Abstract
The aim of this study was to understand the impact of chronic graft-versus-host disease (cGVHD) on the overall health status of hematopoietic cell transplantation (HCT) survivors. Subjects included 584 individuals who had undergone allogeneic HCT between 1976 and 1999, survived 2 or more years, and completed a 255-item health questionnaire. Global assessment of health status was facilitated by measurement of 6 health status domains: general health, mental health, functional impairment, activity limitation, pain, and anxiety/fear. Information regarding diagnosis of cGVHD was abstracted from medical records, and presence of active cGVHD in the preceding 12 months was self-reported. The incidence of cGVHD in participants was 54%, of whom 46% reported active cGVHD. In multivariable analyses, subjects with active cGVHD were more likely to report adverse general health, mental health, functional impairments, activity limitation, and pain than were those with no history of cGVHD. However, health status did not differ between those with resolved cGVHD and those who never had cGVHD. We conclude that active cGVHD has a significant impact on many aspects of the overall health status of HCT survivors and that, most importantly, those successfully treated for cGVHD do not appear to have long-term impairments.
Introduction
Increasing numbers of hematopoietic cell transplantations (HCTs) are now being performed, and improved outcomes are resulting in a growing number of survivors. Chronic graft-versus-host disease (cGVHD) is a relatively common complication after allogeneic HCT, with several series reporting an incidence of 40% to 70%.1 The incidence of cGVHD is likely to increase in the future, secondary to the increasing use of HCT in older patients, transplants from unrelated and mismatched related donors, peripheral blood stem cell transplants, and donor lymphocyte infusions, all of which carry a higher risk for the development of cGVHD.1-3 However, response to the currently available therapeutic options for cGVHD is suboptimal.4 One prospective cohort study documented response rates of 61%, 53%, and 50% at 6 months, 1 year, and 2 years, respectively. In that study, the prevalence of active cGVHD was 33% at 2 years, but only 18% had discontinued immunosuppressive therapy by 2 years and 89% by 4 years.5 cGVHD and/or its treatment has been identified as the leading cause of nonrelapse mortality (NRM) in HCT survivors.6 A large registry-based study demonstrated that acute myeloid leukemia (AML) patients with active cGVHD at 2 years were 3 times more likely to experience NRM than were those without cGVHD and 1.7 times more likely to die from any cause.6 However, the presence of cGVHD is associated with a reduced risk of relapse (relative risk = 0.5-0.6).7
The primary cause of NRM associated with cGVHD is infection, but several studies have also demonstrated an association between cGVHD and reduction in Karnofsky performance scores, poorer quality of life, and later return to work in HCT survivors with cGVHD.6,8-12 These studies, however, have not been designed to examine the impact of cGVHD on specific aspects of overall health. Other studies have documented specific medical late effects following HCT, long-term health-related quality of life after HCT, and the rate of physical and psychological recovery after HCT but have not specifically addressed the impact of cGVHD upon these outcomes.13-15 Additionally, no previous study has addressed the question of the impact of successfully treated or resolved cGVHD on health-related outcomes. The purpose of this study was to gain a better understanding of the impact of cGVHD on the overall health status of HCT survivors and to determine whether any impairment persisted upon resolution of cGVHD.
Patients, materials, and methods
Subjects
The Bone Marrow Transplant Survivor Study (BMT-SS), a collaborative effort between the City of Hope Cancer Center (Duarte, CA) and the University of Minnesota (Minneapolis), examines the long-term outcomes of individuals who have survived 2 or more years after undergoing HCT. The present report from BMT-SS is restricted to individuals who met the following eligibility criteria: (1) allogeneic HCT between 1974 and 1999 at City of Hope or University of Minnesota; (2) age 18 years or older at the time of questionnaire completion; and (3) survival of at least 2 years from HCT. The Human Subjects Committees at the participating institutions approved the BMT-SS protocol. Informed consent was provided according to the Declaration of Helsinki.
