Early signal relay steps upon ligand binding to the receptor tyrosine kinase Flt3 (ie, sites of Flt3 autophosphorylation and subsequent docking partners) are mainly unresolved. By immunoprecipitation of specific tryptic peptides contained in the juxtamembrane region of human Flt3 and subsequent radiosequencing, we identified the tyrosine residues 572, 589, 591, and 599 as in vivo autophosphorylation sites. Focusing on Y589 and Y599, we examined Flt3 ligand (FL)-mediated responses in wild-type-Flt3-(WT-Flt3-), Y589F-Flt3-, and Y599F-Flt3-expressing 32D cells. Compared with WT-Flt3-32D cells upon ligand stimulation, 32D-Y589F-Flt3 showed enhanced Erk activation and proliferation/survival, whereas 32D-Y599F-Flt3 cells hereby displayed substantially diminished responses. Both pY589 and pY599 were identified as association sites for signal relay molecules including Src family kinases and SHP2. Consistently, 32D-Y589F-Flt3 and 32D-Y599F-Flt3 showed decreased FL-triggered activation of Src family kinases. Interference with the Src-dependent negative regulation of Flt3 signaling may account for the enhanced mitogenic response of Y589F-Flt3. Y599 was additionally found to interact with the protein tyrosine phosphatase SHP2 in a phosphorylation-dependent manner. As Y599F-Flt3-32D was unable to associate with and to phosphorylate SHP2 and since silencing of SHP2 in WT-Flt3-expressing cells mimicked the Y599F-Flt3 phenotype, we hypothesize that recruitment of SHP2 to pY599 contributes to FL-mediated Erk activation and proliferation.
Introduction
Fms-like tyrosine kinase 3 (Flt3) functions as a growth factor receptor and is expressed primarily in multipotential hematopoietic stem cells and progenitors as well as in placenta, gonads, and brain. Together with its activating ligand Flt3 ligand (FL) it is a crucial player in assuring normal function of stem cells and the immune system.1-3 Moreover, approximately 30% to 35% of patients with acute myeloid leukemia (AML) carry a mutation in Flt3, rendering Flt3 the most frequently mutated gene in AML.4,5 Flt3 mutations are generally grouped into 2 classes: point mutations in the vicinity of codon 835 or 842 within the tyrosine kinase domain (TKD); or internal tandem duplications (ITDs) of varying lengths within the juxtamembrane domain of Flt3, which sterically represses the intrinsic kinase activity of Flt3 in the absence of ligand.4,6 Both classes of mutations result in constitutive activation of Flt3 but distinct signaling and transforming capacities.7-10 Although debatable as prognostic markers by themselves, ITD and TKD mutations are correlated with poor prognostic features for AML patients, suggesting Flt3 or one of its downstream effectors as potential therapeutic targets.11-15
Flt3 constitutes, together with the receptor for stem cell factor (c-Kit), the receptors for platelet-derived growth factors (PDG-FRs), and colony-stimulating factor-1 (CSF-1), the type III family of receptor tyrosine kinases (RTKs).5 Type III RTKs share a common modular structure consisting of 5 extracellular Ig-like domains, a short transmembrane stretch, a juxtamembrane region followed by a bipartite kinase domain interrupted by the kinase insert, and the carboxyterminal tail.16 Ligand binding causes receptor dimerization, kinase activation, and transphosphorylation of RTK on multiple tyrosine residues.17 These autophosphorylated tyrosine residues together with 3 to 6 adjacent amino acids form high-affinity docking sites for relay molecules possessing either phosphotyrosine binding (PTB) or Src homology 2 (SH2) domains.18 Upon relocation to the receptor, these signaling or adapter molecules become activated in either a phosphorylation-dependent or -independent manner and are thereby capable of transducing the signal downstream. Relay molecules reported to be recruited and/or activated upon Flt3 activation include the p85 subunit of PI3K, Ras-GAP, PLC-γ, Vav, SHIP1, SHP2, ShcA, Grb2, Cbl, and Src family kinases (SFKs) as well as Stat5.19-26 Whereas the immediate signaling steps following ligand binding (ie, binding of signaling molecules to autophosphorylated tyrosines) are well studied in the c-Kit, PDGFRs, and CSF-1 receptor systems,16 not a single in vivo autophosphorylation site of Flt3 or its respective binding partners have been reported. Most ITD isoforms carry a duplication containing at least one of the juxtamembrane tyrosine residues Y589, Y591, Y597, or Y599, which could theoretically add to the aberrant signal relay from the autoactivated receptor.27
Here we report that Y572, Y589, Y591, and Y599 of Flt3 are phosphorylated in vivo in Flt3-expressing cells following ligand stimulation. Furthermore, we demonstrate that phosphorylated Y589 and Y599 are docking sites for signaling intermediates including SFKs. pY589 has a negative regulatory effect most likely due to recruitment and activation of SFKs via pY589. In contrast, pY599 appeared to be one of the major tyrosine residues with a stimulatory function upon FL binding. Mutation of tyrosine 599 to a phenylalanine residue decreased FL-mediated proliferation and/or survival upon interleukin-3 (IL-3) withdrawal in Flt3-32D cells. Apart from a possible contribution of SFKs to the Y599F phenotype, we provide data suggesting that the protein tyrosine phosphatase SHP2 plays a role. In Y599F-Flt3-32D cells, SHP2 binding to Flt3 is dramatically reduced, as is ligand-stimulated tyrosine phosphorylation of SHP2. Furthermore, silencing of SHP2 impaired Erk phosphorylation in ligand-stimulated wild-type Flt3 (WT-Flt3) cells, which is consistent with other reports stressing the importance of SHP2 for Erk activation downstream of RTKs.28 Interestingly, mutated hyperactivated SHP2 has been found in some forms of leukemia including Flt3-ITD-positive AML. 29-32
