Abstract
V617F JAK2 mutation is a reliable molecular marker of polycythemia vera (PV), potentially useful to monitor the effect of treatments in this disease. In a phase 2 study of pegylated (peg) IFN-α–2a in PV, we performed prospective sequential quantitative evaluation of the percentage of mutated JAK2 allele (%V617F) by real-time polymerase chain reaction (PCR). The %V617F decreased in 24 (89%) of 27 treated patients, from a mean of 49% to a mean of 27% (mean decrease of 44%; P < .001), and no evidence for a plateau was observed. In one patient, mutant JAK2 was no longer detectable after 12 months. In 3 patients homozygous for the mutation, reappearance of 50% of wild-type allele was observed during treatment. The results seem to confirm the hypothesis that IFN-α preferentially targets the malignant clone in PV and show that %V617F assessment using a quantitative method may provide the first tool to monitor minimal residual disease in PV. This trial was registered at www.clinicaltrials.gov as #NCT00241241.
Introduction
Polycythemia vera (PV) is a myeloproliferative disorder (MPD) characterized by clonal proliferation of myeloid progenitors leading to increased production of mature hematopoietic cells predominating on the erythroid lineage. The discovery of the constitutively active V617F JAK2 mutation1-4 may explain several characteristics of this disease, such as endogenous erythroid colony (EEC) formation, decreased expression of mpl receptor, increased Bcl-xl expression, and STAT-5 constitutive activation.5 It provides for the first time in PV a reliable and comprehensive molecular marker that is already useful in clinical practice for diagnosis. It may also be used for prognosis as, for example, quantification of the percentage of circulating JAK2-mutated alleles (%V617F) was shown to be significantly higher in patients who developed myelofibrosis.6 The use of %V617F to monitor minimal residual disease and evaluate treatment efficacy has not yet been fully studied, however.
Interferon α (IFN-α), a drug that appears to be nonleukemogenic7 (contrary to 32P, alkylating agents, and possibly other cytotoxic drugs used in PV), may have a preferential activity on the malignant clone in PV, as suggested by cytogenetic remissions obtained in patients treated with IFN-α.8 Jones et al9 recently reported that patients with PV treated with IFN-α had lower %V617F compared to a control group that included patients treated by phlebotomy, hydroxyurea, or anagrelide, or who were untreated. However, this retrospective study included only 7 PV patients treated with IFN-α, at variable dosages (from 1 MU 3 times weekly to 3 MU daily) and for very variable periods (13-132 months). Moreover, in the absence of pretreatment samples (except for a single patient), they could not precisely evaluate the impact of IFN-α on %V617F.
We recently completed a multicenter phase 2 trial of pegylated (peg) IFN-α–2a (Pegasys; Roche Laboratories, Neuilly-sur-Seine, France) in younger PV patients (PVN1 study), whose objectives included prospective sequential evaluation of molecular markers. This trial was designed because pegylated forms of IFN-α allow weekly administration, potentially improving compliance,10 and appear to be more effective than standard IFN-α in patients with hepatitis C.11 In addition, 2 trials have tested peg-IFN-α-2b in patients with essential thrombocythemia (ET),12,13 showing good efficacy but no clear advantage in terms of toxicity compared to standard IFN-α, whereas peg–IFN-α–2a has not been previously tested in MPD, to our knowledge.
Study design
A summary of the clinical PVN1 study is available on the www.clinicaltrials.gov website (identifier: NCT00241241). Inclusion criteria were PV diagnosis according to Polycythemia Vera Study Group (PVSG) criteria, age 18 to 65 years, no previous treatment or only phlebotomies, or cytoreductive treatment for less than 2 years. The primary end point was response rate of PV patients to peg–IFN-α–2a; secondary end points were evaluation of toxicity and %V617F during peg–IFN-α–2a therapy. Hematologic complete response (CR) was defined by a hematocrit (Ht) lower than 45% in men and 42% in women without phlebotomy for more than 3 months, absence of splenomegaly, and normal white blood cell (WBC) and platelet counts. Hematologic partial response (PR) was defined by a Ht lower than 45% in men and 42% in women for more than 3 months, but with persistent splenomegaly or elevated platelet counts. Failure was defined by persistent phlebotomies to maintain Ht in the target range. The PVN1 study was approved by the Institutional Review Board at the Hopital R. Ballanger (Aulnay-Sous-Bois, France) and by the French Health Products Safety Agency (AFSSAPS). Informed consent was provided according to the Declaration of Helsinki. The trial was designed, conducted, and analyzed by the French academic “PV-Nord” group. Roche, the manufacturer of peg–IFN-α–2a, provided 1 year of treatment for patients but was otherwise not involved in the trial.