Data collection
Participants completed the BMT-SS questionnaire, a 255-item survey assessing medical late effects, current medical conditions, medication use, health status, health behaviors, pregnancy history, demographic characteristics, socioeconomic indicators, insurance coverage, and other information. The BMT-SS questionnaire specifically addresses functional impairment and activity limitations and their impact upon daily life at home, school, or work. The questionnaire was originally developed for use by the Childhood Cancer Survivor Study16 and was subsequently modified to address topics specifically related to the HCT survivor population. The questionnaire has a yes/no/don't know format for the majority of questions or a Likert scale or ordinal response to score degree of impairment or dysfunction. The BMT-SS questionnaire was validated on a random sample of 100 HCT survivors, and the agreement with medical records was excellent (percentage agreement adjusted for chance, kappa > 0.8) for musculoskeletal, cardiovascular, pulmonary, and endocrine impairments and for GVHD, and moderate (kappa 0.4-0.7) for second cancers, central nervous system disorders, and eye problems.17 Data on patient and treatment characteristics were prospectively collected, and presence or absence of cGVHD was abstracted from these databases. The presence or absence of active cGVHD in the preceding 12 months was self-reported. Subjects also self-reported whether or not they had taken immune suppressant therapy for at least 1 month during the 2 years prior to questionnaire completion.
Outcomes
The outcomes measured in this analysis were 6 domains of health status previously defined by Hudson et al18 from the Childhood Cancer Survivor Study. We measured these same 6 domains of health status including (1) general health, (2) mental health, (3) functional impairment, (4) activity limitation, (5) pain as a result of HCT or primary diagnosis, and (6) fear/anxiety as a result of HCT or primary diagnosis. Participants were considered to have an adverse outcome with regard to their general health if they responded that it was “fair or poor” as opposed to “good, very good, or excellent.” Mental health status was measured using the 18-item Brief Symptom Inventory (BSI-18).19,20 This measure provides a global severity index and symptom-specific subscales for depression, somatization, or anxiety. Participants were considered to have had an adverse outcome with respect to their mental health if they scored in the lowest 10% of population norms on any of the 3 symptom-specific subscales. Questionnaire items selected to measure functional impairment and activity limitation were adapted from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System Survey questionnaire.21,22 Participants were considered to have had an adverse functional outcome if they answered “yes” to any of 3 questions asking whether they currently had any health problem that resulted in (1) needing help with personal care needs, such as eating, bathing, dressing, or getting around their home; (2) needing help in handling routine needs, such as everyday household chores, doing necessary business, shopping, or getting around for other purposes; or (3) being unable to hold a job or attend school. Activity limitations were recorded if participants responded that during the last 2 years their health had limited them for more than 3 months in any of the following 3 areas: (1) the kinds or amount of moderate activities they could perform, like moving a table, carrying groceries, or bowling; (2) walking upstairs or climbing a few flights of stairs; or (3) walking one block. Participants were considered to have had an adverse pain outcome related to their HCT or primary diagnosis if they responded that they currently had a medium amount, a lot of, or excruciating pain as opposed to none or a small amount. Similarly, participants were considered to have had an adverse fear/anxiety outcome related to HCT or their primary diagnosis if they reported a medium amount of, a lot of, or extreme anxiety as opposed to none or a small amount.
Independent variables
Sociodemographic variables considered in the analysis included sex, ethnicity, age at interview, level of education, insurance coverage, and annual household income. Disease- and treatment-related variables considered included diagnosis, stem cell source, donor type, conditioning regimen, age at transplantation, time since transplantation, year of transplantation, and institution. Continuous variables were categorized as shown in Table 1.
Data analysis
Descriptive statistics including means, standard deviations, frequencies, percentages, and ranges were calculated for sociodemographic and treatment variables for the eligible study population, stratified by participant and cGVHD status represented as a 3-level variable: (1) no history of cGVHD; (2) history of cGVHD (resolved); and (3) active cGVHD in past 12 months. Two sample t tests for continuous variables and Chi-squared tests for dichotomous variables were used to test for significant differences between participants and nonparticipants and between survivors with active cGVHD, those with resolved cGVHD, and those with no history of cGVHD for transplant-related variables and sociodemographic factors.
