Comment on Savoldo et al, page 2942
Epstein-Barr virus (EBV)–infected B lymphocytes and T cells acheive a homeostatic balance in healthy individuals. Increasing virus-specific cytotoxic T lymphocyte (CTL) numbers by adoptive immunotherapy may be effective as antitumor therapy but not affect the viral load in peripheral blood lymphocytes.
In this issue of Blood, Savoldo and colleagues report on their experience expanding and infusing autologous CTLs targeting EBV in solid organ transplantation (SOT) patients at high risk for EBV lymphoproliferative disease or with established EBV lymphoproliferative disease.
The authors report no barrier to the expansion of EBV-specific cells from patients on posttransplantation immunosuppression but note that although the infused cells expand in vivo, the expansion is much more limited than that achieved in the bone marrow or hematopoietic stem cell transplantation setting, perhaps reflecting availability of lymphoid space in the marrow or hematopoietic stem cell transplantation settings. Both observations are important for the application of adoptive cellular immunotherapy.FIG1
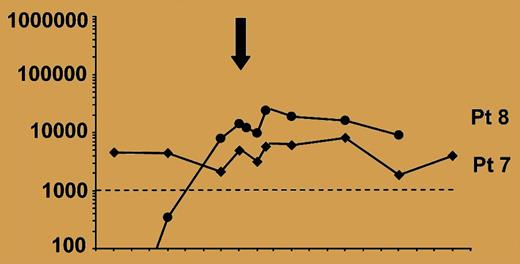
Monitoring of EBV load in SOT recipients after adoptive transfer of EBV-CTLs.
Monitoring of EBV load in SOT recipients after adoptive transfer of EBV-CTLs.
Of special interest to those concerned with the nature of viral persistence is the observation that the infusion of cytotoxic T lymphocytes (CTLs) targeting EBV often does not affect viral genome copy number in peripheral blood lymphocytes (see figure)—even when these CTLs are active in killing localized tumor. This likely reflects the very limited viral gene expression in peripheral blood lymphocytes that harbor virus. Although in the laboratory, infection of resting B lymphocytes leads to the outgrowth of lymphoblastoid cell lines expressing many viral genes and continuously proliferating, it is now clear that in healthy EBV-seropositive individuals and even in many patients on immunosuppressive agents or with human immunodeficiency virus infection, EBV genomes are predominantly harbored in resting memory B cells that express few if any viral antigens. This ability to persist in resting cells with limited viral gene expression is part of the explanation for life-long persistence of EBV infection. As the present report indicates, even when EBV CTL numbers are increased by adoptive immunotherapy, genome copy numbers in these cells are not changed. The virus can hide in plain sight. The good news for patients is that adoptive T-cell therapy can be effective against tumor cells expressing a spectrum of antigens even if these virus-infected lymphocytes in the blood evade the CTLs. ▪