Abstract
Severe congenital neutropenia (SCN) is characterized by neutropenia, recurrent bacterial infections, and maturation arrest in the bone marrow. Although many cases have mutations in the ELA2 gene encoding neutrophil elastase, a significant proportion remain undefined at a molecular level. A mutation (Leu270Pro) in the gene encoding the Wiskott-Aldrich syndrome protein (WASp) resulting in an X-linked SCN kindred has been reported. We therefore screened the WAS gene in 14 young SCN males with wild-type ELA2 and identified 2 with novel mutations, one who presented with myelodysplasia (Ile294Thr) and the other with classic SCN (Ser270Pro). Both patients had defects of immunologic function including a generalized reduction of lymphoid and natural killer cell numbers, reduced lymphocyte proliferation, and abrogated phagocyte activity. In vitro culture of bone marrow progenitors demonstrated a profound reduction in neutrophil production and increased levels of apoptosis, consistent with an intrinsic disturbance of normal myeloid differentiation as the cause of the neutropenia. Both mutations resulted in increased WASp activity and produced marked abnormalities of cytoskeletal structure and dynamics. Furthermore, these results also suggest a novel cause of myelodysplasia and that male children with myelodysplasia and disturbance of immunologic function should be screened for such mutations.
Introduction
Severe congenital neutropenia (SCN) is typically characterized by severe neutropenia, recurrent bacterial infections, and a maturation arrest at the promyelocyte/myelocyte stage in the bone marrow.1 The majority of autosomal dominant and sporadic cases of SCN have been shown to result from heterozygous mutations in the ELA2 gene encoding neutrophil elastase.2,3 There is proof-of-principle evidence for a causative role of the mutation in the disease,4 but the underlying cause in a significant proportion of SCN patients is unknown. However, a novel mutation in the Wiskott-Aldrich syndrome protein (WASp) resulting in an X-linked form of SCN has been reported in a single large kindred.5
Mutations in the WAS gene encoding WASp are more commonly identified as resulting in the Wiskott-Aldrich syndrome (WAS), which in its classic and severest form is characterized by immunodeficiency, microthrombocytopenia, and eczema.6,7 WASp is a 502–amino acid hematopoietic-specific member of a conserved family of proteins that participates in the dynamic regulation of actin polymerization, primarily through activation of the actin-related protein 2/3 (Arp2/3) complex.8,9 Interaction between the C-terminal VCA domain of WASp, which consists of a verprolinhomology domain (amino acid residues 420-446), a central basic connecting region (469-484), and a terminal acidic region (485-502), and the Arp2/3 complex is essential for actin nucleation, which is itself the rate-limiting step in polymerization of monomeric G-actin. Recent data indicate that the activity of WASp is regulated by the adoption of an autoinhibited conformation in which the VCA domain forms a hydrophobic interaction with the GTPase binding domain (GBD, residues 230-288) and adjacent C-terminal residues.10 Binding of GTP-loaded Cdc42 and phosphatidylinositol 4,5-bisphosphate (PIP2) appears to cooperatively disrupt this interaction, thereby freeing the C-terminus for binding or activation of the Arp2/3 complex.11 Other factors have also been shown to influence this process, including phosphorylation of tyrosine 291 that is adjacent to the GBD.12
Nearly 150 unique mutations in the WAS gene have been described in WAS and the related disorder X-linked thrombocytopenia. They are distributed throughout the gene and include missense, nonsense, and frameshift mutations, and insertions, deletions, and mutations of splice sites.13-15 They invariably lead to reduced or completely absent WASp levels and/or activity and diminished actin polymerization in hematopoietic cells.16 As a result, many aspects of hematologic and immune cell function are abrogated, including cell signaling, migration, and phagocytosis. In contrast, the missense mutation Leu270Pro in the kindred with X-linked SCN is in the conserved GBD and results in constitutive activation of WASp in vitro, probably through disruption of autoinhibition.5
In order to determine whether WAS mutations may account for the severe neutropenia in our cohort of patients lacking an ELA2 mutation, we screened the WAS GBD and C-terminal domains in 14 male children, 8 with classic SCN and 6 with evidence of myelodysplasia and/or immunologic abnormalities in addition to neutropenia. Two novel mutations were identified in the GBD and were then further examined in functional assays.
Patients, materials, and methods
Patients
Fourteen male children with apparent congenital neutropenia and no evidence of mutations in the ELA2 gene were recruited. Eight were apparently classic cases of SCN (absolute neutrophil count consistently < 0.5 × 109/L as the sole abnormality); the remainder had a more variable phenotype with evidence of myelodysplasia and/or immunologic abnormalities in addition to severe neutropenia. All 6 of the latter group were lymphopenic and 2 also showed myelodysplastic changes in the bone marrow. One child came from a family in which 3 brothers were affected by an illness characterized by severe neutropenia and lymphopenia, progressive anemia, and probable autoimmune enteropathy. The study was approved by the Local Research Ethics Committee of Great Ormond Street Hospital for Children NHS Trust. Informed consent was provided in accordance with the Declaration of Helsinki.