V617F JAK2 mutation analysis was performed at the time of inclusion in the trial and then planned every 3 to 6 months during the first year and every 6 months thereafter. V617F mutation was detected by single-nucleotide polymorphism genotyping assays performed in DNA samples extracted from purified granulocytes using real-time polymerase chain reaction (PCR)–based mutation detection (TaqMan ABI Prism 7700).14 This PCR assay allows quantitative evaluation of %V617F with a sensitivity of about 1% of HEL DNA diluted in nonmutated DNA, and a sensitivity of 2% to 4% of a homozygously mutated patient DNA diluted in normal DNA, as described.14
For continuous variables, mean ± SDs were given. Mean decrease of %V617F over time was tested using a mixed effect model, accounting for intrapatient correlation of serial measures. All statistical tests were 2-sided, with P values at or below .05 denoting statistical significance. Analysis was performed using SAS (SAS, Cary, NC) and R (http://www.r-project.org/) software packages.
Results and discussion
The 40 planned patients were enrolled between September 2004 and October 2005. Median follow-up for the entire cohort was 11 months (range, 3-18 months). Among them, the first 30 patients, who received peg–IFN-α–2a for at least 6 months (median follow-up, 13 months; range, 6-18 months), were already evaluable for hematologic response and all of them responded, including 25 CR (83%) and 5 PR (17%). Three patients were negative for V617F JAK2 mutation at diagnosis (including one PR), and serial samples for %V617F analysis were available in the remaining 27 positive patients (median number of follow-up samples, 2; range, 1-5).
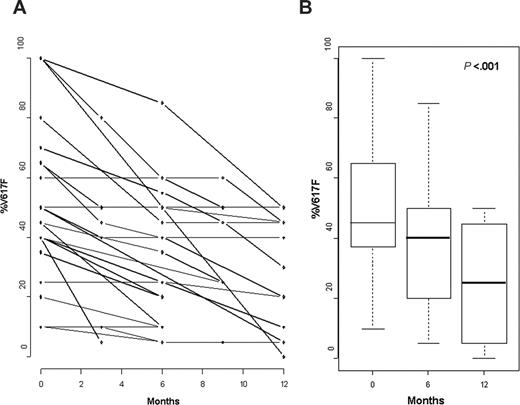
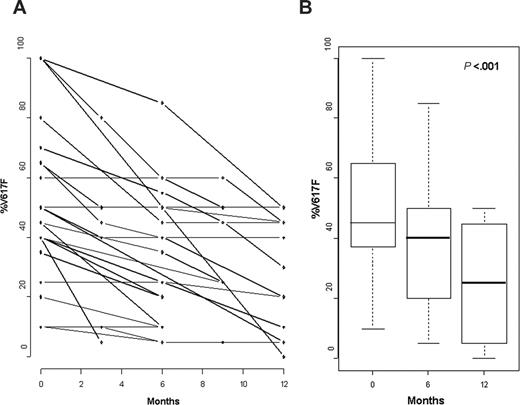
During treatment, %V617F decreased in 24 of 27 patients (89%) and remained stable in 3 of 27 patients (11%; Table 1; Figure 1A). The mean %V617F was 49% ± 25% (range, 10%-100%) in the initial samples, and the mean minimal %V617F during treatment with peg–IFN-α–2a was 27% ± 17% (range, 0%-50%; P < .001; Figure 1B), that is, a mean decrease of 44% ± 26% (range, 0%-100%). Among the 24 patients with molecular response during treatment, a more than 50% reduction of %V617F was seen in 14 patients (58%), including one patient in whom mutant JAK2 was no longer detectable after 12 months (patient 6, Table 1). However, in 10 of the 12 patients treated for more than 1 year and with 3 or more follow-up samples, we observed no evidence for a plateau in molecular response because the last samples showed a %V617F lower than in the previous ones (Figure 1A). In 10 patients, a rapid molecular response (≥ 50% decrease within 6 months) was observed, whereas 14 patients had a slower molecular response (< 50% decrease after 6 months of treatment). In 5 of them, where subsequent evaluation was available, %V617F, however, still continued to decrease. Although no difference in hematologic response (HR) could be observed between rapid and slow molecular responders (90% hematologic CR after a median time of 3 months in both groups), rapid molecular responders had significantly lower mean platelet counts (518 × 109 ± 185 × 109/L) and WBC counts (7.9 × 109 ± 2.4 × 109/L) at inclusion than slow molecular responders (mean platelet counts 892 × 109 ± 339 × 109/L, and mean WBC counts 14.2 × 109 ± 4.4 × 109/L; P = .02 and .004, respectively, by nonparametric Wilcoxon rank sum test). On the other hand, initial %V617F was not significantly different in rapid (mean 40% ± 27%) and slow molecular responders (mean, 58% ± 25%; P = .14). Longer follow-up will be required to determine the clinical significance of molecular response kinetics in PV. In the 3 patients (patients 4, 7, and 9 in Table 1) with homozygous JAK2 mutation (ie, 100% V617F) before treatment, %V617F lowered to 45%, 45%, and 50%, respectively, during treatment.
