Abstract
Promyelocytic NB4 leukemia cells undergo differentiation to granulocytes following retinoic acid treatment. Here we report that tissue transglutaminase (TG2), a protein cross-linking enzyme, was induced, then partially translocated into the nucleus, and became strongly associated with the chromatin during the differentiation process. The transglutaminase-catalyzed cross-link content of both the cytosolic and the nuclear protein fractions increased while NB4 cells underwent cellular maturation. Inhibition of cross-linking activity of TG2 by monodansylcadaverin in these cells led to diminished nitroblue tetrazolium (NBT) positivity, production of less superoxide anion, and decreased expression of GP91PHOX, the membrane-associated subunit of NADPH oxidase. Neutrophils isolated from TG2–/– mice showed diminished NBT reduction capacity, reduced superoxide anion formation, and down-regulation of the gp91phox subunit of NADPH oxidase, compared with wild-type cells. It was also observed that TG2–/– mice exhibited increased neutrophil phagocytic activity, but had attenuated neutrophil chemotaxis and impaired neutrophil extravasation with higher neutrophil counts in their circulation during yeast extract–induced peritonitis. These results clearly suggest that TG2 may modulate the expression of genes related to neutrophil functions and is involved in several intracellular and extracellular functions of extravasating neutrophil.
Introduction
Transglutaminases are a family of Ca2+-dependent enzymes that can mediate covalent cross-linking of proteins by forming isodipeptide bonds between glutamines and the ϵ-amino groups of lysine residues (transglutamylation).1 Several distinct transglutaminases have been described in vertebrates. Among them, TG2, also referred to as tissue transglutaminase, is a multifunctional protein and the only member of the transglutaminase family expressed in a wide variety of tissues and cell types.2 In addition to cross-linking protein, TG2 can modify proteins by amine incorporation, deamination, and by acting as an isopeptidase in a Ca2+-dependent manner.3 Although TG2 cross-links several intracellular and extracellular proteins, the biologic significance of its enzymatic functions is still far from being completely understood. It has been shown in several experimental models that TG2 facilitates apoptosis and clearance of dead cells in response to stimuli that result in its increased expression and activation of transamidating activity.3 TG2 is also known as a cell surface adhesion mediator. Integrin-bound TG2 on the cell surface provides a binding site for fibronectin and facilitates adhesion and spreading of cells.4,5 It plays a significant role in wound healing and angiogenesis, as well as in the assembly, remodeling, and stabilization of the extracellular matrix in various tissues.6,7 The GTP-binding form of TG2, which mediates intracellular signaling by α1B and α1D adrenergic receptors, has a signaling function apart from its transamidating activity.8,9 Data suggest that TG2 dysregulation may contribute to the pathology of neurodegenerative conditions. In Alzheimer and Huntington disease, the enzyme polymerizes proteins with polyglutamine expansions such as huntingtin, and binds to and cross-links β-amyloid peptides forming insoluble protein polymers.10 In celiac disease, the uncontrolled activation of TG2 can generate T-cell stimulatory gluten peptides through deamination of specific glutamines inducing T-cell–mediated autoimmune response and IgA-type autoantibodies against TG2.11 TG2–/– mice are viable and phenotypically normal, but in adult life they show moderate glucose intolerance, defective clearance of apoptotic cells, and decreased fibroblast function and wound healing.12-14
Phagocytes are myeloid-derived leukocytes that are specialized in exiting the blood vessels, migrating through the connective tissues, and finding large, particular targets such as bacteria and fungi to ingest. Myeloid cells committed to mature toward neutrophils spend about 14 days in the bone marrow, where they mature completely to a terminally differentiated state. During this time period, the cells undergo remarkable morphologic and functional changes. A larger portion of the chromatin condenses and associates with the nuclear envelope to form filament-like structures. Meanwhile, the cytosol is filled up with proteins involved in nonoxidative (eg, lactoferrin and gelatinase in specific granules) as well as oxidative (eg, the NADPH oxidase system) killing and in destruction of microbes.15-19
Acute promyelocytic leukemia (APL), characterized by differentiation arrest of granulopoiesis at the promyelocytic stage, is the first human malignancy that can be efficiently treated with a cell differentiation inducer, all-trans retinoic acid (ATRA).20 The retinoid-responsive APL and the human myeloid leukemic cell line NB4 appear to be blocked at an early stage of myeloid differentiation. The therapeutic effect of ATRA relies on the induction of leukemia blast cell differentiation into neutrophil granulocytes that subsequently die by apoptosis.21,22 The NB4 cell line provides an in vitro cell system to study neutrophil maturation, function, and death.23-25
Here we report that TG2 translocates into the nucleus during differentiation of NB4 cells to neutrophil granulocytes. Inhibition of transamidation activity of TG2 during the differentiation process led to a decreased amount of protein cross-links in the nucleus and inhibited the development of certain neutrophil cellular functions such as superoxide anion production. The mRNA levels of GP91PHOX of NADPH oxidase were decreased in the presence of TG2 inhibitor in maturing NB4 cells, and the lack of TG2 activity in TG2–/– mouse neutrophils was accompanied by a significant decrease in gp91phox mRNA and protein expression, diminished NBT positivity, and superoxide anion production with an enhanced phagocytic activity. Here we also report that neutrophils of TG2–/– mice showed decreased extravasation to the peritoneal cavity in response to inflammatory stimuli and exhibited deficient migration.
Materials and methods
Mice, cell lines, and cultures
Wild-type and TG2 KO mice were generated by De Laurenzi and Melino, University of Rome Tor Vergata, Rome, Italy.26
Culture conditions of the t(15;17) promyelocytic leukemia cell line, NB4, have been described previously.23 Briefly, cells were cultured in RPMI 1640 supplemented with 10% (vol/vol) fetal calf serum and 2 mM glutamine (GIBCO, Paisley, Scotland). Cultures were maintained at 3 to 6 × 105 cells/mL by a daily adjustment of cell number, which involved adding fresh culture medium with supplements. Neutrophil differentiation was evaluated by morphology using May-Grünwald-Giemsa staining and by nitroblue tetrazolium (NBT; Sigma, St Louis, MO) reduction assays carried out as described previously.23
Transglutaminase assay
The crude cytoplasmic and nuclear fractions were prepared from untreated and 1 μM ATRA–treated NB4 cells. Cultured cells were lysed in buffer A (250 mM sucrose, 1 mM DTT, 80 mM KCl, 15 mM NaCl, 5 mM EDTA, 15 mM PIPES, pH 7.4, 1 mM PMSF at 4°C) containing 0.1% nonionic detergent nonidet P-40 and homogenized with 8 to 10 strokes in dounce homogenizer. The completeness of lysis was determined by microscopy using May-Grünwald-Giemsa staining. The nucleus and cytosol content of cells were separated by centrifugation at 1100g for 15 minutes at 4°C. Cytosol was centrifuged at 13 400g for 15 minutes at least 3 times to remove the remaining nuclei. The nuclear fractions were washed 3 times in buffer A containing 0.1% NP-40, then lysed in 50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 15 mM β-MEA, 0.1% Triton X-100, 0.5 mM PMSF. TG2 activity was measured in a reaction mixture with a total volume of 100 μL consisting of 50 μL (2 mg/mL) crude cell fraction homogenate, 10 μL N, N-dimethylcasein (40 mg/mL), 20 μL [1,4(n)-3H] putrescine (30 Ci[37 MBq]/mmol), 10 μL 250 mM Tris-HCl, pH 7.5, containing 150 mM β-MEA, and 10 μL CaCl2 (50 mM). The reaction was initiated by the addition of CaCl2, incubated at 37°C for 5 minutes, and then 25 μL of sample was removed, dropped on filter paper, precipitated in cold trichloroacetic acid, and washed intensively with 10% and 5% TCA and ethanol, respectively. The radioactivity of the filter paper was measured in a liquid scintillation β-counter.
Western blot analysis of tissue transglutaminase in lysates of cell fractions
Cells were treated and lysed as described under “Transglutaminase assay.” Lysates containing 2 mg/mL protein were mixed with equal volumes of lysis buffer (0.125 M Tris-HCl, pH 6.8, containing 4% SDS, 20% glycerol, 10% mercaptoethanol, and 0.02% bromophenol blue) and incubated at 100°C for 10 minutes. Protein (25 μg) was electrophoresed on 8% SDS–polyacrylamide gels and electroblotted onto a PVDF membrane. The blot was first saturated with 5% BSA in TTBS. Then, monoclonal antibody CUB7402 (Neomarkers, Fremont, CA) against TG2, diluted in 0.5% BSA in TTBS 1:2000 or 1:8000, was added and incubated at 4°C overnight, or at room temperature for 2 hours, followed by incubation with horseradish peroxidase (HRP)–labeled affinity-purified goat anti–mouse IgG (Sigma) overnight at 4°C, or at room temperature for 1 hour. Each step was followed by 3 15-minute washes in TTBS. Transglutaminase bands were visualized by ECL Kit (Amersham, Little Chalfont, United Kingdom).