Materials and methods
Plasmids, antibodies, antisera, and GST fusion proteins
pMSCV-neo vector containing human Flt3 cDNA was a kind gift from Dr G. Gilliland (Brigham and Women's Hospital, Harvard Medical School, Boston, MA). For transfections of adherent cells, polymerase chain reaction (PCR)-amplified cDNA of human Flt3 was subcloned into KpnI-BamHI restriction sites of pcDNA 3.1 expression vector (Invitrogen, Carlsbad, CA). Murine SHP2 SMARTpool siRNA duplexes (proprietary target sequences) were obtained from Dharmacon (Lafayette, CO). Recombinant human FL and murine IL-3 were purchased from Prospec Tany (Rehovot, Israel). The anti-Flt3 antibody was raised against a synthetic peptide corresponding to amino acids (aa's) 740-757 of human Flt3 and affinity purified as described.33 Peptide-specific antibodies against individual tyrosine phosphorylation sites in Flt3 were raised by immunizing rabbits with synthetic peptides corresponding to aa's 583-597 (CTGSSDNE(p)YF-(p)YVDFRE; pY589/pY591) and aa's 591-605 (CYVDFRE(p)YE(p)YDLK-WEF; pY597/pY599) of human Flt3 and affinity purified as described.34 Specificity of the antibodies was confirmed in immunoblots with WT and the respective Y→F mutant of Flt3. A rabbit antiserum recognizing Erk-2 has been described elsewhere.35 Anti-pErk (p42/p44) antibody was from Cell Signaling Technology (Beverly, MA). Anti-SHP2, anti-Fgr, anti-Lyn, anti-Hck, and anti-PY99 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). HRP-coupled secondary antimouse and antirabbit antibodies were from Pierce (Rockford, IL). GST fusion proteins containing the SH2 domains of either Src or SHP2 were kind gifts from Dr T. Pawson (Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, ON, Canada).
Site-directed mutagenesis
To mutate specific tyrosines to phenylalanines, the QuikChange mutagenesis XL kit (Stratagene, La Jolla, CA) was employed according to the manufacturer's instructions. All mutations were subsequently confirmed by sequencing.
Cell culture
Porcine aortic endothelial (PAE) cells36 were cultured in Ham F-12 medium, whereas Cos-1 and EcoPack cells were cultured in Dulbecco modified essential medium (DMEM; Gibco Invitrogen, Carlsbad, CA). Media were supplemented with 10% FCS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Promyeloid 32D cells (German Collection of Microorganisms and Cell Cultures [DSMZ], Braunschweig, Germany) were maintained in RPMI-1640 medium plus 10% heat-inactivated FCS, 10 ng/mL recombinant murine IL-3, 100 units/mL penicillin, and 100 μg/mL streptomycin.
The 32D cells were starved by withdrawing growth factors for 4 hours. All other cell lines were starved by maintaining them overnight in medium with only 0.5% serum. Flt3 was stimulated with 100 ng/mL FL for the indicated periods of time unless stated otherwise.
Inhibitor concentrations for the appropriate experiments were 100 μg/mL cycloheximide (Sigma-Aldrich, St Louis, MO), 10 μM PP1, or 15 μM PP2 (Calbiochem, EMD Biosciences, Darmstadt, Germany), respectively.
The 32D cells were metabolically labeled by incubation in methionine/cysteine-free DMEM medium (Gibco Invitrogen) with 100 μCi (3.7 MBq) Promix (Amersham Biosciences, Uppsala, Sweden) at 37°C for 4 hours before lysis and further processing.
Transient and stable transfection
Adherent cells were transfected using Lipofectamine/PLUS or Lipofectamine 2000 reagents (Invitrogen) according to the manufacturer's instructions. In order to establish 32D cells (no endogenous Flt3) stably expressing WT or mutant Flt3, packaging EcoPack cells were transfected with the corresponding pMSCV-Flt3 constructs, and virus-containing supernatants were collected 72 hours after transfection. Retroviral infection of 32D cells was followed by a 2-week selection in 0.2 mg/mL G418. For all experiments, pools of retrovirally infected cells were used in order to average out possible interclonal variation.
Silencing of SHP2 in Flt3-32D cells was achieved by electroporation (300V, 1500 μF) in a Gene PulserII (Bio-Rad, Hercules, CA) in the presence of 100 nM SHP2-siRNA and incubation for another 72 hours before experiments were performed.
Flow cytometry
Flt3-32D transfectants were labeled with PE-anti-human CD135 or PE-mouse IgG1 κ (BD Biosciences Pharmingen, Heidelberg, Germany) as isotype control according to standard protocols. Flow cytometry was performed with a FACSort instrument (BD Biosciences, San Jose, CA).
Assessment of cell proliferation/survival
To assess the number of living Flt3-32D cells upon IL-3 withdrawal in the absence and presence of FL, cells were washed twice in IL-3-free medium, seeded in 96-well plates (30 000 cells/100 μL IL-3-free medium and well), and treated with different concentrations of FL for 48 hours. Then an MTT assay (Sigma-Aldrich) was performed according to the manufacturer's instructions. Triplicate samples from 3 individual experiments were subjected to analysis of variance (ANOVA; GraphPad Prism, 4.0; San Diego, CA).
Immunoprecipitation, GST pulldown, and Western blotting
Immunoprecipitation, GST pulldown, and Western blotting were conducted as described elsewhere.34,35 Immunodetection was performed by enhanced chemiluminescence using Super Signal Dura reagent (Pierce) and a CCD camera (LAS-3000; Fujifilm, Tokyo, Japan). Bands were quantified by MultiGauge software (Fujifilm).