Molecular diagnostics
The polymerase chain reaction (PCR) was performed on DNA from peripheral blood leukocytes to amplify the GBD, VCA domain, and C-terminus of WAS (exons 7-9, 11, and 12); primers were modified slightly from those published by Schwartz et al18 (exon 7, forward: GGTTGGTAAGTGGGTCAATGAG, reverse: CCTCTGCCCCCAGGAATCTGT; exons 8, 9, and 11 as published; exon 12, forward: CACCAACCTCCCAGGGCATCT, reverse: AGCACAGGGCAGCAAGTAACT), and reaction conditions were as previously described.17,18 Products were bidirectionally sequenced using an Applied Biosystems 310 Analyser with BigDye version 2 terminator chemistry (Applied Biosystems, Foster City, CA). Positive results were confirmed by restriction endonuclease digestion using the enzymes as specified. X-chromosome inactivation patterns of family members of the 2 index cases were analyzed using the human androgen receptor assay (HUMARA) as previously described19 except that the primer was labeled with a fluorescent dye and the products were analyzed using the CEQ 8000 DNA Genetic Analysis System (Beckman Coulter, Fullerton, CA).
Cellular assays
Immunologic function tests. Proliferation was assessed using standard thymidine incorporation assays. Briefly, fresh blood was diluted 1:20 and incubated in the presence or absence of phytohemagglutinin (PHA-HA16; Biostat Diagnostic Systems, Stockport, United Kingdom) at a concentration of 1, 2, 4, and 8 μg/mL for 3 days at 37°C with 5% CO2. Proliferation was determined by incorporation of [3H]thymidine after 4 hours of incubation. CD3-induced proliferation was assessed in a similar fashion but with a 1:10 dilution of blood and 4 days of incubation with an anti-CD3 antibody (M1654; Sanquin, Amsterdam, the Netherlands).
Neutrophil function tests. Phagocytosis was assessed using the PHAGOTEST kit (ORPEGEN Pharma, Heidelberg, Germany) according to the manufacturer's instructions. Briefly, FITC-labeled opsonized E coli were incubated with whole blood from the test subject for 10 minutes at 37°C. The experiments were then cooled and quench solution was added to strip the FITC from extracellular bacteria. Oxidative burst was measured using the PHAGOBURST kit (ORPEGEN Pharma) according to the manufacturer's instructions, with an additional 3-minute incubation of the blood with 3 μg/mL cytochalasin B prior to the addition of N-formyl-Met-Leu-Phe (fMLP).20 Briefly, whole blood was incubated with fMLP, phorbol 12-myristate 13-acetate (PMA), or opsonized E coli for 10 minutes at 37°C followed by the addition of dihydrorhodamine 123 (DHR123) and a further 10-minute incubation. For both assays, samples were analyzed on an EPICS XL flow cytometer using Expo2 software (Beckman Coulter, High Wycombe, United Kingdom) by collecting 10 000 events in the granulocyte gate.
Apoptosis. Apoptosis in lymphocytes was measured by incubating the mononuclear cell fraction from density-centrifugation–separated peripheral blood for 48 hours in RPMI/10% fetal calf serum (FCS; both from Invitrogen, Paisley, United Kingdom) containing interleukin-2 (IL-2; 60 IU/mL) and PHA (10 mg/mL). Apoptosis restricted to the Fas pathway was induced by the addition of 1 mg/mL IgM anti-CD95 antibody (CH11 clone; Kamiya Biomedical, Seattle, WA). A negative control, medium only, was included to assess spontaneous cell death. After a further 48-hour culture, cells were washed and resuspended in annexin buffer (Pharmingen, Oxford, United Kingdom) prior to staining with annexin-V FITC and 7AAD/propidium iodide (PI) to determine apoptosis and death levels. Ten thousand cells were read by Beckman Coulter XL FACS within 30 minutes of staining. Spontaneous apoptosis in bone marrow was measured by incubating density-centrifugation–separated cells for 15 minutes at room temperature with annexin-V FITC and PI to a final concentration of 1 μg/mL for each. The signal was then quenched in annexin buffer and analyzed immediately. Cells had been prelabeled by incubation with an antibody to either CD34, CD33, or CD15 for 30 minutes and then washed with phosphate-buffered saline (PBS).
Bone marrow culture
Bone marrow (BM) mononuclear cells (MNCs) were obtained by Ficoll-Hypaque density centrifugation and cultured in semisolid, methylcellulose-based media with 20% Iscoves modified Dulbecco medium (IMDM), in the presence of stem cell factor (SCF, 10 ng/mL), IL-3 (30 ng/mL), granulocytemonocyte–colony-stimulating factor (GM-CSF, 25 ng/mL), granulocyte–colony-stimulating factor (G-CSF, 25 ng/mL), and erythropoietin (3 U/mL) for 14 days as previously described.21 CD34+ cells were isolated using the MiniMACS system (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions and cultured in IMDM, 20% FCS in the presence of SCF (20 ng/mL), IL-3 (20 ng/mL), and G-CSF (100 ng/mL) for 14 days as previously described.21 Briefly, 105 cells were added to a 1-mL volume of culture media and the cultures subsequently split to keep the cell density below 8 × 105/mL. Experiments were performed in triplicate.
