%; P = .001), and the diffuse areas tended to have fewer Tregs than the follicular areas (7.6% vs 10.6%, P = .063). Of interest, the DLBCL had a significant decrease in mean Treg numbers (2.2%) compared with the FL grades 1 and 2 (9.6%) and 3 (10.5%) (P = .006). The low numbers of Treg in these DLBCLs at relapse was similar to the number of Tregs in the DLBCL component detected in 2 FLs at diagnosis.
Sequential samples at diagnosis and at first relapse were available for 18 patients. Twelve cases maintained the same FL grade in both samples, 2 cases progressed from an FL grade 1 and 2 to an FL grade 3, 1 case showed a downgrading from an FL grade 3 to an FL grade 1, and 3 cases transformed from an FL grade 2 (1 case) or FL grade 3 (2 cases) to a DLBCL (Table 6). The number of Tregs was similar at diagnosis and at relapse in the cases with either no modification of the tumor grade or downgrading, but decreased in both the progressed grade 3 FL (mean: 18.7% vs 15.1%) and in the transformed DLBCL cases (mean: 15.5% vs 4.9%) (Table 6). The median survival from the first relapse was 4.4 years. No significant correlation was found between Treg number (both total and follicular) and survival from relapse.
Overall, in this study we have examined 7 transformed DLBCLs, 2 observed at diagnosis concomitantly with FL (one with grade 1 and one with grade 3) and 5 at first relapse. The total number of infiltrating Tregs in these tumors was significantly lower (mean: 3.3%) than in the FL at diagnosis (grade 1 and 2, mean: 12.1%; grade 3, mean: 9%) or at first relapse (grade 1 and 2, mean: 9.6%; grade 3, mean: 10.5%) (P < .02) (Figure 2).
Discussion
The tumor cell microenvironment is now thought to play a critical role in the biology of a number of B-cell lymphomas.15-18 Several studies have indicated the important influence of tumor cell microenvironment in the pathogenesis of FL. First, these tumor cells are difficult to grow in vitro unless they are stimulated with stromal cells and a combination of CD40 and cytokine signals19 or cocultured with CD4 T cells recognizing lymphoma alloantigens.20 Another important observation is the ability of patients with FL to undergo spontaneous tumor regression, highlighting the role of immunosurveillance mechanisms in this disease.21
Gene expression studies of FL have also emphasized the impact of immunoregulatory cells on the clinical behavior and response to therapy of these tumors.3,22-24 In particular, an expression signature characterized by a combination of genes mainly expressed by T cells and macrophages has been associated with good prognosis, whereas an expression signature composed of genes highly expressed in macrophages and dendritic cells has been correlated with shorter survival.3 Although the microarray studies suggest that the relationship between the macrophage/dendritic cell population and prognosis may be complex, a recent immunohistochemical study has confirmed the impact of the total number of macrophages in the aggressive clinical evolution of FL.4 The T-cell population(s) that may influence the behavior of FL tumor cells is not clear. The microarray studies indicated that the T-cell signature associated with good prognosis was not a direct consequence of the total number of tumor-infiltrating T cells.3 Similarly, the immunohistochemical detection of the most common T-cell–associated markers did not reveal any particular subpopulation of cells related to survival in FL.4
In the present study, we demonstrate that the number of tumorinfiltrating FOXP3-positive Tregs is a predictor of survival in patients with FL, independently of the FLIPI, and that the number of these cells decreases during the transformation to DLBCL. In common with other studies,3,4 total T-cell numbers had no prognostic significance in this series. These results suggest that Tregs may be an important subset of cells in the tumor microenvironment modulating the host immune response and biologic behavior of FL. Tregs are a subpopulation of T lymphocytes that play a crucial role in the control of T-cell–mediated autoimmunity by suppressing the proliferation and cytokine production of other T cells.5,25,26 These cells seem also to participate in the regulation of the immune response against tumor cells, representing an important element of the cancer microenvironment. Thus Tregs may suppress tumor-specific T-cell immunity, thereby contributing to cancer cell growth.9 Concordantly, the presence of a high number of these cells in the solid tumors has been correlated with a poor prognosis of patients.9,11,27 However, most of these studies have been performed on carcinomas, with the role of Tregs in hematologic malignancies being less established. A recent study in patients with B-CLL identified the highest numbers of CD4+CD25+ Tregs in untreated or progressing patients presenting with extended disease, and both numbers and suppressive function were decreased on treatment with fludarabine.28 However, this study in B-CLL investigated Treg numbers only in the peripheral blood. Contrary to these observations, we report here that FL patients with higher Treg numbers in their tumors had a better response to therapy and improved overall survival. In this series of FL patients, the FLIPI effectively segregated the patients into prognostically relevant groups. Of interest, the prognostic value of the number of Tregs was independent of the FLIPI score, suggesting that this parameter reflects a different biologic aspect of the tumors. These data are similar to previous findings in Hodgkin lymphoma, where a high number of FOXP3-positive Tregs was also associated with improved patient survival.29 Taken together, these studies suggest that the role of Tregs in the pathogenesis of these B-cell lymphomas is different than in carcinomas.