Affinity fishing of proteins with immobilized peptides
Peptide synthesis and purification were performed as described.37 An additional cysteine residue was added in the amino terminus to allow coupling to KLH using m-maleinimido-benzoyl-N-hydroxy-succinimide ester (MBS) coupling. The following peptides were synthesized: Y589 (CTGSSDNEYFYVDFRE, corresponding to aa's 583-597 of human Flt3), pY589 (CTGSSDNE(p)YFYVDFRE), Y599 (CYVDFREYEYDLKWEF, corresponding to aa's 591-605 of human Flt3), and pY599 (CYVD-FREYE(p)YDLKWEF). As control for specificity, a peptide corresponding to the PI3-kinase binding site in human c-Kit, pY721, was used (CSDST-NE(p)YMDMKPGV). Peptides were dissolved at 1 mg/mL in DMSO and immobilized on Affigel-10 (Bio-Rad) according to the manufacturer's instructions. Slurry (100 μL) was incubated in the cold for 2 hours with precleared 32D cell lysates. For competition experiments, 0.5 mg/mL of the indicated soluble peptide was included to the reaction mixture. Peptide-bound proteins were either subjected to autoradiography (35S-labeled cells) or processed for immunoblotting.
In vitro kinase activity of Flt3 and Lyn
In vitro kinase activity of Flt3 and Lyn were conducted essentially as described in Voytyuk et al34 and Lennartsson et al.35 After SDS-polyacrylamide gel electrophoresis (SDS-PAGE) separation and electroblotting, radioactive proteins were localized on a PhosphorImager (FLA3000; Fujifilm) and densitometrically quantified by MultiGauge software when needed.
In vivo 32P-orthophosphate labeling of cells, immunoprecipitation of tryptic phosphopeptides for sequencing, and 2-dimensional phosphoamino acid analysis
Flt3-PAE cells were 32P labeled in phosphate-free DMEM (Gibco Invitrogen) supplemented with 0.5% dialyzed FCS, 10 mM HEPES, and 2 to 6 mCi (74-222 MBq) 32P-orthophosphate (PBS-43; Amersham) for 6 to 7 hours at 37°C and subsequently treated as described elsewhere.33
Results
Y572, Y589, Y591, and Y599 in the juxtamembrane region of Flt3 are in vivo autophosphorylation sites
In order to investigate autophosphorylated tyrosine residues in the juxtamembrane region of Flt3 (aa's 572-603) we labeled Flt3-PAE cells with [32P]orthophosphate, isolated Flt3 after ligand stimulation, and performed 2-dimensional phosphopeptide mapping as described previously.33 However, the obtained maps showed a phosphoserine content as high as 95% that interfered with the identification of any tyrosine as autophosphorylation site (data not shown). Therefore, we chose to selectively immunoprecipitate the tryptic peptides of interest, aa's 572-595 and aa's 596-602, from a complete tryptic digest of labeled Flt3 using peptide-specific antibodies. Each isolated tryptic fragment was subjected to 2-dimensional phosphoamino acid analysis and automated Edman degradation, and the radioactivity released in each cycle was quantified. Phosphoamino acid analysis of the tryptic peptide consisting of aa's 572-595 of Flt3 revealed the presence of phosphoserine and phosphotyrosine (Figure 1Ai). Radiosequencing of the peptide showed a peak of radioactivity at positions 1 and 3. In order to analyze potential phosphotyrosine residues lying in the C-terminal of amino acid 581, the material was further digested with AspN, which cleaves the N-terminal of aspartic acid residues. Sequence analysis of AspN-cleaved material pooled with non-cleaved material displayed peaks at positions 1, 3, 4, and 6, providing the in vivo evidence for autophosphorylation of Y572, Y589, and Y591 in addition to S574 as phosphorylation sites in human Flt3. In vivo labeling of the respective Y→F mutants of Flt3 and subsequent disappearance of the corresponding radioactivity peak upon Edman degradation confirmed the identity of pY572, pY589, and pY591 as in vivo autophosphorylation sites (Figure 1A). The tryptic peptide covering aa's 596-602 of WT-Flt3 had a phosphotyrosine at position 4 that was not detected in Y599F-Flt3, demonstrating in vivo phosphorylation of Y599 (Figure 1B).
Y589F and Y599F-Flt3 show distinct FL-dependent phenotypes in stable 32D transfectants
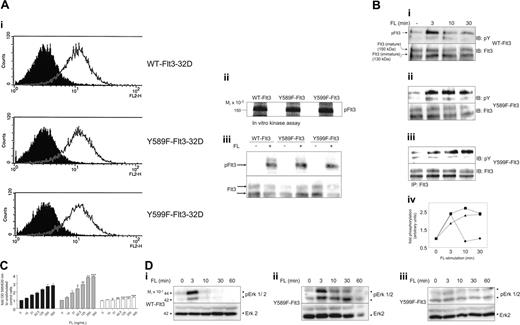
To investigate the role of individual phosphotyrosines, we focused on pY589 and pY599. pY589 is homologous to pY568 in c-Kit and pY579 in PDGFR-β where they have been reported to bind and activate SFKs and to be pivotal for ligand-stimulated proliferation, differentiation, and migration.35,38-40 To elucidate the impact of phosphorylation at Y589 and Y599 on Flt3-mediated signaling, we mutated each tyrosine residue individually to phenylalanine and stably introduced WT and mutant Flt3 isoforms into promyeloid 32D cells and examined FL-induced responses. Equal surface expression of each isoform in the obtained pools of transfectants was shown by flow cytometry (Figure 2A). Intact kinase activity upon ligand stimulation was confirmed by in vitro kinase assays of immunoprecipitated active Flt3 with Flt3 as endogenous substrate (Figure 2Aii) and a comparable phosphotyrosine content in immunoprecipitated Flt3 from living FL-stimulated cells (Figure 2Aiii).