Measurement of protein-bound Nϵ-(γ-glutamyl)-lysine linkage
The amount of the Nϵ-(γ-glutamyl)-lysine cross-link content was measured in homogenized samples according to Tarcsa and Fesus.27 Briefly, the protein content of cytosolic and nuclear cell fractions was precipitated and lyophilized. After rehydration, extensive enzymatic digestion, and derivatization with phenylisothiocyanate the obtained Nϵ-(γ-glutamyl)-lysine isodipeptide derivative was separated on a cation exchanger resin and then on a silica high-pressure liquid chromatography column, and finally, quantified after reversed-phase high-pressure liquid chromatography.
In situ labeling of TG2 activity
Cells were labeled with 6 mM 5-(biotinamido)-pentylamine (BPNH2; Molecular Probes, Eugene, OR) for 12 hours, harvested, and fractionated into nuclear and cytosolic fractions. To visualize the proteins into which the 5-(biotinamido)-pentylamine had been incorporated, samples (25 μg of protein) were electrophoresed on 8% SDS–polyacrylamide gels, transferred to PVDF membrane, and probed with horseradish peroxidase–conjugated streptavidin (Amersham) at 1:1000 final dilution in TTBS with 0.5% BSA, at room temperature for 1 hour. The blots were washed and developed as described under “Western blot analysis of tissue transglutaminase in lysates of cell fractions.”
Immunolabeling of cells
Cells were fixed with (1) 4% paraformaldehyde in HEPES (4°C; 10 minutes), (2) 8% paraformaldehyde in HEPES (4°C; 50 minutes); (3) 4% paraformaldehyde in HEPES (4°C; 20 minutes), (4) 2% paraformaldehyde in PBS (15 minutes), and then (5) methanol (–20°C; 20 minutes). After fixation, cells were incubated in 25 mM glycine in PBS (20 minutes); permeabilized with 0.1% Triton X-100 in PBS (20 minutes); washed 5 times for 20 minutes in TTBS; blocked with TTBS, 5% BSA, or milk powder, pH 7.4 (20 minutes); incubated (2 hours) with mouse monoclonal antibody against TG2; washed 5 times for 30 minutes in TTBS; incubated with Alexa Fluor 633 goat anti–mouse IgG (Molecular Probes) (1 hour); washed 5 times for 30 minutes in TTBS; and then rinsed 3 times in PBS before coverslips were mounted in Mowiol (Vector Labs, Burlingame, CA) dissolved in glycerol.
Images were collected using a Zeiss LSM 510 confocal microscope (63× Plan-Apochromat objective, numeric aperture: 1.4; Carl Zeiss, Heidelberg, Germany). PI and Alexa Fluor 633 were excited by 543-nm and a 633-nm HeNe lasers, and fluorescence emission was detected through a 560- to 610-nm bandpass, and a 650 long-pass filter, respectively. Sequential excitation was used to avoid crosstalk between the detection channels. Images were recorded at identical instrument settings to allow direct comparison of intensities. Micrographs were low-pass filtered to reduce noise using the data acquisition software of the LSM 510.
Determination of superoxide anion production by isoluminol and luminol-amplified chemiluminescence
Cells were centrifuged at 200g for 5 minutes at 4°C. A measuring vial (1.0 mL) was prepared containing 0.1 mL (106) cells and 0.1 mL isoluminol or luminol (0.5 mM) in 0.8 mL modified Krebs-Ringer buffer (120 mM NaCl, 5 mM KCl, 1.7 mM KH2PO4, 8.3 mM Na2HPO4, 10 mM glucose, 1 mM CaCl2, and 1.5 mM MgCl2; pH 7.3). The samples were kept for 5 minutes at 37°C and activated by addition of 2 μL PMA (50 nM).
Mouse peritoneal polymorphonuclear neutrophils' O2– production was measured by a chemiluminescence assay using L-012 dye. The reaction volume of 500 μL contained 1 × 105 cells and 5.0 μL L-012 (100 μM). After measuring the background signal, 2 μL PMA (50 nM) was added and incubated for 5 minutes, and then the chemiluminescence was counted in a MOONLIGHT 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA) at intervals of 10 seconds. Production of light was recorded in relative luminometer units (RLUs).28,29
Northern blotting
Total RNA was extracted with TRI Reagent (Sigma) according to the supplier's instruction; 30 μg was electrophoresed in 1.5% agarose gel containing 0.2 M formaldehyde, transferred to a supported nitrocellulose membrane (Hybond-c Extra; Amersham), and UV linked. The membrane was prehybridized with Perfect Hyb Plus (Sigma) for 30 minutes and hybridized with 32P-labeled probes (HexaLabel DNA Labeling Kit; MBI, Fermentas, Vilnius, Lithuania) overnight at 68°C. cDNA clone MGC: 45153 IMAGE: 5505170 corresponding to GP91PHOX was used as a probe.30 Membrane was washed twice at room temperature in 2 × SSC containing 0.1% SDS for 5 minutes, and twice for 20 minutes at 65°C in 0.1 × SSC containing 0.1% SDS. Wrapped blot was exposed to x-ray film (Kodak X-Omat AR; Rochester, NY) using an intensifying screen at –70°C for 5 days.
Real-time quantitative polymerase chain reaction (Q-PCR)
Total RNA was isolated from cells using Trizol Reagent (Invitrogen, Paisley, Scotland) according to the manufacturer's instructions. Extracted total RNA was treated with RNAse-free DNAse (Promega, Madison, WI). Transcript quantitation was performed by quantitative real-time RT (reverse transcriptase) PCR using Taqman probes. Every sample was assayed in triplicate using a no-RT control parallel lacking reverse transcriptase enzyme to detect genomic DNA contamination. For RT reaction, 20 to 100 ng total RNA, specific reverse primer, and Superscript II Reverse Transcriptase (Invitrogen) were used performing 42°C for 30 minutes and 72°C for 5 minutes. Real-time monitoring was carried out using an ABI Prism 7900 (Applied Biosystems, Singapore) performing 40 cycles of 95°C for 12 seconds and 60°C for 1 minute. Transcript levels were normalized to the level of cyclophilin D. Sequences of primers and Taqman probes used in transcript quantitation are the following: hgp91phox+: GTGGCATGGATGATTGCACTT; hgp91phox–: TGACTCGGGCATTCACACA; mgp91phox+: ACAGGAACCTCACTTTCCATAAGATG; mgp91phox–: AACGTTGAAGAGATGTGCAATTGT; hCyclophilin+: ACGGCGAGCCCTTGG; hCyclophilin–: TTTCTGCTGTCTTTGGGACCT; mCyclophilin+: CGATGACGAGCCCTTGG; mCyclophilin–: TCTGCTGTCTTTGGAACTTTGTC; hCyclophilin: FAM-CGCGTCTCCTTTGAGCTGTTTGCA-TAMRA; mCyclophilin: FAM-CGCGTCTCCTTCGAGCTGTTTGCA-TAMRA; hgp91phox: FAM-ACTCTGCGATTCACACCATTGCACATCT-TAMRA; mgp91phox: FAM-TGGATGATAGCACTGCACACCGCC -TAMRA.
Recruitment of peritoneal neutrophils from mice
Wild-type and TG2-deficient mice were injected intraperitoneally with 1 mL 10% yeast extract (Sigma).26 At 4 hours, mice were injected intraperitoneally with 3 mL RPMI-1640 medium, their abdomens were massaged, and total lavage fluid was withdrawn. Peritoneal cells were washed in sterile saline, centrifuged (200 g, 10 minutes, 25°C), and resuspended in RPMI-1640 culture medium. To create a monolayer of PMN cells, the granulocytes were allowed to adhere for a half hour, followed by gentle washing of the monolayer with culture medium to remove nonadherent cells. Following cytospin and May-Grünwald-Giemsa staining, the percentage of neutrophil granulocytes and macrophages was determined.
Chemotaxis assay
The chemotaxis assay was performed by using a 24-well BioCoat Matrigel Invasion Chamber technique. Briefly, Matrigel Invasion Chambers (BD Biosciences, Bedford, MA; the inserts contained 8-μm pore size PET membranes with a thin layer of Matrigel Basement Membrane Matrix and plate) were allowed to rehydrate in serum-free RPMI 1640 for 2 hours in humidified tissue culture incubator at 37°C in 5% CO2 atmosphere. Cell suspension (500 μL; 5 × 104 neutrophil cell/mL) was added to each insert and 500 μL RPMI 1640 supplemented with 10% mouse serum, 100 nM fMLP, and 15 μM monodansylcadaverine (MDC) to wells, when it was needed. Cells were incubated at 37°C for 4 or 8 hours. Eight to 10 randomly selected areas of the cell culture were then photographed, and cells were counted.
Phagocytosis assay
The phagocytic capacity of neutrophils from TG2+/+ and TG2–/– mice was determined by using the Phagotest kit (OPREGEN, Heidelberg, Germany) according to the supplier's instructions. Briefly, 100 μL of whole blood sample was mixed with 20 μL fluorescein isothiocyanate (FITC)–labeled Escherichia coli cells at 0°C. Mixtures of heparinized whole blood and bacteria were incubated at 37°C for 30 minutes. As a control, whole blood and FITC-labeled E coli samples were incubated at 0°C. The fluorescence of bacteria attached to the cell surface was quenched by adding 100 μL Coomassie brilliant blue. After 2 washes, erythrocytes were lysed by incubation in lysing solution for 20 minutes at room temperature. Then propidium iodide was added to stain the DNA of the bacteria and the cells. Neutrophils were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Lincoln Park, NJ). First, cells were gated out according to their DNA content, and then granulocytes were identified on a forward scatter–side scatter plot and the fluorescence (FL1) of the cells was measured. Ten thousand neutrophils were counted in each sample.