Macrophage culture and immunostaining
Peripheral blood (PB) MNCs were obtained by Ficoll-Hypaque density centrifugation and monocytes isolated by plastic adherence in 25-cm2 tissue culture flasks in RPMI supplemented with 10% FCS, penicillin (100 IU/mL), and streptomycin (100 μg/mL). After 2 hours at 37°C in 5% CO2, nonadherent cells were discarded and fresh media were added containing 10 ng/mL recombinant human macrophage–colony-stimulating factor (rHum-CSF; R&D Systems, Abingdon, United Kingdom), and incubation was continued. Typically this protocol produces at least 80% purity of macrophages when assessed by CD14 expression. On day 6, 105 macrophages were transferred to 13-mm diameter glass coverslips, fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton, and blocked with PBS containing 1% bovine serum albumin for 30 minutes. Macrophages were incubated with rhodamine phalloidin (Molecular Probes, Leiden, the Netherlands) for 20 minutes to visualize F-actin and then with antivinculin antibody (anti–human VIN-1 mouse IgG1 monoclonal, ascitic fluid used at 1:100 dilution [Sigma, Poole, United Kingdom]). Antibody binding was detected with an antimouse antibody conjugated to Cy-5 (Jackson Immunoresearch Europe, Soham, United Kingdom). Images were recorded using a confocal Leica TCS-SP2 microscope equipped with a 100×/1.40-0.7 oil-immersion objective lens (Heidelberg, Germany) to obtain projections of 8-12 z sections and processed with Adobe Photoshop version 5.5 (Adobe Systems, San Jose, CA).
Live-cell analysis
Phase images of PB-derived macrophages were taken using a Zeiss Axiovert 35 microscope (Carl Zeiss, Welwyn Garden City, United Kingdom) and Panasonic wv-BL200 camera (Panasonic UK, Bracknell, United Kingdom). Cell silhouettes were delineated using Photoshop and used to determine the area of spreading and the elongation and dispersion parameter22 using a customized Mathematica (notebook provided by Dr G. A. Dunn [Randall Centre, Kings College London, United Kingdom]). Student t test was used to assess statistical significance. PB-derived human macrophages were plated on clean glass coverslips and mounted in viewing chambers in culture medium (RPMI, 10% FCS, penicillin 100 IU/mL, and streptomycin 100 μg/mL) containing 10 ng/mL rHuM-CSF. Interference reflection microscopy (IRM) images were collected using a Zeiss Standard 18 microscope fitted with an incident light fluorescence attachment. For IRM, exciter and barrier filters were removed from the LP420 reflector and replaced with a narrow band pass filter to isolate the 546-nm line of the fitted mercury arc light source. IRM images were observed using a Zeiss 63 × Neofluar Antiflex oil immersion objective, NA 1.25, and frame grabbing was executed using a Pulnix TM-765 CCD camera (Pulnix Europe, Basingstoke, United Kingdom) and a Matrox Meteor PCI frame grabber (Matrox Graphics, Dorval, QC, Canada) working under in-house software. Single-frame TIFF images were extracted from each TIFF stack and processed using Adobe Photoshop 6 subroutines written in-house. Briefly, 5 images taken 60 seconds apart were overlapped using the difference function of Photoshop to analyze the persistence of adhesion points. A composite image was obtained with 5 grayscale levels representing dynamic adhesions (light gray) and static or persistent adhesions (dark gray and black). The histogram function of Photoshop was used to quantify the percentage of pixels per image corresponding to each grayscale level. The turnover index was calculated by dividing the percentage of pixels present in only one of the frames by the percentage present in all frames. Student t test was used to assess statistical significance. (For more information on methods, see Document S1, available at the Blood website; see the Supplemental Methods link at the top of the online article.)
WASp protein production and actin polymerization assay
Wild-type (WT) and mutant WASp were cloned into the EF-Bos-GST expression vector. COS-7 cells were transfected with GST-WASp-WT, GST-WASp-Ile294Thr or GST-WASp-Ser272Pro, or GST-vector alone, the cells were lysed, and the fusion proteins from 3 different lysate dilutions purified with glutathione–Sepharose 4B beads as previously described.12 U937 cells (1 × 107) were lysed in 1 mL polymerization buffer, PB (1% NP40, 130 mM NaCl, 20 mM Tris-HCl [pH 7.4], 1 mM EDTA, 1% aprotinin, 10 μg/mL leupeptin, 1 mM phenylmethanesulfonyl fluoride), and clarified by centrifugation at 20 000g for 10 minutes. WASp-coated beads (25 μL) were incubated with 250 μL U937-PB supplemented with 5 mM MgCl2 at room temperature for 1 hour with rotation. As a negative control, 2 μM cytochalasin D was added to the lysate to inhibit F-actin polymerization. Beads were washed twice with PB/MgCl2, resuspended in NuPAGE LDS sample buffer (Invitrogen), and heated to 70°C for 10 minutes. Bound proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 4% to 12% NuPAGE Bis-Tris gels (Invitrogen), blotted, and stained with antibodies against GST (B-14: sc-138 monoclonal mouse anti-GST; Santa Cruz Biotechnology, Santa Cruz, CA) to reflect the WASp, or actin (AC-15 monoclonal mouse anti–β-actin; Sigma). Secondary antibody for both was sheep anti–mouse Ig-HRP (Amersham Biosciences, Chalfont St Giles, United Kingdom), and the enhanced chemiluminescence (ECL) Western detection system (Amersham Biosciences) was used for detection. Images were captured with the Uvichemi image documentation system, and densitometry was performed using Uviband software (UVItec, Cambridge, United Kingdom). Each assay was repeated 6 times. For each experiment, mutant actin polymerization activity relative to wild-type WASp was calculated from densitometry signals (DSs) as follows: actin polymerization activity (× WT activity) = actin DSMutant × WASp DSWT/WASp DSMutant × actin DSWT. Statistical significance for each mutant was analyzed using a one-sample t test.