Mapping of autophosphorylation sites. (A) Identification of Y572, Y589, and Y591 as sites of in vivo autophosphorylation: PAE cells expressing WT or the indicated Y→F mutant of Flt3 were labeled with 2 to 6 mCi (74-222 MBq) [32P]-orthophosphate for 6 hours before they were stimulated with FL for 10 minutes, and labeled Flt3 was immunoprecipitated (IP) and subsequently digested with trypsin. Tryptic fragments of Flt3 containing aa's 572-595 were precipitated with 1 μg of a peptide-specific antibody described in “In vivo 32P- or- thophosphate labeling of cells” and subjected to phosphoamino acid analysis and Edman-based degradation (after an additional digest with AspN when indicated). The amount of radioactivity released in each cycle of Edman degradation was quantitated using a PhosphorImager and MultiGauge software (Fujifilm, Tokyo, Japan). (B) Identification of Y599 as in vivo autophosphorylation site: PAE cells expressing WT or Y599F-Flt3 were treated as described for panel A. The tryptic peptide containing aa's 596-602 of Flt3 was immunoprecipitated and processed as mentioned for panel A.
Mapping of autophosphorylation sites. (A) Identification of Y572, Y589, and Y591 as sites of in vivo autophosphorylation: PAE cells expressing WT or the indicated Y→F mutant of Flt3 were labeled with 2 to 6 mCi (74-222 MBq) [32P]-orthophosphate for 6 hours before they were stimulated with FL for 10 minutes, and labeled Flt3 was immunoprecipitated (IP) and subsequently digested with trypsin. Tryptic fragments of Flt3 containing aa's 572-595 were precipitated with 1 μg of a peptide-specific antibody described in “In vivo 32P- or- thophosphate labeling of cells” and subjected to phosphoamino acid analysis and Edman-based degradation (after an additional digest with AspN when indicated). The amount of radioactivity released in each cycle of Edman degradation was quantitated using a PhosphorImager and MultiGauge software (Fujifilm, Tokyo, Japan). (B) Identification of Y599 as in vivo autophosphorylation site: PAE cells expressing WT or Y599F-Flt3 were treated as described for panel A. The tryptic peptide containing aa's 596-602 of Flt3 was immunoprecipitated and processed as mentioned for panel A.
FL-induced responses in WT-Flt3, Y589F-Flt3, and Y599F-Flt3-32D cells. (A) The 32D cells were retrovirally infected with WT-Flt3, Y589F-Flt3, or Y599F-Flt3. Bulk populations obtained after selection in 0.2 g/L G418 were tested for their surface expression of Flt3 isoforms by flow cytometry (i: open curve, anti-Flt3; filled curve, isotype control). Kinase activity of the expressed Flt3 isoform was confirmed by immunoprecipitation of FL-stimulated Flt3 and a subsequent in vitro kinase assay with Flt3 as endogenous substrate (ii) as described in “In vitro kinase activity of Flt3 and Lyn” as well as by immunoprecipitation of activated Flt3 from stimulated cells, followed by probing with a phosphotyrosine antibody and subsequent reprobing with a Flt3 antibody to verify equal loading (iii). (B) Time-dependent autophosphorylation of Flt3 isoforms was examined by immunoprecipitation of Flt3 at the indicated time points upon FL stimulation. After SDS-PAGE and immunoblotting (IB), membranes were probed for phosphotyrosine and Flt3, respectively. The intensity of the bands was quantified by densitometric analysis. The ratio of pFlt3 to Flt3, normalized to time point zero, is depicted for each Flt3 isoform (• indicates WT-Flt3; ▪, Y589F-Flt3; ▴, Y599F-Flt3). (C) Dose-dependent rescue from IL-3 withdrawal in the Flt3-32D transfectants by FL was determined by an MTT assay. Thirty thousand cells/well (96-well, triplicates) were starved from IL-3 in the presence of different concentrations of FL and 2% serum. After 48 hours the MTT dye was added for another 4 hours before absorbance of the converted dye was read at 595/630 nm. OD indicates optical density. Bars indicate fold increase of metabolic activity compared with control cells (no IL-3, no FL). ▪ indicates WT-Flt3;  , Y589F-Flt3; and □, Y599F-Flt3. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001. (D) To examine FL-induced Erk phosphorylation, 32D transfectants were starved from cytokines for 4 hours and then stimulated with 100 ng/mL FL for the indicated periods of time. After lysis, total cell lysates were subjected to a Western blot analysis for pErk and total Erk, respectively. All experiments were performed at least 3 times with consistent results.
, Y589F-Flt3; and □, Y599F-Flt3. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001. (D) To examine FL-induced Erk phosphorylation, 32D transfectants were starved from cytokines for 4 hours and then stimulated with 100 ng/mL FL for the indicated periods of time. After lysis, total cell lysates were subjected to a Western blot analysis for pErk and total Erk, respectively. All experiments were performed at least 3 times with consistent results.