Results
Neutrophil granulocyte differentiation is accompanied by the induction of TG2 and its translocation into the nucleus
Previously, it was demonstrated that the treatment of NB4 cells with ATRA can dramatically increase the expression of TG2 mRNA concomitant with neutrophil granulocyte differentiation.31 Treatment of NB4 cells with ATRA decreased cell proliferation and moderately increased NBT positivity after 2 days of treatment, resulting in approximately 90% NBT positivity in 3 to 5 days (not shown). To gain more insight into the function of TG2 during maturation of neutrophils, its distribution in cellular compartments was analyzed. Transglutaminase activity, which was determined in cell lysates by Ca2+-dependent incorporation of [3H]putrescine into dimethylcasein, showed a rapid and strong increase in the cytosol fractions reaching a steady-state level at day 5 and remaining elevated until day 7 (Figure 1A).
Activity could also be detected in the nuclear fraction after 2 days of treatment, which further increased for up to 6 days in differentiated neutrophils (Figure 1A). In accordance with the enzymatic activity measurements, control NB4 cells did not contain TG2 as demonstrated by immunoblot analysis. The enzyme appeared in these cells upon initiation of differentiation, and its amount strongly increased both in the cytosol and the nucleus while NB4 cells underwent the differentiation process (Figure 1B). The enzymatic activity of TG2 was lower in the nucleus than in the cytosol, although both fractions contained similar amounts of TG2 protein as detected by Western blots. The explanation for this contradiction might be the different endogenous polyamines and GTP contents of the 2 compartments or so-far-unrevealed interactions of TG2 with nuclear proteins.
Neutrophil differentiation induces TG2 expression both in the cytosol and the nucleus of NB4 cells. (A) Transglutaminase activity in cells lysates. Cells were treated with 1 μM ATRA for 7 days during which TG2 enzymatic activity was measured in their cytosolic and nuclear fractions. Activity was measured by detecting incorporation of [3H]putrescine into casein. The amount of incorporated [3H]putrescine was determined in a beta-counter. Bars depict the means of 3 separate experiments each performed in duplicate. Error bars indicate standard deviation (SD). (B) Western blot analysis of TG2 in NB4 cells. Cytosolic and nuclear fractions were separated by SDS-PAGE. Total protein (25 μg) was loaded in each lane, and blots were developed with CUB7402 monoclonal antibody against TG2.
Neutrophil differentiation induces TG2 expression both in the cytosol and the nucleus of NB4 cells. (A) Transglutaminase activity in cells lysates. Cells were treated with 1 μM ATRA for 7 days during which TG2 enzymatic activity was measured in their cytosolic and nuclear fractions. Activity was measured by detecting incorporation of [3H]putrescine into casein. The amount of incorporated [3H]putrescine was determined in a beta-counter. Bars depict the means of 3 separate experiments each performed in duplicate. Error bars indicate standard deviation (SD). (B) Western blot analysis of TG2 in NB4 cells. Cytosolic and nuclear fractions were separated by SDS-PAGE. Total protein (25 μg) was loaded in each lane, and blots were developed with CUB7402 monoclonal antibody against TG2.
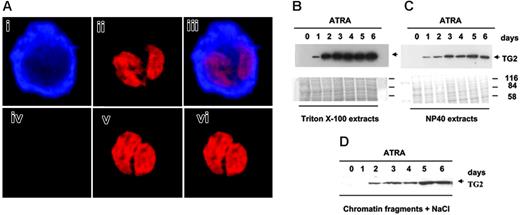
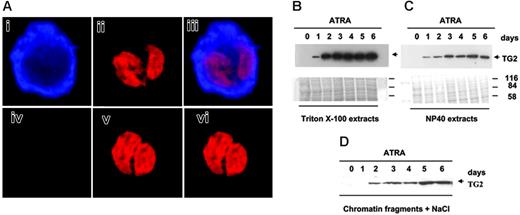
The distribution of TG2 in nuclear fractions of maturing NB4 cells. (A) Localization of TG2 by confocal microscopy. NB4 promyelocytes were cultured in the presence of 1 μM ATRA for 6 days. (i-iii) Cells were fixed, permeabilized, and labeled for TG2 with monoclonal antibodies followed by Alexa Fluor 633–tagged GAMIG (i, blue), and were also stained with PI (ii, red). Panel iii is the overlay image. Note that TG2 is present in the nucleus. In the control, unspecific labeling by Alexa Fluor 633-GAMIG was checked by omitting anti-TG2 antibody (iv). Panel v shows the nucleus of the same control cell, and panel vi, the overlay image of panels iv and i. The thickness of the optical sections shown is 800 nm. (B-D) Extraction of TG2 from nuclei of NB4 cells treated with ATRA. Nuclear samples were fractionated into (B) Triton X-100, (C) NP40-soluble, and (D) nuclear matrix compartments as described in “Materials and methods.” Briefly, the Triton X-100–soluble lipids and proteins were extracted, and then NP40-soluble fractions were obtained. The remaining insoluble protein remnant was suspended in nuclear buffer and sonicated for a short time to get the soluble DNA and protein fraction. Last, with the help of 5 M NaCl, the high salt–soluble protein was extracted from this suspension. The last 2 fractions contained mostly the nuclear matrix, chromatin, and associated proteins.32 From each fraction, 25 μg protein was analyzed by Western blotting using monoclonal antibody to detect TG2. To confirm that the fractions were free of cytoplasmic contamination and that they contained a different protein pattern, the blotted membranes were stained by Coomassie blue and immunoblotted for β-tubulin (not shown).
The distribution of TG2 in nuclear fractions of maturing NB4 cells. (A) Localization of TG2 by confocal microscopy. NB4 promyelocytes were cultured in the presence of 1 μM ATRA for 6 days. (i-iii) Cells were fixed, permeabilized, and labeled for TG2 with monoclonal antibodies followed by Alexa Fluor 633–tagged GAMIG (i, blue), and were also stained with PI (ii, red). Panel iii is the overlay image. Note that TG2 is present in the nucleus. In the control, unspecific labeling by Alexa Fluor 633-GAMIG was checked by omitting anti-TG2 antibody (iv). Panel v shows the nucleus of the same control cell, and panel vi, the overlay image of panels iv and i. The thickness of the optical sections shown is 800 nm. (B-D) Extraction of TG2 from nuclei of NB4 cells treated with ATRA. Nuclear samples were fractionated into (B) Triton X-100, (C) NP40-soluble, and (D) nuclear matrix compartments as described in “Materials and methods.” Briefly, the Triton X-100–soluble lipids and proteins were extracted, and then NP40-soluble fractions were obtained. The remaining insoluble protein remnant was suspended in nuclear buffer and sonicated for a short time to get the soluble DNA and protein fraction. Last, with the help of 5 M NaCl, the high salt–soluble protein was extracted from this suspension. The last 2 fractions contained mostly the nuclear matrix, chromatin, and associated proteins.32 From each fraction, 25 μg protein was analyzed by Western blotting using monoclonal antibody to detect TG2. To confirm that the fractions were free of cytoplasmic contamination and that they contained a different protein pattern, the blotted membranes were stained by Coomassie blue and immunoblotted for β-tubulin (not shown).
Confocal microscopy and immunostaining of permeabilized untreated promyelocytes as well as differentiated NB4 cells confirmed that TG2 accumulates in the cytosol of differentiated cells. Double staining for DNA and TG2 also revealed that TG2 colocalizes with the overall ultrastructural organization of the nucleus of differentiating NB4 cells (Figure 2A).
To determine which part of the nucleus contained TG2 protein, intact nuclei were fractionated by various detergents and analyzed by Western blotting (Figure 2B). These experiments revealed that the amount of TG2 was increased in each fraction obtained on consecutive days of differentiation, and a significant amount of nuclear TG2 was found in the Triton X-100, NP40, chromatin, and matrix fractions (Figure 2B-D).