Results
Clinical features and identification of patients with WAS mutations
Novel mutations were detected in the WAS GBD of 2 of the 14 male patients screened.
Patient 1. Patient 1 (P1) was of Zairian parentage and was unusual for SCN in that he did not come to hospital medical attention until the age of 4 years when he presented with prolonged pyrexia of undetermined origin. There was no documented excess of neutropenia or excessive bacterial infections prior to this event. Initial investigations revealed severe neutropenia (absolute neutrophil count, 0.28 × 109/L) and normal hemoglobin level and platelet count but platelet anisocytosis, including large platelets (mean platelet volume, 11.3-13.7 fL; upper limit of normal, 11 fL) (Table 1). Over a period of 3 years, lymphocyte numbers were intermittently reduced, but a reversal of the normal CD4+/CD8+ lymphocyte ratio, reduced natural killer cell (CD3–CD16/56+) numbers, and impaired lymphocyte proliferative responses to CD3 stimulation were invariable (Table 1). Proliferative response to PHA was normal. Neutrophil NADPH-oxidase activation by Fc or fMLP receptor ligation (but not in response to PMA stimulation) and phagocytosis of opsonized bacteria were also markedly impaired (Table 1).
At the time of referral to our institution, BM examination revealed trilineage dysplasia including bizarre megakaryocytic nuclear morphology with both abnormal giant megakaryocytes and micromegakaryocytes, hypogranular and markedly reduced granulopoiesis, and an excess of blasts (9% of nonerythroid cells), consistent with primary myelodysplasia. Cytogenetic analysis at presentation was normal. Analysis 6 months later revealed loss of one copy of chromosome 13 in 3 of 16 metaphase cells analyzed by GTG-band analysis, one of which also had monosomy 14. Interphase fluorescent in situ hybridization using probes to Rb and BCR, however, did not reveal monosomy 13. Three further analyses at yearly intervals have been normal. Apart from occasional sepsis, the patient has remained well on prophylactic antibiotics and is thriving. His clinical condition has not warranted the use of G-CSF. His current bone marrow morphology, although still abnormal, is considerably less dysplastic than originally observed.
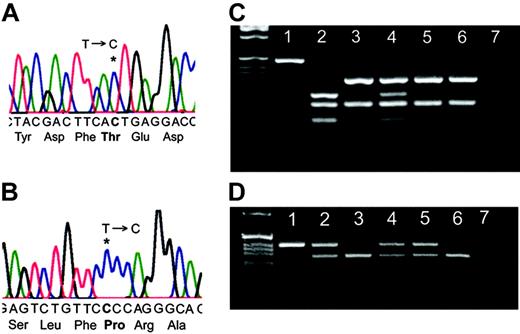
WAS screening showed that P1 had a T-to-C substitution leading to Ile294Thr in the GBD (Figure 1A). Presence of the mutation was confirmed by DdeI restriction enzyme nuclease digestion; his mother was a carrier and his clinically normal sister was unaffected (Figure 1C). The mutation was not detected in 100 randomly chosen healthy controls and 100 individuals of African origin. X-chromosome inactivation patterns of his mother using the trinucleotide repeat HUMARA showed a mean ratio of 79%:21% expression of the 2 X alleles, with no significant differences between the pattern in purified neutrophils and CD3+ cells (data not shown). The androgen receptor gene locus is closely linked with the WAS gene,5 and therefore it was possible to determine that this reflected 79% expression of the wild-type (WT) allele.
Patient 2. Patient 2 (P2) presented as a typical case of SCN in early infancy with severe neutropenia (absolute neutrophil count, 0.19 × 109/L), maturation arrest at the promyelocyte stage in the BM, and recurrent bacterial infections. Hemoglobin level was on the low side of normal. Platelet numbers were usually within the normal range, although large-sized platelets were noted to be present. There was no BM dysplasia, and cytogenetic analysis has been consistently normal. Immunologic analysis revealed low natural killer cell (CD3–CD16/56+) numbers and impaired lymphocyte proliferative responses to CD3 stimulation (Table 1). Neutrophil NADPH-oxidase activation by Fc or fMLP receptor ligation and phagocytosis of opsonized bacteria were also markedly impaired. The patient was treated with G-CSF at a dosage of around 3 μg/kg and has shown excellent clinical and laboratory response with normal neutrophil counts (typically in the range 1.5-2.9 × 109/L). The family is not resident in the United Kingdom, and, therefore, there has been only limited access to material for in vitro studies.
WAS screening showed that P2 had a T-to-C substitution leading to Ser272Pro in the GBD (Figure 1B). Presence of the mutation was confirmed by BpmI restriction endonuclease digestion; his mother, maternal aunt, and maternal grandmother were all carriers (Figure 1D). The mutation was not detected in 50 healthy controls. The HUMARA showed that his mother, maternal aunt, and maternal grandmother all had apparent nonrandom X-inactivation with 98%, 85%, and 79% expression, respectively, of the WT allele (data not shown).