FL-induced responses in WT-Flt3, Y589F-Flt3, and Y599F-Flt3-32D cells. (A) The 32D cells were retrovirally infected with WT-Flt3, Y589F-Flt3, or Y599F-Flt3. Bulk populations obtained after selection in 0.2 g/L G418 were tested for their surface expression of Flt3 isoforms by flow cytometry (i: open curve, anti-Flt3; filled curve, isotype control). Kinase activity of the expressed Flt3 isoform was confirmed by immunoprecipitation of FL-stimulated Flt3 and a subsequent in vitro kinase assay with Flt3 as endogenous substrate (ii) as described in “In vitro kinase activity of Flt3 and Lyn” as well as by immunoprecipitation of activated Flt3 from stimulated cells, followed by probing with a phosphotyrosine antibody and subsequent reprobing with a Flt3 antibody to verify equal loading (iii). (B) Time-dependent autophosphorylation of Flt3 isoforms was examined by immunoprecipitation of Flt3 at the indicated time points upon FL stimulation. After SDS-PAGE and immunoblotting (IB), membranes were probed for phosphotyrosine and Flt3, respectively. The intensity of the bands was quantified by densitometric analysis. The ratio of pFlt3 to Flt3, normalized to time point zero, is depicted for each Flt3 isoform (• indicates WT-Flt3; ▪, Y589F-Flt3; ▴, Y599F-Flt3). (C) Dose-dependent rescue from IL-3 withdrawal in the Flt3-32D transfectants by FL was determined by an MTT assay. Thirty thousand cells/well (96-well, triplicates) were starved from IL-3 in the presence of different concentrations of FL and 2% serum. After 48 hours the MTT dye was added for another 4 hours before absorbance of the converted dye was read at 595/630 nm. OD indicates optical density. Bars indicate fold increase of metabolic activity compared with control cells (no IL-3, no FL). ▪ indicates WT-Flt3;  , Y589F-Flt3; and □, Y599F-Flt3. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001. (D) To examine FL-induced Erk phosphorylation, 32D transfectants were starved from cytokines for 4 hours and then stimulated with 100 ng/mL FL for the indicated periods of time. After lysis, total cell lysates were subjected to a Western blot analysis for pErk and total Erk, respectively. All experiments were performed at least 3 times with consistent results.
, Y589F-Flt3; and □, Y599F-Flt3. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001. (D) To examine FL-induced Erk phosphorylation, 32D transfectants were starved from cytokines for 4 hours and then stimulated with 100 ng/mL FL for the indicated periods of time. After lysis, total cell lysates were subjected to a Western blot analysis for pErk and total Erk, respectively. All experiments were performed at least 3 times with consistent results.
When comparing ligand-dependent receptor activation over time, Y589F-Flt3-32D and Y599F-Flt3-32D cells showed sustained receptor phosphorylation compared with WT-Flt3-expressing cells (Figure 2B). Y589F-Flt3-32D cells also showed a prolonged ligand-mediated p42/44-Erk activation (Figure 2D). Moreover, Y589F-Flt3-32D cells displayed consistently higher numbers of living cells in response to FL treatment than WT-Flt3-32D cells (Figure 2C), employing the MTT assay as readout for cell proliferation and/or survival upon IL-3 withdrawal. Y599F-Flt3-32D cells, however, were impaired in their response to FL treatment in terms of IL-3-independent proliferation (Figure 2C-D) and Erk activation. Parental 32D cells expressing no Flt3 did not show any rescue by FL from IL-3 withdrawal (data not shown).
Both pY589 and pY599 are recruiting several proteins including Src family kinases
Intrigued by the distinct phenotype of the Y589F and Y599F mutants of Flt3, we wanted to elucidate binding partners of pY589 and pY599 that account for the observed phenotype in the Y→F mutants. For this we chose an affinity chromatography approach employing immobilized synthetic phosphopeptides covering aa's 583-pY589-597 (pY589) or aa's 591-pY599-605 (pY599) of Flt3, respectively. As negative control for unspecific, phosphorylation-independent binding we used immobilized peptides without phosphorylation on the tyrosine residues of interest. As additional control for specificity, a phosphopeptide corresponding to the PI3-kinase binding site in human c-Kit (pY721) was used (data not shown). Lysates of [35S]-labeled 32D cells were incubated with the corresponding peptides, and bound proteins were visualized by the PhosphorImager after separation by SDS-PAGE. Proteins of the approximate sizes of 45, 52, and 55 kDa were found to interact in a phosphospecific manner with pY589 (Figure 3A, proteins a-c), whereas pY599 showed specificity for proteins of approximately 45, 52, 55, and 70 kDa (proteins d-g). For subsequent identification of the potential interaction partners of pY589 and pY599, we employed candidate immunoblotting. As pY589 is homologous to the Src-binding site Y568 in c-Kit, we were prompted to investigate whether Src also binds to pY589 of Flt3. From the SFK members mainly expressed in 32D cells (Lyn, Fgr, and Hck), all were found to interact with pY589 (Figure 3Bi). Surprisingly, pY599 was also able to recruit Lyn, Fgr, and Hck despite the absence of the pYEEI consensus motif for the SH2 domain of Src.41 However, Src interaction with both sites within the entire Flt3 protein was confirmed by pulldown experiments employing a fusion protein of GST and the SH2 domain of Src. Phosphorylation-dependent binding of Src-SH2 to Flt3 was weakened if either Y589 or Y599 of Flt3 were mutated to a phenylalanine residue (Figure 3C). Coimmunoprecipitation of endogenous Lyn, Fgr, or Hck, respectively, with the WT-Flt3 upon ligand stimulation verified the observed interaction in living cells. In contrast, association was greatly reduced in 32D cells expressing either the Y589F mutant or the Y599F mutant of Flt3 (Figure 3D). Interestingly, Fgr, and to some extent Hck, showed preference for binding to Y589. In order to study the functional consequences of this interaction, 32D cells expressing either the wild-type, Y589F, or Y599F Flt3 were stimulated with FL and lysed. Lyn, being the most prominent representative of SFKs in 32D cells and the SFK evenly recruited to Y589 and Y599, was immunoprecipitated and subjected to an in vitro kinase assay with acid-denatured enolase as exogenous substrate. In cells expressing wild-type Flt3, Lyn kinase activity was stimulated approximately 3-fold (Figure 3E). In contrast, Lyn immunoprecipitated from 32D cells expressing either Y589F or Y599F mutant of Flt3 showed a dramatic decrease in the degree of Lyn kinase activation. Consistently, phosphorylation of Cbl, a well known target of SFK,42 was impaired in FL-stimulated Y589F-Flt3 and Y599F-Flt3 cells (Figure 3F).