The induced TG2 is active in maturing NB4 cells
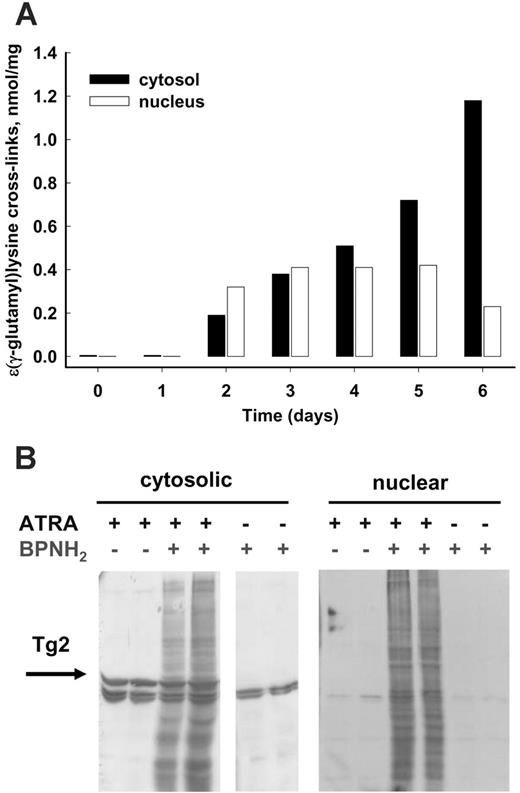
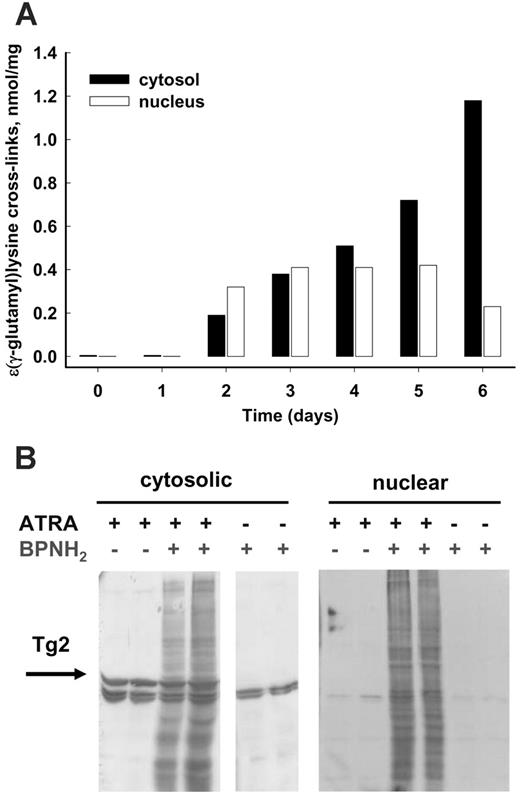
TG2, similarly to other mammalian transglutaminases, requires Ca2+ to become a catalytically active transamidating enzyme. To determine whether the induced TG2 in differentiating NB4 cells was indeed activated, the amount of protein-bound Nϵ-(γ-glutamyl)-lysine was determined. It was found that ATRA treatment not only increased the amount of TG2 protein in NB4 cells, but it also led to increasing levels of protein-bound Nϵ-(γ-glutamyl)-lysine cross-links in both the cytosolic and the nuclear fractions (Figure 3A).
To determine whether tissue transglutaminase was active on primary amines such as polyamines as well, an in situ transglutaminase assay was applied. As a probe for endogenous tissue transglutaminase activity, the biotin-labeled polyamine 5-(biotinamido)-pentylamine was used to detect in situ transglutaminase activity. High numbers of proteins were modified by biotinylated polyamine in the nucleus and the cytosol of intact cells, as shown in Figure 3B.
In ATRA-treated NB4 cells, both cytosolic and nuclear TG2 are active. (A) Protein-bound Nϵ-(γ-glutamyl)-lysine cross-links in differentiating NB4 cells. NB4 cells were treated with 1 μM ATRA for 6 days, then cytosolic and nuclear fractions were separated. The Nϵ-(γ-glutamyl)-lysine cross-link content from each fraction containing 1 to 2 mg protein was determined as described in “Materials and methods.” (B) In situ labeling of proteins of NB4 cells by TG2. Following 1 μM ATRA treatment for 4 days, cells were incubated in the presence of 6 μM 5-(biotinamido)-pentylamine for an additional 12 hours and then separated into cytosolic and nuclear fractions. BPNH2-labeled proteins were analyzed by SDS-PAGE following immunoblotting with horseradish peroxidase (HRP)–conjugated streptavidin. To detect TG2 in the cytosol, the same blot was probed with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. Untreated NB4 control cells revealed the endogenous biotinylated proteins. The parallel lanes represent 2 independent experiments.
In ATRA-treated NB4 cells, both cytosolic and nuclear TG2 are active. (A) Protein-bound Nϵ-(γ-glutamyl)-lysine cross-links in differentiating NB4 cells. NB4 cells were treated with 1 μM ATRA for 6 days, then cytosolic and nuclear fractions were separated. The Nϵ-(γ-glutamyl)-lysine cross-link content from each fraction containing 1 to 2 mg protein was determined as described in “Materials and methods.” (B) In situ labeling of proteins of NB4 cells by TG2. Following 1 μM ATRA treatment for 4 days, cells were incubated in the presence of 6 μM 5-(biotinamido)-pentylamine for an additional 12 hours and then separated into cytosolic and nuclear fractions. BPNH2-labeled proteins were analyzed by SDS-PAGE following immunoblotting with horseradish peroxidase (HRP)–conjugated streptavidin. To detect TG2 in the cytosol, the same blot was probed with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. Untreated NB4 control cells revealed the endogenous biotinylated proteins. The parallel lanes represent 2 independent experiments.
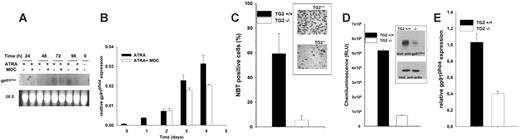
Inhibition of TG2 transamidation activity decreases both amine incorporation into proteins and the protein cross-link content in the cytosol and the nucleus of maturing NB4 cells. NB4 cells were cultured for 96 hours in the presence of 1 μM ATRA or 1 μM ATRA plus 15 μM MDC. (A) Transglutaminase activity in MDC-treated cells. Total cell lysate (50 μg) was used for assaying TG2 activity. Activity was measured by incorporation of [3H]putrescine into casein. The means of 3 separate experiments performed in duplicate are shown. (B) Western blot analysis of TG2 in NB4 cells. The amount of TG2 in ATRA- and ATRA plus MDC–treated samples was determined by SDS-PAGE following immunoblotting with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. (C) Cytosolic and nuclear Nϵ-(γ-glutamyl)-lysine cross-link content in the absence or presence of TG2 inhibitor on day 4 of differentiation. Results are expressed as the mean ± SD of 3 independent experiments.
Inhibition of TG2 transamidation activity decreases both amine incorporation into proteins and the protein cross-link content in the cytosol and the nucleus of maturing NB4 cells. NB4 cells were cultured for 96 hours in the presence of 1 μM ATRA or 1 μM ATRA plus 15 μM MDC. (A) Transglutaminase activity in MDC-treated cells. Total cell lysate (50 μg) was used for assaying TG2 activity. Activity was measured by incorporation of [3H]putrescine into casein. The means of 3 separate experiments performed in duplicate are shown. (B) Western blot analysis of TG2 in NB4 cells. The amount of TG2 in ATRA- and ATRA plus MDC–treated samples was determined by SDS-PAGE following immunoblotting with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. (C) Cytosolic and nuclear Nϵ-(γ-glutamyl)-lysine cross-link content in the absence or presence of TG2 inhibitor on day 4 of differentiation. Results are expressed as the mean ± SD of 3 independent experiments.
Inhibition of TG2 transamidation activity by MDC reduces the level of protein-bound cross-links in differentiating NB4 cells and decreases their capacity to generate superoxide anion
To determine the role of TG2 in differentiating neutrophils, maturing NB4 cells were treated with monodansylcadaverine (MDC), a competitive inhibitor of transglutaminase. The applied concentration of MDC did not cause any phenotypic changes or toxicity in NB4 cells. However, on days 4 and 5 after MDC treatment, a 3- to 4-fold decrease of transglutaminase activity measured in cell lysates was observed, while the level of its expression did not change (Figure 4A-B).
The effect of MDC treatment on the concentration of protein-bound Nϵ-(γ-glutamyl)-lysine cross-links in NB4 cells differentiated for 4 days was then analyzed. It resulted in a significant decrease of the cross-link content both in the cytosol and the nucleus (Figure 4C).
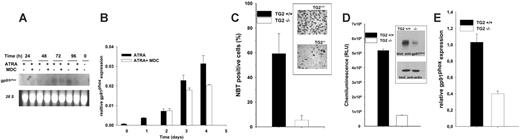
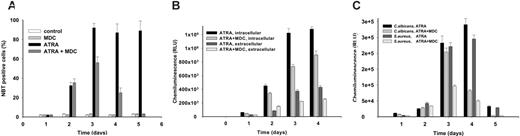
To test the ability of MDC-treated maturing NB4 cells to produce superoxide (O –2), the cells were stimulated by phorbol myristate acetate (PMA) and evaluated for their ability to reduce NBT. Microscopic analysis of cells revealed that 80% of ATRA-treated NB4 cells were NBT positive after 4 days, whereas only approximately 30% of ATRA + MDC–treated NB4 cells showed any detectable formasan precipitates, and only a few NBT-positive cells were formed after 5 days of MDC treatment (Figure 5A).
To measure the production of O –2, luminol- and isoluminol-amplified chemiluminescence assays were performed. The PMA-stimulated oxygen metabolites measured by both luminol and isoluminol yielded results similar to those of the NBT tests. PMA-induced O –2 levels were lower in ATRA + MDC–treated NB4 cells than in cells treated with ATRA alone (Figure 5B). In addition, superoxides triggered by complement-opsonized Staphylococcus aureus or Candida albicans were also significantly decreased in the MDC-treated maturing NB4 cells, indicating that intracellular transglutaminase activity was linked to the regulation of the NADPH oxidase system (Figure 5C).