A recent study has shown that Treg numbers are increased in B-cell non-Hodgkin lymphoma tissues compared with reactive tonsil or lymph nodes, probably being recruited via CCL22 secreted by the malignant B cells. Of importance, these intratumoral FOXP3-positive Tregs from lymphoma patients were demonstrated to be functionally capable of suppressing the proliferation and cytokine production of CD4+ T cells.7
Generally, the suppressive activity of CD4+CD25+ Tregs is dependent on T-cell receptor activation or T-cell priming. T-cell receptor activation could regulate not only the effector function of Tregs but also their migratory behavior. Thus, upon T-cell activation, Tregs may migrate from the T-cell–rich zone to the germinal centers by switching the chemokine receptor CCR7 expressed by the interfollicular Tregs to the CXCR5, the specific receptor for the follicle-homing chemokine CXCL13.30 Of interest, intrafollicular Tregs suppress germinal center T helper (GC-Th) cells and the GC-T–induced B-cell responses such as immunoglobulin (Ig) production and expression of activation-induced cytosine deaminase (AID).30 In addition, GC-Tregs also suppress the GC-Th–cell–dependent survival of B cells.30 Moreover, Tregs may have a direct effect on B cells.30-32 Thus, a recent report has described that, under T-cell–free activation conditions, FOXP3-positive Tregs are able to directly suppress B cells by reducing the expression of AID and inhibiting their Ig class switch recombination and Ig production.32 Besides, Tregs may suppress B-cell proliferation by inducing cell death mediated by a cytotoxic-dependent pathway.33 All these observations on the specific direct and indirect B-cell inhibitory functions of the GC-Tregs allow us to hypothesize that the favorable clinical impact of a high number of Tregs in FL patients may be due to their inhibitory effects on the tumor cells. In this sense, it is interesting that in our study the number of Tregs in the follicular compartment, but not in the interfollicular area, was a predictor of a better outcome in patients.
The natural history of FL is characterized by continuous relapses that become progressively less sensitive to chemotherapy.34 The analysis of prognostic factors at relapse should be useful to select patients for whom inclusion in experimental trials is warranted. Several clinical parameters, including response duration and FLIPI among others, seem to be of prognostic significance in FL at first relapse.34-36 However, the predictive significance of biologic markers at relapse in FL has not been well examined. In this study, we have assessed the prognostic value of Treg numbers in FL at first relapse. Contrary to the results in the samples at diagnosis, the number of Tregs at first relapse did not predict survival. However, the number of patients was relatively low to draw definitive conclusions. Of interest, we could examine sequential samples in 18 patients obtained at diagnosis and first relapse. The Treg infiltrate was similar in tumors that relapsed with the same cytologic grade and decreased slightly in tumor progressing from grade 1 or 2 to grade 3. However, the number of these cells was dramatically reduced in cases that transformed to DLBCL. These extremely low numbers of Tregs were also observed in the 2 DLBCL components present at diagnosis and in 2 additional cases diagnosed at the first relapse in which the biopsy at diagnosis could not be examined. These observations indicate that transformation of FL to DLBCL is associated with a marked reduction in the Treg infiltrate. Although the low numbers of Tregs may facilitate the progression of the tumors by releasing inhibitory signals upon the tumor B cells, further studies are needed to determine the potential role of these cells in the progression of FL.
In summary, the results of this study show that high numbers of Tregs are not related to the total numbers of CD3+ and CD4+ cells and predict improved overall survival in FL independently of the FLIPI. In addition, transformation to DLBCL is associated with a marked reduction in the number of associated Tregs, suggesting that these cells may also play a role in tumor progression. These findings support the idea that tumor-infiltrating Tregs may be an important subset of cells modulating the tumor microenvironment and influencing the biologic behavior of FL. A better understanding of the biologic role of FOXP3-positive Tregs in these tumors should assist in the development of therapeutic strategies based on the immunomodulation of the tumor microenvironment.
Prepublished online as Blood First Edition Paper, July 6, 2006; DOI 10.1182/blood-2006-04-018218.
E.C. and A.H.B. contributed equally to the study.
Supported by the Spanish Ministry of Education and Science (SAF 05/5855), Instituto de Salud Carlos III, Redes Temáticas de Investigación Centros de Cáncer (C03/10) and Red de Estudio de Linfomas (G03/179), and the Leukaemia Research Fund.
J.C. was responsible for producing the database, histopathologic revision of the samples, quantifying the immune marker, data analysis, and preparing the final manuscript; A.L.-G. was responsible for acquiring of clinical data and the statistical analyses; B.C.F. performed the immunohistochemical staining; L.C. was responsible for the histopathologic revision of the samples, data acquiring, and data analysis; A.M. contributed to the data analysis; G.R. produced and characterized the anti-FOXP3 monoclonal antibody; E.M. was responsible for evaluating the clinical data; and E.C. and A.H.B. contributed to the design of the project, data analysis, and writing of the paper. All authors reviewed and approved the final version.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.