Additionally, an observed interaction between purified GST-Src (SH2) and immobilized pY589 or pY599 (data not shown) suggested that SFKs are recruited directly to both pY589 and pY599.
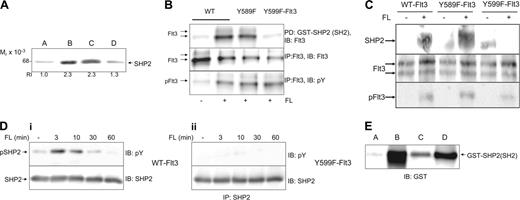
The protein tyrosine phosphatase SHP2 associates with Y599 in Flt3 in a phosphorylation-dependent manner
Due to the divergent proliferative behavior of the Y589F and Y599F mutants, SFKs seemed unlikely candidates evenly accounting for both observed phenotypes unless we assume site-selective action of SFKs. For a variety of different receptor systems, the protein tyrosine phosphatase SHP2 has been reported to be crucial for Erk activation and thus to be involved in proliferation and survival.28 This led us to probe for SHP2 among the phosphopeptide pY599-binding proteins and to reveal phosphospecific interaction of SHP2 with pY599 (Figure 4A). The band of about 70 kDa pulled down by pY599 from 35S-labeled cell lysate (Figure 3A, protein d) is therefore suggested to be SHP2. Interaction of SHP2 with pY599 of the entire Flt3 protein was confirmed by a pulldown experiment using a fusion protein of GST with the N- and C-terminal SH2 domains of SHP2. WT-Flt3 and Y589F-Flt3 interact with SHP2 in a ligand-dependent manner, whereas Y599F-Flt3 cannot bind to SHP2-SH2 (Figure 4B). Interaction between SHP2 and Flt3 in living cells was confirmed by coimmunoprecipitation of SHP2 with Flt3 from FL-stimulated 32D cells expressing WT-Flt3 (Figure 4C). In contrast, no interaction was seen between SHP2 and Flt3 in 32D cells expressing the Y599F mutant of Flt3, whereas it was intact in the Y589F mutant. Furthermore, Figure 4D shows lack of SHP2 phosphorylation in Y599F-Flt3 cells that occurs in WT-Flt3 cells. This again stresses the specific importance of pY599 for SHP2 recruitment and action. Y589 and Src-inhibited WT-Flt3-32D cells were able to phosphorylate SHP2 to a comparable level upon FL stimulation as seen in WT-Flt3-32D cells (data not shown). Thus, a contribution of SFK to SHP2 phosphorylation is unlikely. The sequence surrounding Y599 is different from the consensus sequence for the SH2 domain of SHP2 (pYIXV41 vs 599 pYDLK in Flt3). However, the alternative consensus sequence for binding of SHP2, as proposed by Rönnstrand et al43 (pYAD/EI/L), is similar to the sequence surrounding Y599, although shifted one position. There are other examples in the literature where the peptide sequence in a protein binding to an SH2 domain has been shifted one position compared with the predicted consensus.44 We propose that binding of SHP2 to pY599 is direct, as we observed an interaction of purified SHP2-GST fusion protein with immobilized pY599 that could be competed by an excess of soluble pY599 peptide (Figure 4E).
pY589 and pY599 are binding sites for Src family kinases and are involved in activation of Lyn. (A) The 32D cells were metabolically labeled with [35S] methionine/[35S] cysteine for 4 hours. Lysates were subjected to an affinity chromatography with the indicated immobilized peptides as described in “Affinity fishing of proteins with immobilized peptides.” Bound proteins were eluted, separated by SDS-PAGE, and detected by exposure on a PhosphorImager. The letters a, b, and c highlight proteins of the sizes 55, 52, and 45 kDa, respectively, showing phosphospecific interaction with pY589, whereas d, e, f, and g point to proteins of the sizes 70, 55, 52, and 45 kDa, respectively, that specifically interact with pY599. (B) Total 32D cell lysate was incubated with the following immobilized synthetic peptides: Y589 (lane A), pY589 (lanes B-D), Y599 (lane E), or pY599 (lanes F-H). To prove phosphoselectivity of found interactions, soluble phosphorylated or unphosphorylated peptides (0.5 mg/mL, Y589, lane C; pY589, lane D; Y599, lane G; pY599, lane H) were included in the reactions to compete for binding proteins. Bound proteins were eluted, separated on a 10% SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, and Hck. Each lane was densitometrically evaluated and relative intensities (RIs) are depicted under each lane. Data of 1 of 3 performed experiments with consistent results are displayed. (C) The 32D cells were incubated in the presence or absence of FL, followed by lysis and pulldown (PD) with a GST fusion protein containing the SH2 domain of Src. Samples were separated by SDS-PAGE, electrotransferred to Immobilon P, and probed with an antibody against GST. In parallel, an aliquot of each cell lysate was immunoprecipitated with an antibody against Flt3, separated by SDS gel electrophoresis, electrotransferred to Immobilon P, and probed with an antibody against Flt3 to verify equal loading. (D) Lysates from 32D cells expressing either wild-type, Y589F, or Y599F mutant Flt3 were stimulated with FL for 10 minutes, lysed, and subjected to immunoprecipitation with an antibody against Flt3. After washing of immunoprecipitates, proteins were separated by SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, or Hck, respectively. The filter was consecutively stripped and reprobed with an Flt3 antibody to ensure equal loading and a phosphotyrosine antibody to ensure equal ligand stimulation. (E) WT-Flt3, Y589F-Flt3, and Y599F-Flt3-32D cells were starved followed by stimulation for 7 minutes with FL (100 ng/mL) and subsequent lysis. Lyn was immunoprecipitated from the cell lysates and subjected to an in vitro kinase assay with enolase as exogenous substrate. Phosphorylated enolase was run out on a 10% gel, blotted, and visualized on a PhosphorImager as well as quantified densitometrically. Results are means of 3 independent experiments and depicted as fold Lyn activation upon ligand binding (▪) compared with unstimulated cells (□). Error bars indicate SEM of 3 different experiments. (F) Phosphorylation of Cbl is impaired in Y589F-Flt3 and Y599F-Flt3 32D cells. The 32D infectants were starved and stimulated with FL (100 ng/mL) for the indicated periods of time. After lysis, Cbl was immunoprecipitated, run on a gel, and probed against phosphotyrosine. To ensure equal protein loading, the membrane was stripped and reprobed for Cbl.