Identification of 2 WAS mutations. (A) Sequence analysis of P1 demonstrating 36201T>C leading to Ile294Thr. (B) Sequence analysis of P2 demonstrating 36143T>C leading to Ser272Pro. (C) Confirmation of the presence of the T>C mutation in P1 by PCR and DdeI restriction endonuclease digestion. Lane 1: undigested PCR product, lane 2: P1, lane 3: patient's sister (clinically healthy), lane 4: mother, lanes 5 and 6: healthy controls, and lane 7: water. (D) Confirmation of the T>C mutation in P2 by PCR and BpmI restriction endonuclease digestion. Lane 1: P2, lane 2: mother, lane 3: father, lane 4: maternal aunt, lane 5: maternal grandmother, lane 6: maternal uncle, and lane 7: DDW.
Identification of 2 WAS mutations. (A) Sequence analysis of P1 demonstrating 36201T>C leading to Ile294Thr. (B) Sequence analysis of P2 demonstrating 36143T>C leading to Ser272Pro. (C) Confirmation of the presence of the T>C mutation in P1 by PCR and DdeI restriction endonuclease digestion. Lane 1: undigested PCR product, lane 2: P1, lane 3: patient's sister (clinically healthy), lane 4: mother, lanes 5 and 6: healthy controls, and lane 7: water. (D) Confirmation of the T>C mutation in P2 by PCR and BpmI restriction endonuclease digestion. Lane 1: P2, lane 2: mother, lane 3: father, lane 4: maternal aunt, lane 5: maternal grandmother, lane 6: maternal uncle, and lane 7: DDW.
The WAS mutations lead to a profound in vitro reduction in neutrophil production and increased levels of BM apoptosis
In vitro ability to produce neutrophils from CD34+ BM cells was examined using a liquid culture system. By day 14 of culture, CD34+ cells from 2 healthy donors expanded 97- and 147-fold, and more than 90% were morphologically postmitotic (ie, metamyelocytes or neutrophils). CD34+ cells from 2 more typical SCN children (one with a Gly56Glu ELA2 mutation and the second a likely autosomal recessive child) expanded 43- and 53-fold; 29% and 37% of cells, respectively, were postmitotic. Samples from P1 expanded 5- and 8-fold on 2 separate occasions, and P2 expanded 9-fold (Figure 2A); 17% to 24% of their day-14 cells were postmitotic. No myeloid colonies were produced in semisolid culture of BM MNCs from both P1 and P2, whereas formation of erythroid colonies was preserved (data not shown).
In both P1 and P2, the level of anti-CD95–induced apoptosis in cultured PB lymphocytes was increased, 34% and 47%, respectively, compared with a mean of 16% for 33 control samples (Figure 2B). The levels of spontaneous apoptosis in freshly derived BM cells at different stages of maturation from 2 healthy donors and P1 were therefore determined by flow cytometric measurement of annexin V positivity in prelabeled bone marrow CD34+, CD33+, and CD15+ cells. Approximately 4-fold higher levels of apoptosis were observed in the patient cells. For CD34+ cells, 52% of P1 cells were annexin V+ compared with a mean of 14% in the controls; for CD33+ cells the values were 53% and 15%, respectively; for CD15+, 15% and 5%, respectively (Figure 2C). Bone marrow was not available from P2 for the above experiment. Taken together, these findings indicate an intrinsic failure of myeloid cell maturation and a propensity of precursor cells to premature apoptosis.
Presence of the Ile294Thr or Ser272Pro WASp mutation profoundly impaired myelopoiesis and led to increased levels of hematopoietic cell apoptosis. (A) CD34+ cell expansion after 14 days in liquid culture. (B) Annexin V–positive cells following anti-CD95 administration to cultured PB lymphocytes. Error bar indicates 1 SD from 33 healthy donors. (C) Spontaneous apoptosis in bone marrow progenitor cells.
Presence of the Ile294Thr or Ser272Pro WASp mutation profoundly impaired myelopoiesis and led to increased levels of hematopoietic cell apoptosis. (A) CD34+ cell expansion after 14 days in liquid culture. (B) Annexin V–positive cells following anti-CD95 administration to cultured PB lymphocytes. Error bar indicates 1 SD from 33 healthy donors. (C) Spontaneous apoptosis in bone marrow progenitor cells.
Morphologic and dynamic abnormalities of the actin cytoskeleton
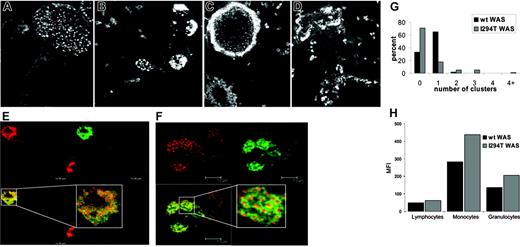
PB-derived macrophages from a healthy donor and P1 were stained for F-actin using rhodamine phalloidin. Normal macrophages were polarized, with podosomes evenly distributed at the leading edge (Figure 3A). Mutant macrophages exhibited a loss of polarization and uneven podosome distribution. Overall in P1, the numbers of cells with podosomes were reduced; however, where present, they were abnormally arranged in multiple clusters or ring structures (Figure 3B-D). Staining with antivinculin antibody revealed a ring of vinculin around an actin core23 for both normal and patient cells (Figure 3E-F), indicating that once assembled they were of normal structure. Fluorescent microscopy of cultured patient macrophages was indicative of increased levels of actin polymerization in all cells, irrespective of the formation of podosomes (data not shown). This finding was confirmed in both patients by immunostaining of freshly isolated PB cells with rhodamine phalloidin and measuring the mean fluorescent intensity (MFI) by flow cytometry (Figure 3G). Mutant cells exhibited a higher MFI than the normal cells, and this was especially pronounced in the granulocyte and monocyte lineages (Figure 3H). Western blotting of lysates from peripheral blood cells of both patients demonstrated that this was not due to increased WASp expression (Document S1).