Inhibition of TG2 activity decreases superoxide production in differentiating NB4 cells. NB4 cells were treated with 1 μM ATRA or 1 μM ATRA plus 15 μM MDC for 5 days. (A) Ratio of NBT-positive cells following treatment with MDC. At the indicated days, cell smears were fixed and analyzed for nitroblue tetrazolium reduction (at least 300 cells were scored for each experimental condition). (B-C) Chemiluminescence reaction of stimulated cells. (B) In NB4 cells (106), extracellular and intracellular NADPH-oxidase activity was induced by 50 nM PMA and measured in the presence of isoluminol (0.5 mM) or luminol (0.5 mM), respectively. (C) NB4 cells (106) were stimulated by opsonized S aureus and C albicans. Extracellular and intracellular NADPH-oxidase response was determined in the presence of isoluminol (0.5 mM) or luminol (0.5 mM), respectively. In contrast to luminol-amplified chemiluminescence reactions, which measure reactive oxygen species (ROSs) both in the extracellular and the intracellular compartments, isoluminol clearly detects the intracellular oxidase activity only.28 Results are the mean ± SD of 3 experiments.
Inhibition of TG2 activity decreases superoxide production in differentiating NB4 cells. NB4 cells were treated with 1 μM ATRA or 1 μM ATRA plus 15 μM MDC for 5 days. (A) Ratio of NBT-positive cells following treatment with MDC. At the indicated days, cell smears were fixed and analyzed for nitroblue tetrazolium reduction (at least 300 cells were scored for each experimental condition). (B-C) Chemiluminescence reaction of stimulated cells. (B) In NB4 cells (106), extracellular and intracellular NADPH-oxidase activity was induced by 50 nM PMA and measured in the presence of isoluminol (0.5 mM) or luminol (0.5 mM), respectively. (C) NB4 cells (106) were stimulated by opsonized S aureus and C albicans. Extracellular and intracellular NADPH-oxidase response was determined in the presence of isoluminol (0.5 mM) or luminol (0.5 mM), respectively. In contrast to luminol-amplified chemiluminescence reactions, which measure reactive oxygen species (ROSs) both in the extracellular and the intracellular compartments, isoluminol clearly detects the intracellular oxidase activity only.28 Results are the mean ± SD of 3 experiments.
Inhibition or loss of TG2 activity leads to down-regulation of GP91PHOX, the major subunit of the NADPH oxidase system, and TG2–/– neutrophils are deficient in generating superoxide anions
It has been previously documented that neutrophils deficient in the transcription factor PU.1 failed to transcribe the gp91phox gene, which encodes the gp91phox subunit of the NADPH oxidase system, and failed to generate superoxide anions.33 To determine whether the decreased function of this subunit was responsible for the decreased superoxide levels in the MDC-treated differentiating NB4 cells, Northern blot analysis was performed to verify the level of GP91PHOX mRNA. As shown in Figure 6A, the MDC-treated cells expressed a lower amount of GP91PHOX mRNA after 72 hours and 96 hours compared with ATRA-only–treated differentiating NB4 cells. Real-time quantitative PCR analysis also confirmed the expression pattern of GP91PHOX mRNA seen on Northern blot analysis (Figure 6B).
To determine the role of TG2 in neutrophil function in vivo, we compared NBT positivity and superoxide anion generation in neutrophils derived from either TG2+/+ or TG2–/– mice.26 Neutrophils were isolated from peritoneal exudates of mice previously injected with yeast extract. In accordance with the NB4 cell culture model, neutrophils of TG2–/– mice showed a 10-fold decrease in NBT-positive cells compared with those of TG2+/+ mice (Figure 6C). To quantify the production of O2 specifically, a luminol analog L-012, by which superoxide can be detected as a chemiluminescence signal with a high sensitivity at lower cell numbers, was used. The neutrophils of TG2–/– mice generated one order of magnitude less superoxide anion than those of TG2+/+ mice when they had been stimulated with PMA (Figure 6D).
Neutrophils with decreased TG2 activity express lower levels of GP91PHOX mRNA and protein, and generate less superoxide. (A-B) Northern blot and real-time quantitative PCR (Q-PCR) analysis of GP91PHOX mRNA expression in NB4 cells. (A) NB4 cells were treated with 1 μM ATRA or 1 μM ATRA plus 15 μM MDC for 3 days. RNA was prepared and subjected to Northern blot analysis as described in “Materials and methods.” (B) NB4 cells were incubated with MDC from day 1 in the presence of ATRA and harvested from following days. Relative expression of GP91PHOX was normalized to the expression of human cyclophilin. (C) NBT positivity of TG2–/– and TG2+/+ mouse neutrophils. Wild-type and TG2–/– mice were injected intraperitoneally with 1 mL 10% yeast extract. Four hours after the injection, the peritoneal lavage fluid was withdrawn and washed. Granulocytes were allowed to adhere for 0.5 hours, and were then analyzed for NBT reduction. Inset images are NBT-stained neutrophils in a 96-well microplate. Visualization was done using an inverted microscope (Axiovert 135; Zeiss, Oberkochen, Germany), an Achrostigmat 20×/0.45 Ph2 objective, adapter ring VAD-S70, and a DSC-S70 digital still camera (Sony, Tokyo, Japan). Film was imaged in AlphaImager 2200 (Alpha Innotech, San Leandro, CA). (D) Superoxide generation and gp91phox protein level of TG2–/– and TG2+/+ mouse neutrophils. Wild-type and TG2–/– mouse neutrophils were collected and separated as previously described. The reaction volume of 500 μL contained 1 × 105 cells and 5.0 μL L-012 (100 μM). ROSs were generated by adding 50 nM PMA. Chemiluminescence was detected as described in “Materials and methods.” Inset: Proteins of wild-type and TG2–/– mouse neutrophils were analyzed by Western blotting using an antibody against the gp91phox and beta-actin. (E) Q-PCR analysis of gp91phox mRNA expression of TG2–/– and TG2+/+ mouse neutrophils; gp91phox mRNA levels were normalized to mouse cyclophilin. Data in panels B-D are representative of 2 independent experiments; error bars represent SD.
Neutrophils with decreased TG2 activity express lower levels of GP91PHOX mRNA and protein, and generate less superoxide. (A-B) Northern blot and real-time quantitative PCR (Q-PCR) analysis of GP91PHOX mRNA expression in NB4 cells. (A) NB4 cells were treated with 1 μM ATRA or 1 μM ATRA plus 15 μM MDC for 3 days. RNA was prepared and subjected to Northern blot analysis as described in “Materials and methods.” (B) NB4 cells were incubated with MDC from day 1 in the presence of ATRA and harvested from following days. Relative expression of GP91PHOX was normalized to the expression of human cyclophilin. (C) NBT positivity of TG2–/– and TG2+/+ mouse neutrophils. Wild-type and TG2–/– mice were injected intraperitoneally with 1 mL 10% yeast extract. Four hours after the injection, the peritoneal lavage fluid was withdrawn and washed. Granulocytes were allowed to adhere for 0.5 hours, and were then analyzed for NBT reduction. Inset images are NBT-stained neutrophils in a 96-well microplate. Visualization was done using an inverted microscope (Axiovert 135; Zeiss, Oberkochen, Germany), an Achrostigmat 20×/0.45 Ph2 objective, adapter ring VAD-S70, and a DSC-S70 digital still camera (Sony, Tokyo, Japan). Film was imaged in AlphaImager 2200 (Alpha Innotech, San Leandro, CA). (D) Superoxide generation and gp91phox protein level of TG2–/– and TG2+/+ mouse neutrophils. Wild-type and TG2–/– mouse neutrophils were collected and separated as previously described. The reaction volume of 500 μL contained 1 × 105 cells and 5.0 μL L-012 (100 μM). ROSs were generated by adding 50 nM PMA. Chemiluminescence was detected as described in “Materials and methods.” Inset: Proteins of wild-type and TG2–/– mouse neutrophils were analyzed by Western blotting using an antibody against the gp91phox and beta-actin. (E) Q-PCR analysis of gp91phox mRNA expression of TG2–/– and TG2+/+ mouse neutrophils; gp91phox mRNA levels were normalized to mouse cyclophilin. Data in panels B-D are representative of 2 independent experiments; error bars represent SD.
Western blot analysis has revealed that the TG2–/– neutrophils expressed a significantly lower amount of gp91phox protein than TG2+/+ neutrophils (Figure 6D insert). In order to establish that the lower amount of gp91phox protein was the consequence of decreased mRNA level, real-time quantitative PCR analysis was carried out that clearly verified that TG2–/– neutrophils expressed a significantly lower amount of gp91phox mRNA than TG2+/+ neutrophils (Figure 6E).