pY589 and pY599 are binding sites for Src family kinases and are involved in activation of Lyn. (A) The 32D cells were metabolically labeled with [35S] methionine/[35S] cysteine for 4 hours. Lysates were subjected to an affinity chromatography with the indicated immobilized peptides as described in “Affinity fishing of proteins with immobilized peptides.” Bound proteins were eluted, separated by SDS-PAGE, and detected by exposure on a PhosphorImager. The letters a, b, and c highlight proteins of the sizes 55, 52, and 45 kDa, respectively, showing phosphospecific interaction with pY589, whereas d, e, f, and g point to proteins of the sizes 70, 55, 52, and 45 kDa, respectively, that specifically interact with pY599. (B) Total 32D cell lysate was incubated with the following immobilized synthetic peptides: Y589 (lane A), pY589 (lanes B-D), Y599 (lane E), or pY599 (lanes F-H). To prove phosphoselectivity of found interactions, soluble phosphorylated or unphosphorylated peptides (0.5 mg/mL, Y589, lane C; pY589, lane D; Y599, lane G; pY599, lane H) were included in the reactions to compete for binding proteins. Bound proteins were eluted, separated on a 10% SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, and Hck. Each lane was densitometrically evaluated and relative intensities (RIs) are depicted under each lane. Data of 1 of 3 performed experiments with consistent results are displayed. (C) The 32D cells were incubated in the presence or absence of FL, followed by lysis and pulldown (PD) with a GST fusion protein containing the SH2 domain of Src. Samples were separated by SDS-PAGE, electrotransferred to Immobilon P, and probed with an antibody against GST. In parallel, an aliquot of each cell lysate was immunoprecipitated with an antibody against Flt3, separated by SDS gel electrophoresis, electrotransferred to Immobilon P, and probed with an antibody against Flt3 to verify equal loading. (D) Lysates from 32D cells expressing either wild-type, Y589F, or Y599F mutant Flt3 were stimulated with FL for 10 minutes, lysed, and subjected to immunoprecipitation with an antibody against Flt3. After washing of immunoprecipitates, proteins were separated by SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, or Hck, respectively. The filter was consecutively stripped and reprobed with an Flt3 antibody to ensure equal loading and a phosphotyrosine antibody to ensure equal ligand stimulation. (E) WT-Flt3, Y589F-Flt3, and Y599F-Flt3-32D cells were starved followed by stimulation for 7 minutes with FL (100 ng/mL) and subsequent lysis. Lyn was immunoprecipitated from the cell lysates and subjected to an in vitro kinase assay with enolase as exogenous substrate. Phosphorylated enolase was run out on a 10% gel, blotted, and visualized on a PhosphorImager as well as quantified densitometrically. Results are means of 3 independent experiments and depicted as fold Lyn activation upon ligand binding (▪) compared with unstimulated cells (□). Error bars indicate SEM of 3 different experiments. (F) Phosphorylation of Cbl is impaired in Y589F-Flt3 and Y599F-Flt3 32D cells. The 32D infectants were starved and stimulated with FL (100 ng/mL) for the indicated periods of time. After lysis, Cbl was immunoprecipitated, run on a gel, and probed against phosphotyrosine. To ensure equal protein loading, the membrane was stripped and reprobed for Cbl.
Overall, our data imply that both Y589 and Y599 are autophosphorylation sites that constitute sites of receptor interaction for several signal transduction molecules including SFKs and SHP2.
SHP2 recruitment to pY599 contributes to FL-mediated Erk activation in Flt3-32D cells
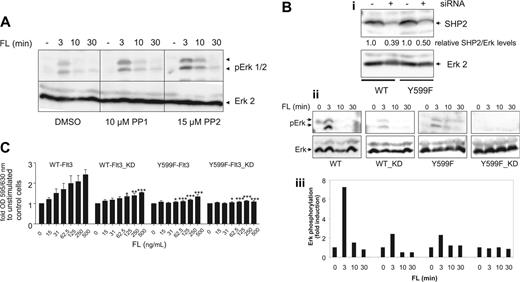
As demonstrated earlier, Y599 plays an important role for FL-mediated Erk activation and growth in Flt3-32D cells. We next aimed to elucidate the individual impact of SFKs and SHP2 on the pY599 phenotype. Employing the Src-selective inhibitors PP1 or PP2, we could not observe any major negative effect on FL-triggered Erk activation in WT-Flt3-32D cells (Figure 5A). In contrast, PP1 and PP2 even slightly prolonged Erk activation in FL-stimulated WT-Flt3-32D cells as seen in Y589F-Flt3 cells. Furthermore, treatment of cells with the MEK inhibitor U0126 decreased FL-mediated cell growth, demonstrating an importance of activation of the Erk pathway for FL-induced cell growth (data not shown). These data suggest that SFKs do not play a dominant role for FL-triggered and Erk-mediated growth-promoting signals in Flt3-expressing 32D cells.
Parallel Trypan Blue exclusion counting of the different cell lines under the culture conditions described confirmed the obtained MTT data (data not shown).