Macrophages from P1 had abnormal podosome clustering and increased levels of F-actin. (A) Normal macrophages were polarized with podosomes evenly distributed at the leading edge. (B-D) Macrophages from P1 exhibited a loss of polarization and uneven podosome distribution; typically podosomes were found in multiple clusters or ring structures. (E and F) Staining with antivinculin antibody revealed a ring of vinculin around an actin core for both normal and patient cells, respectively. Insets show a magnified view of an individual podosome. (G) Quantification of podosome clusters per cell. (H) Quantification of F-actin in normal and patient cells.
Macrophages from P1 had abnormal podosome clustering and increased levels of F-actin. (A) Normal macrophages were polarized with podosomes evenly distributed at the leading edge. (B-D) Macrophages from P1 exhibited a loss of polarization and uneven podosome distribution; typically podosomes were found in multiple clusters or ring structures. (E and F) Staining with antivinculin antibody revealed a ring of vinculin around an actin core for both normal and patient cells, respectively. Insets show a magnified view of an individual podosome. (G) Quantification of podosome clusters per cell. (H) Quantification of F-actin in normal and patient cells.
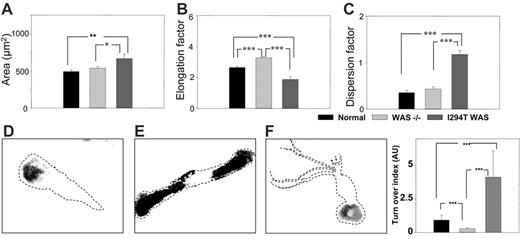
Three morphologic parameters were used to measure the possible differences in cell morphology between macrophages from healthy donors, WASp-deficient (null mutants), and WASp-Ile294Thr patients: area of spreading, elongation, and dispersion factors. Normal macrophages elongated and polarized, displaying a distinctive leading edge and a contracting tail, whereas WASp-deficient macrophages appeared more elongated, displaying a significant (40.3%) increase in the elongation factor (P < .001), and failed to polarize normally (Figure 4A-C). Macrophages from P1 were less elongated and showed an increase in area compared with normal (35.6% increase, P < .01) or WASp-deficient (24.2% increase, P < .05) cells. They also displayed numerous membrane extensions that were absent in normal or WASp-deficient cells, and this was reflected in a 200% increase in the value of the dispersion factor compared with normal (P < .001) and a 174% increase compared with WASp-deficient (P < .001) cells.
Quantification of podosome turnover in live PB-derived macrophages was determined using a method developed by Holt et al (Mark R. Holt, Y.C., Deborah H. Sutton, David R. Critchley, G.E.J., and Graham A. Dunn; “Quantifying cell-matrix adhesion dynamics in living cells using interference reflection microscopy,” manuscript submitted June 2006; also see Document S1). In both normal control and mutant (P1) macrophages, the major area of contact with the substratum was mediated by podosomes, and podosome dynamics were analyzed from 5 images taken 1 minute apart using IRM. Representative composites of macrophages from a healthy control, a WASp-deficient patient, and P1 are shown in Figure 4D, E, and F, respectively. In normal macrophages, 41% of the podosomes were sustained for a mean of 5 minutes, whereas 25% of these disassembled between 1 to 2 minutes. In comparison, in P1 the adhesion sites of macrophages were significantly more dynamic, with only 8% of the adhesions stable for 5 minutes and 35% being disassembled within 1 to 2 minutes (Movies S1-S3). Quantification of the percentage of pixels representing the stability of the adhesions allowed calculation of an adhesion turnover index (Figure 4G). The adhesion sites of P1 macrophages were significantly more dynamic compared with normal cells, with a 10-fold increase in the adhesion index (turnover index for normal cells = 0.93, for P1 cells = 9.14). Attachment of WASp-deficient macrophages is not mediated by podosomes, and the residual adhesion points in WASp-deficient macrophages were significantly more stable compared with both normal and P1 cells (turnover index = 0.29). These findings indicate significant abnormalities of the actin cytoskeleton in P1 macrophages and are suggestive of hyperactivity.
Live WASp-Ile294Thr macrophages exhibited abnormal shape and motility. (A-C) Comparison of area, elongation factor, and dispersion factor for normal, WASp-deficient (–), and WASp-Ile294Thr macrophages. (D,E,F) Representative composite images of normal, WASp-deficient, and WASp-Ile294Thr macrophages, respectively. Dashed lines represent the outline of the cell body. (G) Quantification of podosome turnover. *P < .05; **P < .01; ***P < .001. Error bars indicate 1 SE.