Frequencies and percents were calculated for each adverse health status outcome among HCT survivors, both as totals and stratified by suspected transplantation and sociodemographic risk factors. Proportions of adverse health status outcomes were compared between those with active cGVHD, those with resolved cGVHD, and those without a history of cGVHD in multivariable models, one that included treatment-related risk factors and the other that included sociodemographic risk factors.23 Odds ratios (ORs) and 95% confidence intervals (CIs) are reported from unconditional logistic regression models.24 Models that evaluated the influence of cGVHD on adverse health status while accounting for transplantation-related variables included sex, age at interview, time since transplantation, donor type, and conditioning regimen. Models that evaluated the influence of cGVHD on adverse health status while accounting for sociodemographic risk factors included sex, race, age at interview, education, insurance, and annual household income. Stem cell source and treating institution were not found to be independent predictors of the outcomes nor did they appreciably alter the risk estimates, so they were not included in the final models. Two-way interaction terms for both the treatment-related risk factors and the sociodemographic risk factors were also evaluated. Confounding was examined for each variable, looking at the strength and the precision of the estimate in both full and reduced models.25 SAS version 9.1 was used for all analyses (SAS Institute, Cary, NC).
Results
Recruitment and subjects
Nine hundred ninety-seven HCT survivors were eligible for our analysis. Among these survivors, 280 (28.1%) refused participation, 123 (12.3%) could not be contacted, 6 (0.6%) enrolled but did not complete the health questionnaire, and 4 (0.4%) were pending data collection. This final analysis included 584 HCT survivors, 58.6% of those presumed eligible and 66.8% of those successfully contacted. Table 1 describes the characteristics of study participants and provides a comparison between them and nonparticipants. Participants were slightly more likely than nonparticipants to have a history of cGVHD (53.6% versus 46.7%; P = .04).
Chronic graft-versus-host disease
The characteristics of study participants according to their cGVHD status are summarized in Table 2. Overall, 313 (53.8%) subjects were diagnosed with cGVHD, of whom 144 (24.6%) reported active cGVHD within the previous 12 months. The prevalence of active cGVHD was 54.9% in those interviewed 2 to 5 years from their HCT, 29.2% in those interviewed 6 to 10 years after their HCT, and 16% in those who received transplants 11 to 28 years prior to the interview. Table 3 shows that the prevalence of reporting taking immune suppressant therapy for at least 1 month during the preceding 2 years in the active, resolved, and no cGVHD groups was 88.6%, 36.5%, and 30.8%, respectively, 2 to 5 years following HCT; 81.0%, 15.5%, and 5.8% 6 to 10 years following HCT; and 39.1%, 1.7%, and 4.8% at least 11 years from the time of HCT.
Adverse health outcomes
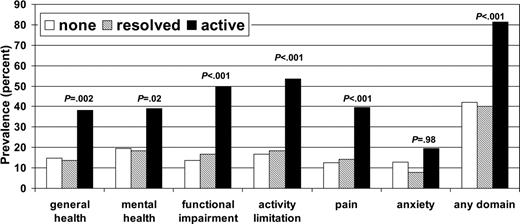
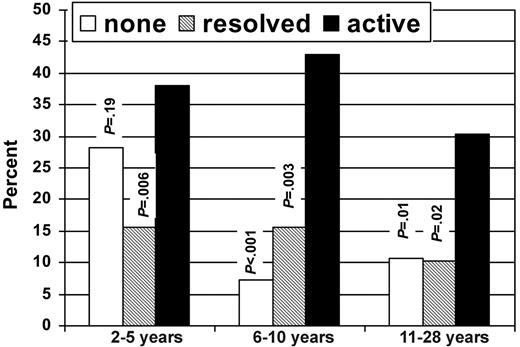
Figure 1 shows the prevalence of HCT survivors with adverse health outcomes according to cGVHD status. Overall, 81.3% of subjects with a history of active cGVHD had an adverse outcome in at least one domain, compared with 40.2% of subjects with resolved cGVHD and 42.1% of subjects with no history of cGVHD. There were no significant differences in frequency of adverse outcomes in any of the domains between those with no history of cGVHD and those with resolved cGVHD. Figure 2 shows the percentage of subjects who reported poor general health according to time since HCT and cGVHD status.