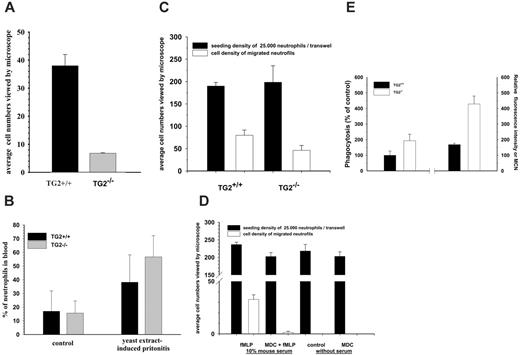
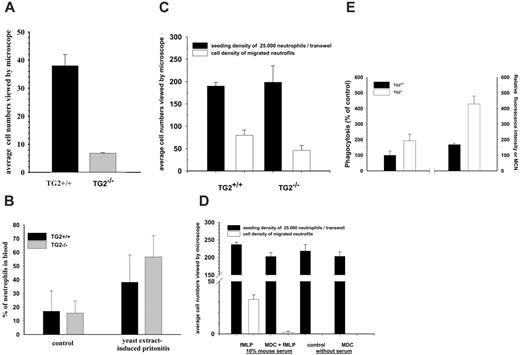
Extravasation, chemotaxis, and phagocytosis capacity of neutrophils in TG2+/+ and TG2–/– mice. (A) Accumulation of neutrophils in the peritoneum when wild-type and TG2-deficient mice were injected intraperitoneally with 1 mL 10% yeast extract. At 4 hours, mice were injected intraperitoneally with 3 mL RPMI-1640 medium and total lavage fluid was withdrawn. To create a monolayer of PMN cells, the granulocytes were allowed to adhere for 30 minutes, followed by gentle washing of the monolayer with culture medium to remove nonadherent cells. Cell number was evaluated on 25 to 30 fields of view seen through the eyepieces of the microscope. (B) Changes of neutrophil counts in circulation 4 hours after injection of 1 mL 10% yeast extract. Mice were killed and their blood samples were collected by cardiac puncture and analyzed for neutrophil counts by fluorescence-activated cell sorting (FACS). In panels A and B, the data represent mean ± SD from 6 to 8 mice per group. (C) In vitro evaluation of chemotaxis of TG2+/+ and TG2–/– neutrophils. Wild-type and TG2–/– peritoneal neutrophils were isolated and kept in serum-free medium overnight and then added into Matrigel Invasion upper chambers and allowed to migrate into the lower chambers containing RPMI 1640 supplemented with 10% mouse serum and 100 nM fMLP. The number of neutrophils migrating through the chambers was determined by photographing both the upper and bottom sides of membranes at the beginning and end of migration. The migration time was 4 hours. (D) In vitro evaluation of TG2+/+ neutrophil chemotaxis in the presence or absence of MDC. Peritoneal lavage neutrophils were kept in serum-free medium in the presence or absence of MDC overnight and then added into Matrigel Invasion upper chambers and allowed to migrate into the lower chambers. Where it is indicated, the lower chambers contained RPMI 1640 medium with or without the addition of 10% mouse serum, 100 nM fMLP, and 15 μM MDC. The number of neutrophils that migrated through the chambers was determined as described previously in panel C, with the difference that the migration time was 8 hours. (E) Percentage of phagocytosing neutrophil granulocytes. Mixtures of heparinized whole blood and FITC-labeled E coli were incubated at 37°C and 0°C, respectively, the fluorescence of the attached bacteria on the cell surface was quenched, and then erythrocytes were lysed. Fluorescing cells out of a total of 10 000 granulocytes were counted by a FACSCalibur instrument and expressed in percentage of total cell number. Each experimental group included 2 to 4 mice, and each individual experiment was performed 2 or 3 times in duplicates. Bars depict the means ± SD.
Extravasation, chemotaxis, and phagocytosis capacity of neutrophils in TG2+/+ and TG2–/– mice. (A) Accumulation of neutrophils in the peritoneum when wild-type and TG2-deficient mice were injected intraperitoneally with 1 mL 10% yeast extract. At 4 hours, mice were injected intraperitoneally with 3 mL RPMI-1640 medium and total lavage fluid was withdrawn. To create a monolayer of PMN cells, the granulocytes were allowed to adhere for 30 minutes, followed by gentle washing of the monolayer with culture medium to remove nonadherent cells. Cell number was evaluated on 25 to 30 fields of view seen through the eyepieces of the microscope. (B) Changes of neutrophil counts in circulation 4 hours after injection of 1 mL 10% yeast extract. Mice were killed and their blood samples were collected by cardiac puncture and analyzed for neutrophil counts by fluorescence-activated cell sorting (FACS). In panels A and B, the data represent mean ± SD from 6 to 8 mice per group. (C) In vitro evaluation of chemotaxis of TG2+/+ and TG2–/– neutrophils. Wild-type and TG2–/– peritoneal neutrophils were isolated and kept in serum-free medium overnight and then added into Matrigel Invasion upper chambers and allowed to migrate into the lower chambers containing RPMI 1640 supplemented with 10% mouse serum and 100 nM fMLP. The number of neutrophils migrating through the chambers was determined by photographing both the upper and bottom sides of membranes at the beginning and end of migration. The migration time was 4 hours. (D) In vitro evaluation of TG2+/+ neutrophil chemotaxis in the presence or absence of MDC. Peritoneal lavage neutrophils were kept in serum-free medium in the presence or absence of MDC overnight and then added into Matrigel Invasion upper chambers and allowed to migrate into the lower chambers. Where it is indicated, the lower chambers contained RPMI 1640 medium with or without the addition of 10% mouse serum, 100 nM fMLP, and 15 μM MDC. The number of neutrophils that migrated through the chambers was determined as described previously in panel C, with the difference that the migration time was 8 hours. (E) Percentage of phagocytosing neutrophil granulocytes. Mixtures of heparinized whole blood and FITC-labeled E coli were incubated at 37°C and 0°C, respectively, the fluorescence of the attached bacteria on the cell surface was quenched, and then erythrocytes were lysed. Fluorescing cells out of a total of 10 000 granulocytes were counted by a FACSCalibur instrument and expressed in percentage of total cell number. Each experimental group included 2 to 4 mice, and each individual experiment was performed 2 or 3 times in duplicates. Bars depict the means ± SD.
Impairment of neutrophil extravasation is concomitant with an enhanced phagocytic capacity of opsonized E coli bacteria in TG2–/– mice
Microscopic observation of MDC-treated differentiated NB4 cells revealed that these cells did not move as efficiently as their untreated counterparts on the plate. To assess the potential role of TG2 in the migration of neutrophils to extravasation sites in response to an inflammatory stimulus, wild-type and TG2–/– mice were compared in a yeast extract–induced peritonitis model. Four hours after injection with yeast extract, the mice were killed and neutrophil migration into the peritoneal cavity was quantified. In TG2–/– animals, yeast extract elicited significantly decreased extravasation of neutrophils compared with TG2+/+ mice (Figure 7A). Circulating neutrophil numbers of TG2+/+ and TG2–/– mice were also determined in both normal and inflammatory situations, and it was found that, compared with wild-type mice, TG2–/– mice had higher circulating neutrophil counts in blood (Figure 7B). To determine to what extent TG2 deficiency altered the ability of neutrophils to migrate through extracellular matrix induced by chemoattractant, neutrophil chemotaxis assay through BioCoat Matrigel Invasion Chamber was carried out using isolated peritoneal neutrophils of TG2+/+ and TG2–/– mice. The number of neutrophils migrating across the chambers and accumulating on its lower surface was determined by taking photographic images of the bottom sides of chambers after 4 hours. Considerably less TG2-deficient neutrophils could migrate than wild-type neutrophils, approximately 60% of wild-type neutrophils (Figure 7C). To assess the role of transamidation activity of TG2 in migration of neutrophil in vitro, migration assay was carried out in the presence or absence of MDC. MDC-treated neutrophils showed a significantly reduced capacity to migrate across Matrigel chambers toward the chemoattractant fMLP (Figure 7D). It was our interest to see how the lack of TG2 influences other essential functions of neutrophils such as their ability to phagocyte bacteria. To determine the phagocytic properties of neutrophils of wild-type and TG2-deficient mice, heparinized whole blood was used from TG2+/+ and TG2–/– mice and exposed to FITC-labeled E coli for 30 minutes. The percentage of phagocytosed FITC-labeled E coli by TG2-deficient neutrophils was approximately 70% more than that of the wild-type neutrophils within 30 minutes (Figure 7E). Their phagocytic capacity as shown by relative fluorescence intensity or mean channel number (MCN), which correlates with the number of bacteria per individual neutrophil, was more than 2-fold of the wild-type neutrophils during the same period of time (Figure 7E).
Discussion
The life-long, highly regulated process of hematopoiesis, in which self-renewing hematopoietic stem cells give rise to blood cell lineages, is very suitable to learn the molecular mechanisms that control cell differentiation. Although the retinoid-driven differentiation of promyelocytic leukemia cells to neutrophils is a well-described and clinically used phenomenon, the molecular details of the differentiation program are still not fully understood. Our present study has established the involvement of retinoic acid–induced transglutaminase 2 during the terminal differentiation process in 2 specific aspects: TG2 mediates expression of genes crucial for superoxide anion formation of matured neutrophils and it promotes their migration.