Evolution of %V617F during treatment with peg–IFN-α–2a. (A) Evolution of %V617F in all patients. (B) Mean decrease of %V617F at 6 and 12 months.
Evolution of %V617F during treatment with peg–IFN-α–2a. (A) Evolution of %V617F in all patients. (B) Mean decrease of %V617F at 6 and 12 months.
The 3 patients (patients 3, 11, and 22 in Table 1) in whom %V617F remained unchanged during treatment had, however, achieved clinical CR after 3 to 6 months of treatment. On the other hand, in 4 patients who only achieved clinical PR, %V617F decreased by 36% (± 21%), a decrease not significantly different from the mean 46% (± 27%) decrease found in patients who achieved clinical CR. Furthermore, 20 of the 24 molecular responders had molecular evaluation at the time HR was obtained. In 12 of them, %V617F had started to decrease at the time of HR, whereas in the remaining 8 patients molecular response was only obtained with longer peg–IFN-α–2a treatment (Table 1). Thus, clinical and molecular responses may not be strictly correlated in IFN-treated PV patients.
IFN-α has been shown, in small numbers of patients with PV, to induce cytogenetic remission or reversion from monoclonal to polyclonal patterns of hematopoiesis,8,15 suggesting that the drug could preferentially target the malignant clone. Our molecular results, prospectively obtained in a relatively large cohort of patients homogeneously treated in a phase 2 trial, show that peg–IFN-α–2a significantly decreases the proportion of circulating clonal cells in the majority of PV patients. In one patient, V617F reached undetectable levels after 12 months of treatment, suggesting that at least a very good molecular remission was achieved (it is possible that the clonal population could have still been detectable with more sensitive assays). In addition, in 3 patients where all circulating granulocytes were homozygous for the V617F JAK2 mutation (100% V617F) a reduction of %V617F of 50% was seen after peg–IFN-α–2a treatment. Those results seem to confirm that, in PV (as in chronic myelogenous leukemia [CML]), IFN-α has a preferential effect on the malignant clone and, therefore, a possible impact on long-term outcome. On the other hand, persistence of endogenous erythroid colony formation and bone marrow hyperplasia despite normalization of blood counts have been described in IFN-treated PV patients.7,16 In this work, because we performed no marrow examination and single-cell colony assays, we could not precisely evaluate the size of the JAK2 mutated clone under treatment. Thus, IFN-α may reduce the rate of clonal proliferation without expanding the population of normal cells. On the other hand, Jones et al9 found that %V617F was similar in blood and bone marrow samples from PV patients, suggesting that the decrease of %V617F we observed in circulating granulocytes may have been associated with a reduction of the proportion of mutated cells in the bone marrow.
Until specific targeted therapies against mutant JAK2 are available, peg–IFN-α–2a could be a treatment of choice for patients with PV. In addition, our results suggest that measurement of %V617F may be useful for the assessment of minimal residual disease in treated PV.
Prepublished online as Blood First Edition Paper, May 18, 2006; DOI 10.1182/blood-2006-03-009860.
Supported in part by research funding from Roche France to the PV-Nord group. J.-J.K., B.C., J.-F.B., J.-D.R., C.C., and P.F. designed research; J.-J.K. is the coordinator of the PVN1 study; J.-J.K., S.C., and P.F. analyzed data; J.-J.K. drafted the paper; B.C., M.-L.M., and G.M. performed molecular analyses; P.T., N.C., M.R., S.B., J.-L.D., K.G., P.R., M.-J.G., Y.C., W.V., N.P., and L.A.-A. enrolled patients; and S.C. performed the statistical analysis.
J.-J.K. and B.C. contributed equally to this study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.