Table 4 shows the OR estimates and 95% CIs for adverse outcomes according to cGVHD status in a model adjusted for sex, age at interview, time since transplantation, donor type, and conditioning regimen. Again, there were no statistically significant differences seen in adverse health outcomes between those with no history of cGVHD and those with resolved cGVHD. In contrast, those with active cGVHD have significantly increased risk of adverse outcomes in the domains of general health (OR, 2.7; 95% CI, 1.6-4.6), mental health (OR, 2.7; 95% CI, 1.6-4.4), functional impairment (OR, 5.6; 95% CI, 3.3-9.4), activity limitation (OR, 5.1; 95% CI, 3.1-8.3), and pain (OR, 4.2; 95% CI, 2.4-7.1) but not anxiety related to HCT (OR, 1.5; 95% CI, 0.8-2.6). Other significant associations were seen between functional impairment and female sex (OR, 1.7; 95% CI, 1.1-2.7), age older than 45 years at the time of interview (OR, 1.7; 95% CI, 1.1-2.7), and having an unrelated donor (OR, 2.3; 95% CI, 1.3-3.9). Additionally, activity limitations were significantly associated with conditioning regimen containing radiation (OR, 2.9; 95% CI, 1.2-7.4), adverse general health with having had a HCT 2 to 5 years ago (OR, 1.9; 95% CI, 1.1-3.3), and anxiety related to transplantation with having had the HCT 2 to 5 or 6 to 10 years ago (OR, 2.2; 95% CI, 1.1-4.4) when compared with those who were treated more than 11 years ago.
Table 5 shows the OR estimates and 95% CIs for adverse health outcomes according to cGVHD status in a model adjusted for potential sociodemographic risk factors. This model confirms active cGVHD as the strongest predictor for adverse outcomes in all domains except anxiety related to HCT, with the effect once again most marked in the domains of functional impairment (OR, 6.4; 95% CI, 3.8-11.0), activity limitation (OR, 5.4; 95% CI, 3.3-8.9), and pain (OR, 4.3; 95% CI, 2.5-7.3). As in the previous model, there were no significant differences in adverse outcomes between those with no history of cGVHD and those with resolved disease. This model also demonstrated a significant association between adverse outcomes in the domains of general health (OR, 1.9; 95% CI, 1.1-3.4), mental health (OR, 2.2; 95% CI, 1.3-3.7), functional impairment (OR, 4.3; 95% CI, 2.4-7.6), activity limitation (OR, 2.3; 95% CI, 1.2-4.0), and anxiety related to HCT (OR, 2.0; 95% CI, 1.1-3.8) and having an annual household income of less than $20 000 per year.
Discussion
A previous report from the BMT-SS analyzing late effects in survivors of chronic myeloid leukemia treated with HCT identified cGVHD as the most important predictor of adverse medical late effects and poor overall health among this patient population.26 We undertook this present analysis to better define the impact of cGVHD on the overall health status of all HCT survivors by measuring 6 previously defined health domains. This study demonstrated that cGVHD had a significant impact on the overall health status of HCT survivors. The impact was most marked in the areas of functional impairment, activity limitation, and pain. We also demonstrated that outcomes in survivors with resolved cGVHD were equivalent to those who had never been diagnosed with cGVHD. This finding has not previously been documented and is encouraging but also emphasizes the need for improved therapeutic strategies for treatment of cGVHD.
Despite the strong association between active cGVHD and adverse health outcomes, it is worth noting that even among those subjects with active cGVHD, 61.8% reported their general health as being good, very good, or excellent. There was also some evidence of possible amelioration of effects of cGVHD over time, with 30.4% of subjects with active cGVHD in the cohort 11 to 28 years after HCT reporting only fair or poor general health, compared with 38% and 42.9%, respectively, in the groups 2 to 5 and 6 to 10 years after HCT (Figure 2).
One of the strengths of this study is the large number of HCT survivors who participated and the broad range of information collected from participants. This enabled us, in contrast to previous large registry-based studies,6,8 to measure more descriptive outcomes regarding the overall health status of subjects. The data regarding the incidence of cGVHD and the proportion of subjects continuing immune suppressant therapy at different time points following HCT are consistent with previously published literature.1,4,7 Specifically, the incidence of cGVHD in this cohort was 54%, of whom about half reported active cGVHD within the previous 12 months, with a median time since transplantation of 8.1 years (range, 2-27.7 years). It is unlikely that subjects, all of whom had survived for at least 2 years after HCT, would have been diagnosed with cGVHD after completing the questionnaire because a previous large study showed that the median time to onset of cGVHD was 3.9 months, with the latest onset at 28 months following HCT.4
The results of the study must be interpreted in the context of potential limitations. Participation rate was 58.6% of those presumed eligible and 66.8% of those successfully contacted. There were several slight differences between participants and nonparticipants; most importantly, cGVHD was more common among participants. As a result, our study may have slightly overestimated the impact of cGVHD on overall health status of survivors, but we do not feel that this difference is sufficient to account for the significant discrepancies in outcome between those with and without active cGVHD.