Although TG2 has long been considered to be a cytosolic protein, several lines of evidence suggest that it may be present and have some function in the nucleus as well, despite the fact that TG2 does not have a nuclear localization signal. It has been suggested that TG2 is transported into the nucleus with the help of importin-alpha3.34 In situ transglutaminase activity was first identified in the nuclear compartment of human neuroblastoma SH-SY5Y cells, and the enzyme was also localized to the nucleus by immunochemistry.35 A nuclear GTP-binding protein in rabbit liver nuclei was also identified as a tissue transglutaminase.36 Biochemical evidence of the presence of TG2 activity in nuclei also came from analysis of brain samples from patients with Huntington disease.37 In the sperm of the starfish Asterina pectinifera, histone H2B was found to be cross-linked to H4 through an isodipeptide bond presumably catalyzed by a transglutaminase reaction.38 In apoptotic cells, the retinoblastoma gene product was posttranslationally modified by TG2-catalyzed polymerization.39 TG2 was also shown to cross-link the transcription factor Sp1 in vitro.40,41 In the presented work, we show that the expression of TG2 is increased during ATRA-induced myeloblastic differentiation in NB4 cells at promyelocyte/metamyelocyte stage and elevated further, until the cells reach their terminal maturation stage. After 2 to 3 days of ATRA treatment of NB4 cells, in the postmitotic forms of maturing cells, TG2 is expressed at high levels and the great majority of TG2 resides in the cytoplasm while a significant portion translocates into the nucleus. The intact nucleus fractionated by different detergents contains various kinds of protein-TG2 mixes including high-salt–soluble protein-TG2 complexes. Nuclear localization of TG2 was also confirmed by confocal microscopy, and it was demonstrated that the enzyme was active in situ: it formed protein-bound Nϵ-(γ-glutamyl)-lysine cross-links both in the cytosol and the nucleus. When the exogen polyamine analog 5-(biotinamido)-pentylamine was added to the maturing NB4 cells, the in situ activity of TG2 resulted in the formation of N-mono(γ-glutamyl)-polyamine proteins. To answer the question of whether the induced TG2 activity, particularly its portion appearing in the nucleus, plays a role in the differentiation process of promyelocytic cells along the neutrophil granulocyte lineage, we used a known competitive inhibitor of transglutaminases, MDC, in our cell culture experiments. The MDC-treated, ATRA-differentiated cells showed decreased TG2 enzyme activity as demonstrated by both activity assay and measurement of protein-bound cross-link content of cells. This lower activity could not arise from a decreased TG2 protein level, since both ATRA- and MDC-treated cells expressed the same amount of TG2 protein. It was found that MDC-treated cells showed a significantly reduced NBT reduction capacity, one of the most evident indicators of neutrophil maturation beyond morphologic features, and much lower amounts of superoxide anion when stimulated either with PMA or complement-opsonized bacteria and C albicans. These results suggest that the decreased TG2 cross-linking activity manifested in less protein-bound cross-links is somehow linked to a decreased electron flow via cytochrome b558 (GP91PHOX) in the NADPH oxidase system. One possible explanation of the decreased flow of electrons is the lower expression level of GP91PHOX in MDC-treated cells. To test this assumption, we determined the GP91PHOX mRNA levels in the ATRA- and ATRA + MDC–treated NB4 cells. There was a very low level of GP91PHOX mRNA, detectable only by Q-PCR in the NB4 control cells, which was increased after ATRA treatment by several fold. In the presence of MDC, the elevation of the GP91PHOX mRNA level in maturing cells was markedly reduced. In accordance with these results, neutrophils from TG2–/– mice had both a lower NBT reduction and notably low superoxide anion formation capacity. The GP91PHOX protein was found to be expressed in neutrophils of both TG2–/– and TG2+/+ mice. However, there was a significantly lesser amount of gp91phox mRNA and protein in neutrophils of TG2–/– mice, lending support to the assumption that TG2 is involved in the regulation of gp91phox expression during neutrophil maturation.
Currently, it is not yet possible to explain how TG2 can influence the molecular regulation of gp91phox expression. TG2 is a multifunctional protein; in addition to its ability to modify proteins through their glutamine residues in transglutamylation reactions, it can serve as a G protein, has protein kinase activity, and interacts with fibronectin and cell surface integrins mediating cell attachment and spreading.4,8,9,42,43 It is reasonable to assume that the effect of TG2 on gene expression is mediated by transglutamylation, since GP91PHOX expression could be influenced by monodansyl-cadaverin, a competitive inhibitor of the transglutamylation reaction, and the consequence of MDC treatment in NB4 cells and lack of TG2 in mouse neutrophils was the same in this regard. The previously demonstrated transglutamylation of histones, retinoblastoma protein, and SP1 is an example of transglutaminase-mediated modification of participants in transcriptional regulation. However, so-far-unrecognized mechanisms, including a TG2-mediated polyamination, could also contribute to the observed changes of GP91PHOX transcription in maturing NB4 cells. It has been demonstrated that polyamines can affect gene expression at the transcriptional level, and that effect is most probably due to the direct interaction of polyamines with DNA and/or transacting protein factors, such as polyamine-modulated factor 1 identified as the first transacting protein involved in polyamine-regulated transcription.44 In any case, until now there has been no direct proof of the apparent involvement of TG2 in the regulation of a given gene in relation to a specific cellular function; GP91PHOX seems to be the first example for TG2-modulated genes. Further work is needed to clarify which specific transcriptional regulators of the gp91phox gene are actually modified, and whether there are other target genes influenced by transglutamylation of nuclear proteins.
To define the contribution of TG2 to further neutrophil functions in vivo, a murine model of inflammation was used. The results demonstrate that the rate of extravasation of neutrophils after 4 hours of yeast extract–induced peritonitis was significantly less in mice deficient in TG2 and, as a consequence, higher neutrophil counts were observed in their circulation. Further confirmation that TG2 was necessary for efficient neutrophil migration was provided by the in vitro Matrigel invasion assay. Neutrophils isolated from the peritoneal cavity were also defective in migration through the Matrigel matrix, confirming that TG2 contributes to neutrophil movement. Again, the question arises which biochemical function of TG2 is involved in cell migration. It was previously demonstrated that blocking extracellular interactions of TG2 with a specific antibody leads to inhibition of transendothelial migration of CD8(+) T cells by an unknown mechanism.45 This so-far-unidentified, TG2-specific element of transendothelial movement of cells could play a role in extravasation of neutrophils as well. TG2 acts as an integrin-associated cell surface coreceptor interacting with fibronectin and mediates cell adhesion and spreading; a TG2-specific antibody may disrupt this, explaining the inhibition of transendothelial CD8(+) T cells.4 However, when TG2 was overexpressed in fibroblasts, their migration on fibronectin was decreased as a result of increased adhesion.5 In this particular experiment, cell surface localization, but not transglutaminase activity of the protein, was required for reducing migration. In another study, it was demonstrated that TG2 inhibits fibroblast motility through transglutamylation by either increasing matrix tension or cross-linking cell surface and extracellular matrix components.46 These results clearly suggest that cell surface TG2 anchors fibroblasts to the extracellular matrix in an either nonenzymatic or enzymatic way (or both), preventing their motility—our results showed the opposite effect. There are previous observations, however, that support the notion that TG2 may have a promigratory activity: movement of monocytes on fibronectin was markedly reduced when the adhesive function of TG2 was blocked on the cell surface.4 This clearly shows that the effect of cell surface TG2 on cell migration depends on the cell type. It is very likely that neutrophils use TG2 to migrate in the extracellular space similarly to monocytes/macrophages, which, unlike fibroblasts, also extravasate. It should also be noted that TG2 may act as a promotility protein even in fibroblasts: it was demonstrated that TG2 is required for efficient spreading and migration of fibroblasts on extracellular ligands modulating dynamic adhesion formation, but in contrast to the migration of monocytic cells on fibronectin, this was not affected by antibodies to extracellular TG2 pointing to intracellular functions of the protein in supporting migration.46 The intracellular functions of TG2 could be signaling as a G protein, transamidation, other enzymatic activities, and regulatory interactions. Up-regulation or overexpression of the intracellular G-protein function of TG2 inhibits migratory activity in human aortic smooth muscle and human embryonic kidney cells.47 Therefore, it is unlikely that the role of TG2 in neutrophil migration is linked to its GTPase activity. There are several proteins of the cytoskeleton and its regulatory elements that are transamidation substrates of TG2 including actin, myosin, tubulin, and RhoA, but it is not clear how transamidation, if it happens in migrating neutrophils, might influence motility.48 One may draw a conclusion from our in vitro neutrophil migrational responses carried out in Matrigel Invasion Chamber in the presence or absence of MDC that a significant part of neutrophil motility may depend on extracellular or intracellular TG2-catalyzed transamidation activity in response to the chemoattractant fMLP. Since TG2 signaling via the PLC-PKCα pathway is required for efficient spreading and promoting migration, it is an intriguing possibility that the recently demonstrated protein kinase activity of TG2 may also mediate the promotility effect.42,46 The so-far-demonstrated intracellular interactions of TG2 with calreticulin, BH3-domain containing proteins, glutathion-S-transferase, PLC-δ1, and others do not give a specific clue to explain the promigratory role of TG2.49-52 Therefore, we may conclude from our results that while the involvement of TG2 in gene regulation during neutrophil differentiation as well as in promoting migration is linked to the cross-linking activity of the enzyme, it is not possible to exclude the participation of additional biochemical functions of TG2 either.
Neutrophil extravasation, subsequent phagocytosis, and superoxide anion generation play a crucial role in host defense. Failure in elimination of exogenous or translocated endogenous bacteria due to the decreased superoxide anion production of the host would contribute to the persistent bacterial infection. Our TG2-deficient mice experiments have shown that while the neutrophil respiratory burst was impaired, the phagocytosis capacity of neutrophils was significantly increased. The enhanced phagocytosis activity observed in neutrophils of TG-deficient mice may be due to a shifted reduction-oxidation regulation of transcription factor NF-kB or to an up-regulated compensatory mechanism to preserve TG2-deficient mice in states of impaired respiratory burst.53 Further experiments are needed to explain molecular details of this phenomena.