Live WASp-Ile294Thr macrophages exhibited abnormal shape and motility. (A-C) Comparison of area, elongation factor, and dispersion factor for normal, WASp-deficient (–), and WASp-Ile294Thr macrophages. (D,E,F) Representative composite images of normal, WASp-deficient, and WASp-Ile294Thr macrophages, respectively. Dashed lines represent the outline of the cell body. (G) Quantification of podosome turnover. *P < .05; **P < .01; ***P < .001. Error bars indicate 1 SE.
Enhanced ability of mutant WASp to stimulate actin polymerization in vitro
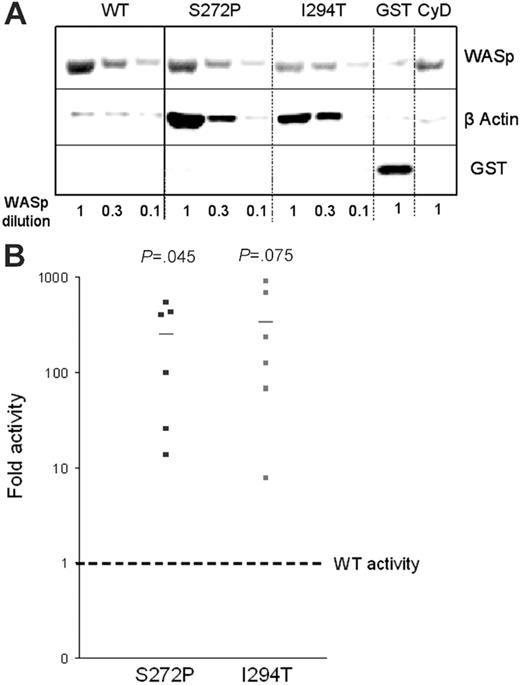
Actin polymerization was assessed in a semiquantitative in vitro assay using Sepharose beads coated with either GST-WASp fusion protein containing either wild-type WASp (WASp-WT), WASp-Ile294Thr, or WASp-Ser272Pro. The beads were incubated with 3 different dilutions of lysates from hematopoietic U937 cells to provide the substrates for actin polymerization, then bound proteins released, resolved by SDS-PAGE, and stained with anti-GST (reflecting WASp) and antiactin antibodies. The level of bound actin was considerably higher using beads coated with either mutant WASp (Figure 5A). Densitometry of GST (WASp) and actin bands was used to calculate the fold increase in actin polymerization compared with that induced by WASp-WT (Figure 5B). Both mutants exhibited increased activity: WASp-Ile294Thr showed a 334-fold increase in polymerization (mean of 6 experiments) and Ser272Pro, a 250-fold increase. The addition of cytochalasin D, which binds to the barbed ends of actin filaments and thus inhibits elongation, abolished polymerization using WASp-Ser272Pro beads (at a U937 lysate dilution of 1) as expected (Figure 5A). Both mutants therefore exhibit an increased capacity to stimulate actin polymerization in vitro.
Discussion
A single kindred has been reported with severe congenital neutropenia and a constitutively activating missense mutation in the GTPase binding domain of WASp.5 We therefore screened the GBD, VCA, and C-terminal domains of the WAS gene in 14 male patients with neutropenia who lacked an ELA2 mutation and identified novel mutations in 2 patients, Ile294Thr and Ser272Pro. Both mutations would be predicted to destabilize the autoinhibited conformation of WASp in which the GBD binds to the C-terminal VCA domain by disrupting the structure of the hydrophobic core, a β hairpin and 5 α-helices arranged in 3 layers.10 Serine residue 272 lies within the α1-helix in the first layer, which would therefore be destroyed by insertion of a proline residue, and this mutation is close to the Leu270Pro mutation identified in the original kindred.5 Similarly, Ile294Thr introduces a polar residue adjacent to Tyr291 on the same face of the α3-helix within the second layer of the hydrophobic core, suggesting that it could disrupt the autoinhibited configuration in an analogous way to phosphorylation of Tyr291. Phosphorylation and dephosphorylation of Tyr291 are thought to be contingent on binding active Cdc42,24 and in the absence of GTP-bound Cdc42, both the phosphorylated and nonphosphorylated forms are protected from covalent modification because of steric occlusion. Destabilization of an autoinhibited conformation, as is likely in the Ile294Thr and Ser272Pro mutants, could make Tyr291 more accessible to both kinases and phosphatases, leading to an enhancement in the kinetics of WASp activity in response to natural input signals from Cdc42 and Src family SH2 domains, the latter of which requires Tyr291 phosphorylation for binding.24 Furthermore, this destabilization would be predicted to lead to dysregulated actin polymerization through uncontrolled activation of the Arp2/3 complex.25-27
Ile294Thr and Ser272Pro mutations enhanced the ability of WASp to stimulate actin polymerization in vitro. Sepharose beads coated with WT or mutant GST-WASp constructs or GST-vector alone were incubated with 3 different dilutions of U937 cell lysates for 1 hour at room temperature. (A) SDS-PAGE of bound proteins stained with anti-GST and antiactin antibodies. (B) Actin polymerization activity for each mutant relative to WT.