The fact that a large proportion of the data were collected by self-report increases the likelihood of misclassification bias. This is further complicated by the facts that no validated quantitative criteria for organ-specific or overall responses exist and reversible disease activity and irreversible damage may be difficult to distinguish. However, validation studies have previously shown excellent correlation between medical records and self-reported active cGVHD,17 and the major variable of interest, presence or absence of cGVHD, was abstracted from data that had been prospectively collected by institutional databases, using standardized protocols. Further validation of the distinction between the active and resolved groups can be seen by the highly significant difference (Table 3) between the proportions of subjects in each of these groups who reported taking immune suppressant therapy. Continuation of immune suppressant medication may be considered as a marker of active disease, although clinical experience suggests that this correlation will not be absolute. This difference is less marked although still significant in the group of subjects who received transplants 2 to 5 years ago because the questionnaire item asked whether medications had been taken during the past 2 years and, therefore, some subjects may have been reporting medications used as GVHD prophylaxis rather than cGVHD treatment. Although the cross-sectional design of this study precludes us from making definitive statements regarding the natural history of cGVHD and the required duration of immunosuppressive therapy, our findings are consistent with previously reported data that have addressed those issues prospectively.4 Recently, new response criteria have been proposed by the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease.27 The proposed criteria incorporate patient self-report as an integral part of response assessment, recognizing its value and further validating its use in this analysis.
Prevalence of HCT survivors with adverse health outcomes by cGVHD status.
Unfortunately, data regarding extent of disease or pattern of organ involvement at the time of the questionnaire were not available. However, there is widespread agreement that the current limited/extensive grading system is of limited use in terms of dividing patients into meaningful prognostic categories,7,28 and new consensus guidelines have recently been proposed to facilitate diagnosis and grading of cGVHD,29,30 and these will be useful for future studies. It should also be noted that data were not collected on potentially eligible subjects who had survived 2 years from the time of HCT but subsequently died from relapse or complications related to therapy including cGVHD. This may have resulted in an underestimation of the potential impact of cGVHD. We have made no effort to distinguish between the effects of cGVHD and its treatment. However, as the majority of survivors with cGVHD require prolonged immune suppressant therapy, we feel that this distinction would be largely artificial because withholding therapy is not a viable option.
Percentage of subjects with poor or fair general health according to time since HCT and cGVHD status.P values are for comparison between active and no cGVHD groups and active and resolved cGVHD groups.
Percentage of subjects with poor or fair general health according to time since HCT and cGVHD status.P values are for comparison between active and no cGVHD groups and active and resolved cGVHD groups.
The validity of the outcome measures also warrants some comment. A single validated instrument for assessment of overall health status in cancer or HCT survivors is not available. The Childhood Cancer Survivor Study selected a variety of measures largely adapted from previously validated measures such as the Brief Symptom Inventory 18, the National Health Interview Survey, and the Behavioral Risk Factor Surveillance System Survey questionnaire to obtain a measure of overall health status.19-22 We have applied the same scoring system to the BMT-SS questionnaire. The items selected appear to have good validity; however, we recognize the inherent difficulties in accurately measuring a construct such as global health status.
In conclusion, this large study has identified active cGVHD as having a significant impact on many aspects of the overall health status of HCT survivors, emphasizing the need for a multidisciplinary approach to the management of these patients. This study has also provided evidence for the first time that resolution of cGVHD results in comparable long-term health outcomes to survivors who were never diagnosed with cGVHD.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-02-003954.
Supported in part by grants from the National Cancer Institute (R01 CA078938; S.B.), the Leukemia Lymphoma Society (2192; S.B.), and the National Institutes of Health (K23 CA85503-01; K.S.B.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.