Clarification of the physiologic and pathologic roles of TG2 is hampered by many difficulties. TG2 has several enzymatic and nonenzymatic biochemical functions and was found to be localized in almost all cell compartments as well as outside of cells; in addition, there are no cell-penetrating active site inhibitors to block the transamidating activity of TG2, and there are at least 9 transglutaminase genes in mammalian species probably contributing or replacing TG2 in several of the presumed functions. Still, dissection of the contributions of TG2 to basic cellular, tissue, or organ functions and various pathologies is crucial both for understanding and influencing them. Based on our results reported here, deficiency in the innate immune response should be added to the so-far-revealed loss-of-function TG2 pathologies, that is, to wound healing, Mody type of diabetes, and development of autoimmune disease.12,13,46 The gain-of-function TG2-related diseases include celiac disease, neurodegeneration, fibrosis, inflammation, and cardiac failure; their possible treatment with future TG2 inhibitors should take into account the consequences on functions that require TG2, such as proper maturation of neutrophils and their migration to inflamed tissue sites.10,11,54-57
Prepublished online as Blood First Edition Paper, June 8, 2006; DOI 10.1182/blood-2004-02-007948.
Supported by a European Union grant (QLk3-CT-2002-02017), the Hungarian National Science Research Foundation (Országos Tudományos Kutatási Alap Grant [OTKA] T 043083, TS 044798, Egészségūgyi Tudományos Tanács [ETT] 115/2003, and Nemzeti Kutatási és Fejlesztés; Programok Grant [NKFP] OM 00427/2004), the ETT 117/2001 (G.V.), and OTKA T 048745. Z.B. was supported by the Békésy fellowship; G.V. was a recipient of the Bolyai fellowship.
Z.B. designed the study, performed the research, and wrote the paper; K.C. performed and analyzed Q-PCR measurement; G.V. designed and performed the confocal microscopy study and contributed to writing “Materials and methods”; A.S. provided guidance for the Q-PCR study, performed FACS analysis, and contributed to writing “Materials and methods”; M.L. contributed to writing the paper; and L.F., in whose lab these studies were performed, provided guidance for the study design and took part in writing the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are indebted to Dr Gerry Melino for providing wild-type and TG2–/– mice. We thank Dr Gyöngyike Májai for excellent flow cytometry analysis. We would also like to thank Mrs Klemm Attiláné, Mr Zsolt Hartman, and Mrs Darai Zoltánné for their excellent technical assistance.

![Figure 1. Neutrophil differentiation induces TG2 expression both in the cytosol and the nucleus of NB4 cells. (A) Transglutaminase activity in cells lysates. Cells were treated with 1 μM ATRA for 7 days during which TG2 enzymatic activity was measured in their cytosolic and nuclear fractions. Activity was measured by detecting incorporation of [3H]putrescine into casein. The amount of incorporated [3H]putrescine was determined in a beta-counter. Bars depict the means of 3 separate experiments each performed in duplicate. Error bars indicate standard deviation (SD). (B) Western blot analysis of TG2 in NB4 cells. Cytosolic and nuclear fractions were separated by SDS-PAGE. Total protein (25 μg) was loaded in each lane, and blots were developed with CUB7402 monoclonal antibody against TG2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2004-02-007948/4/m_zh80180600970001.jpeg?Expires=1765945737&Signature=zGqoLAwoWT5tSnAhiZNa2w7ApeVF04eUYRee-Sv-zdWiisIuOxYUU4EYPfi0YmQmniHlF9JuxEi0s9pNbODpjJz38PTn4W-2uqBKTJN23CdKRAVwAilIWVNi~lkTrmekiuQlYIwTFUjvT3TheFpRTHHbQraCvetJUHmRC1e9As~UCOO0fDLp9qgJBIqm9LcvNM2UKXncmGYkJ0cWzjO1dcE~uYQQqnhlW4bzdlA3UucSCXyM~s68aOnDGhLD79VpeWS83HJwQIMaIuOJ2onbv4tB3tf6PTvTMWk5qqyP5kAqmYrV8ZF1F6NMclf0ywEJX5b4DkurWmpVaXu9hKI4NA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of TG2 transamidation activity decreases both amine incorporation into proteins and the protein cross-link content in the cytosol and the nucleus of maturing NB4 cells. NB4 cells were cultured for 96 hours in the presence of 1 μM ATRA or 1 μM ATRA plus 15 μM MDC. (A) Transglutaminase activity in MDC-treated cells. Total cell lysate (50 μg) was used for assaying TG2 activity. Activity was measured by incorporation of [3H]putrescine into casein. The means of 3 separate experiments performed in duplicate are shown. (B) Western blot analysis of TG2 in NB4 cells. The amount of TG2 in ATRA- and ATRA plus MDC–treated samples was determined by SDS-PAGE following immunoblotting with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. (C) Cytosolic and nuclear Nϵ-(γ-glutamyl)-lysine cross-link content in the absence or presence of TG2 inhibitor on day 4 of differentiation. Results are expressed as the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2004-02-007948/4/m_zh80180600970004.jpeg?Expires=1765945737&Signature=4v2mwONcZ-GQJLzTnc8UHp4Uiotp6zPTy2g3MAiV3v~i88dPdaV~G8NJuQxLljSKTHTBZmDKm28ktHwz-05-iXIxZ-wJuNhQj2W5cqu1iiYfn479B~KD7yxSA0EIhAfUUcIK~BF9iu5DkEi~qcyHAfNG-Qmyslp6GsmByGeZO0zhAkon61YSn-iinSIIVeByDUIY9AsZbf4sjJ7AWEDClKAvuphz3s1IvPfS4pjXnMmT6RfRVrKTErVxHqC-vmlTP6B0flQRSQkSYwhgHL6WETrHSxCDCFMR4OIEVuIzgQihWJBj0buHcsutTYrRrB39zbFmD3zBfqxq8Jzp6en7aw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Neutrophil differentiation induces TG2 expression both in the cytosol and the nucleus of NB4 cells. (A) Transglutaminase activity in cells lysates. Cells were treated with 1 μM ATRA for 7 days during which TG2 enzymatic activity was measured in their cytosolic and nuclear fractions. Activity was measured by detecting incorporation of [3H]putrescine into casein. The amount of incorporated [3H]putrescine was determined in a beta-counter. Bars depict the means of 3 separate experiments each performed in duplicate. Error bars indicate standard deviation (SD). (B) Western blot analysis of TG2 in NB4 cells. Cytosolic and nuclear fractions were separated by SDS-PAGE. Total protein (25 μg) was loaded in each lane, and blots were developed with CUB7402 monoclonal antibody against TG2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2004-02-007948/4/m_zh80180600970001.jpeg?Expires=1765946092&Signature=BBQ1ZedZx9q2qE18al6C1v2HZKjWTNHvvTq-ETGlLQMqRcBZqVmNp~4QVwUWO4b4Pxeg3rBVJbmywv2n7TGg0CWWMJHQkM2cIqK9IqJw2TCHYU9g6e-W3dkIqCf3p3Ij962~1kPvqERvQG2mAqLRiUeEsDp8h-8WP2T0R2ucEjrRsRDvjI-U39EAhcqliP1I9lsxOgmg0gUcbCtoBWkuntyA0Rx3Z98ZwYAprokjmF4nTMoX1Rl7IFIP89ON8Uhhp2Eb0vPrftNCU52B4UcSg6K8WtSwDPLtIkyuWpAOEbhs6d3Tx7SrW3YZcKcbXifYjeGTQTXW7GNrY5UmwlB97Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Inhibition of TG2 transamidation activity decreases both amine incorporation into proteins and the protein cross-link content in the cytosol and the nucleus of maturing NB4 cells. NB4 cells were cultured for 96 hours in the presence of 1 μM ATRA or 1 μM ATRA plus 15 μM MDC. (A) Transglutaminase activity in MDC-treated cells. Total cell lysate (50 μg) was used for assaying TG2 activity. Activity was measured by incorporation of [3H]putrescine into casein. The means of 3 separate experiments performed in duplicate are shown. (B) Western blot analysis of TG2 in NB4 cells. The amount of TG2 in ATRA- and ATRA plus MDC–treated samples was determined by SDS-PAGE following immunoblotting with monoclonal anti-TG2 antibody. The arrow points to the TG2 bands. (C) Cytosolic and nuclear Nϵ-(γ-glutamyl)-lysine cross-link content in the absence or presence of TG2 inhibitor on day 4 of differentiation. Results are expressed as the mean ± SD of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/6/10.1182_blood-2004-02-007948/4/m_zh80180600970004.jpeg?Expires=1765946092&Signature=Ifw8xk9PnlpQ8h8NPhm8971uSuBCHX5m3rzqqTALUx1k3n50etcPdb4hpp-oPcaJ~a7GsfEmSugYRHr14a5DmHBqz2p1QJvlv6CVNUfJ~9BynpwN1-41THpfs0hgd6oMNXr6NATcovvU~InXgseo0VZuybHsVyBmuMOiW7gvE2U8H0QOd0Oao9vbeiWwXoF9YgIEES5ioAKayt3Aqs9fl5ylsmyZXKm9y2MoblKC8kIPOyh60coQ3BDRA7wDlKCA7AhsYnsfaq8lIzQvWMC~2sAs8FhXQ0xERmNkAZqksWQufpPgbfpdso4Zzof2IGL05LdxCIw3UMo5MYonTMsCXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)