Ile294Thr and Ser272Pro mutations enhanced the ability of WASp to stimulate actin polymerization in vitro. Sepharose beads coated with WT or mutant GST-WASp constructs or GST-vector alone were incubated with 3 different dilutions of U937 cell lysates for 1 hour at room temperature. (A) SDS-PAGE of bound proteins stained with anti-GST and antiactin antibodies. (B) Actin polymerization activity for each mutant relative to WT.
In vitro culture of BM and purified CD34+ cells from the 2 patients resulted in an intrinsic disruption of myelopoiesis, with both a severe defect of progenitor cell net proliferation (only one tenth the fold expansion of healthy donor controls) and of maturation (relative percentage of morphologically mature cells approximately one fifth of those in healthy controls). These results were coincident with approximately 3- to 4-fold increased levels of cell death in both patient progenitor cells (CD34+) and more mature myeloid cells (CD33+ and CD15+). The simplest explanation for these observations is that dysregulated actin polymerization has a direct effect on cell growth and/or survival. The actin cytoskeleton plays a crucial role in mitosis,28,29 and pharmacologically induced actin disorganization has been shown to activate a stress-activated mitogen-activated protein kinase pathway and lead to growth arrest.30 Furthermore, cytokinesis requires the regulated formation of a contractile ring of actin and myosin that cleaves the cell in 2. The actin polymerizing activity in this ring is now known to be highly dynamic, with continuous assembly and disassembly, and in the fission yeast Schizosaccharomyces pombe this depends on Arp2/3 complex, the formin Cdc12, profilin, and WASp.31 It is therefore possible that dysregulated WASp activity could compromise cytokinesis, either directly by disturbing normal ring formation, or indirectly by depletion or sequestration of critical substrates for actin polymerization in ectopic cytoskeletal structures. Of interest, there are also clear defects in other lineages (as shown for lymphocytes), indicating that there are global effects on hematopoiesis. The reason for the predominant effect on the myeloid lineage is not clear, although these are shorter lived cells than lymphocytes and are dependent on continual production. It is interesting to note that natural killer cell numbers are similarly depressed, as they also require continual bone marrow production. The fact that the erythroid lineage is relatively spared might suggest that the effect is most profound at relatively late progenitor stages.
In vitro analysis of the WASp mutants indicated that they led to morphologic and dynamic abnormalities of the actin cytoskeleton in patient PB-derived macrophages. In particular, the rate of podosome turnover was markedly increased for WASp-Ile294Thr, suggesting that both assembly and disassembly of podosomes were occurring at increased rates. The degree of WASp activation may also relate directly to the extent to which the autoinhibited configuration is destabilized and/or depend on cooperative interaction with other physiologic activators such as Cdc42, PIP2, and Nck, and in a semiquantitative actin polymerization assay, both mutants showed enhanced WASp activity compared with WT. From experimental models, WASp-Leu270Pro or WASp-Ser272Pro would be predicted to affect binding of Cdc42, whereas Ile294 is just outside this region and therefore Ile294Thr would be predicted to affect only intramolecular interaction with VCA.
The clinical presentation and follow-up of these patients are of interest considering the underlying pathogenesis of their neutropenia. P2 (Ser272Pro) presented as a typical case of SCN that was morphologically indistinguishable from patients carrying a heterozygous ELA2 mutation. In vitro, his progenitor cells showed a proliferative capacity that was poor even when compared with ELA2-mutant+ SCN cells; however, in vivo he has a less severe phenotype than classic SCN and responded to more modest doses of G-CSF. A similar clinical picture has been seen in members of the original WASp-Leu270Pro kindred (Vandenberghe, personal communication). P1 (Ile294Thr) presented with profound myelodysplasia that persisted for several years and, although attenuated, still remains a morphologic feature. The mechanism of this myelodysplasia is unclear; although, of interest, an affected elderly member of the kindred with the WASp-Leu270Pro mutation developed a picture of acute myeloid leukemia (AML) with monosomy 7 while receiving G-CSF therapy (P. Vandenberghe, University of Leuven; personal oral communication, 2004). P1 also had a transient clonal abnormality in the BM (monosomy 13). Together with his highly dysplastic marrow at presentation, this raises the possibility of temporary monoclonal hematopoiesis as has been described in other bone marrow failure syndromes and is associated with an increased risk of frank malignant transformation.32 This further raises concern that these patients may be at particularly high risk of leukemic transformation. In view of this possibility, we would recommend that, where possible, G-CSF be used only in infancy (when the risk of death from neutropenic sepsis is greater) and that prophylactic antibiotics be the mainstay of treatment thereafter.
The diverse clinical presentation of these cases suggests that all young males with myelodysplasia and immunologic abnormalities, in addition to those with molecularly unexplained SCN, should be screened for activating mutations in WASp to enable a better understanding of this disease at a cellular level and also to help management in the clinic.
Prepublished online as Blood First Edition Paper, June 23, 2006; DOI 10.1182/blood-2006-01-010249.
Supported by the Roald Dahl Foundation (P.J.A.), REACH (P.J.A.), Amgen (unrestricted educational grant; P.J.A.), the Primary Immunodeficiency Association (M.P.B., S.B.), the Wellcome Trust (A.J.T., G.E.J., A.W., J.S.), Arthritis Research Campaign (ARC) (Y.C., G.E.J.), the Medical Research Council (MRC) (G.E.J.), and the European Union (A.J.T., M.P.B.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.