Silencing of SHP2 by siRNA led to a reduction of SHP2 levels by approximately 50% to 60% in WT-Flt-32D and Y599F-Flt3-32D cells (Figure 5Bi). Examining ligand-mediated activation of Erk in WT-Flt3-32D, WT-Flt3-(SHP2 knockdown [KD])-32D, Y599F-Flt3-32D, and Y599F-Flt3-KD-32D cells, we observed that reduction of SHP2 levels led to lower levels of pErk, implying the involvement of SHP2 in FL-mediated Erk activation. WT-Flt3-KD and Y599F-Flt3 cells furthermore responded similarly, which corroborates our findings concerning the interaction between SHP2 and pY599 (Figure 5Bii-iii). Looking at FL-triggered proliferation/survival, WT-Flt3-KD and Y599F-Flt3 cells again displayed a comparable behavior, and down-regulation of SHP2 was able to diminish mitogenicity in both WT and Y599F-Flt3-32D cells (Figure 5C).
pY599 is a recruitment site for the protein tyrosine phosphatase SHP2. (A) Total 32D cell lysates were incubated with immobilized peptide Y599 (lane A) and pY599 (lanes B-D). To prove phosphoselectivity of found interactions, soluble phosphorylated or unphosphorylated peptides (0.5 mg/mL, Y599, lane C; pY599, lane D) were included in the reactions to compete for binding proteins. Bound protein was subjected to a Western blot analysis for SHP2. Bands were densitometrically analyzed and relative intensities are depicted under each lane. (B) Interaction of pY599 (but not pY589) of the entire Flt3 protein and SHP2 was shown in a pulldown experiment. For this purpose, Cos-1 cells were transiently transfected with Flt3-WT, Flt3-Y589F, and Flt3-Y599F constructs. After stimulation of the appropriate samples with FL for 10 minutes, cells were lysed and the lysate was incubated with 2 μg GST-SHP2 (N+C)-SH2 fusion protein. Pulled-down proteins were separated by SDS-PAGE on an 8% gel and blotted, and the membranes were probed for Flt3. To confirm efficient transfection and stimulation of each sample, Flt3 was immunoprecipitated from the lysates and immunoblotted for Flt3 and phosphotyrosine. (C) pY599 of Flt3 binds to SHP2 in living cells. The 32D transfectants of either WT- or Y599F-FLt3 were starved for 4 hours from cytokines before they were stimulated with FL (100 ng/mL) for 7 minutes. Cells were lysed and subjected to immunoprecipitation with an antibody against Flt3. Immunoprecipitated proteins were separated by SDS gel electrophoresis, electrotransferred to Immobilon P, followed by probing with an antibody against SHP2. The filter was consecutively stripped and reprobed with a Flt3 antibody to ensure equal loading and a phosphotyrosine antibody to reveal stimulation of Flt3 phosphorylation. (D) To examine SHP2 phosphorylation, 32D transfectants of either WT-Flt3 or Y599F-Flt3 were starved for 4 hours from cytokines before they were stimulated with FL (100 ng/mL) for the indicated periods of time. SHP2 was immunoprecipitated from cell lysates, run out on an 8% gel, and immunoblotted for phosphotyrosine and SHP2, respectively. (E) Direct interaction of pY599 with SHP2-SH2 was demonstrated by incubation of purified GST fusion protein (1 μg) with immobilized Y599 peptide (lane A) or pY599 (lanes B-D) and 0.5 mg/mL of competing soluble pY599 (lane C) or Y599 (lane D) peptide, respectively. After 2-hour incubation end-over-end, immobilized peptide beads were boiled in Laemmli buffer and subjected to a Western blot probing for GST. One representative experiment is shown of at least 2 performed with consistent results.
pY599 is a recruitment site for the protein tyrosine phosphatase SHP2. (A) Total 32D cell lysates were incubated with immobilized peptide Y599 (lane A) and pY599 (lanes B-D). To prove phosphoselectivity of found interactions, soluble phosphorylated or unphosphorylated peptides (0.5 mg/mL, Y599, lane C; pY599, lane D) were included in the reactions to compete for binding proteins. Bound protein was subjected to a Western blot analysis for SHP2. Bands were densitometrically analyzed and relative intensities are depicted under each lane. (B) Interaction of pY599 (but not pY589) of the entire Flt3 protein and SHP2 was shown in a pulldown experiment. For this purpose, Cos-1 cells were transiently transfected with Flt3-WT, Flt3-Y589F, and Flt3-Y599F constructs. After stimulation of the appropriate samples with FL for 10 minutes, cells were lysed and the lysate was incubated with 2 μg GST-SHP2 (N+C)-SH2 fusion protein. Pulled-down proteins were separated by SDS-PAGE on an 8% gel and blotted, and the membranes were probed for Flt3. To confirm efficient transfection and stimulation of each sample, Flt3 was immunoprecipitated from the lysates and immunoblotted for Flt3 and phosphotyrosine. (C) pY599 of Flt3 binds to SHP2 in living cells. The 32D transfectants of either WT- or Y599F-FLt3 were starved for 4 hours from cytokines before they were stimulated with FL (100 ng/mL) for 7 minutes. Cells were lysed and subjected to immunoprecipitation with an antibody against Flt3. Immunoprecipitated proteins were separated by SDS gel electrophoresis, electrotransferred to Immobilon P, followed by probing with an antibody against SHP2. The filter was consecutively stripped and reprobed with a Flt3 antibody to ensure equal loading and a phosphotyrosine antibody to reveal stimulation of Flt3 phosphorylation. (D) To examine SHP2 phosphorylation, 32D transfectants of either WT-Flt3 or Y599F-Flt3 were starved for 4 hours from cytokines before they were stimulated with FL (100 ng/mL) for the indicated periods of time. SHP2 was immunoprecipitated from cell lysates, run out on an 8% gel, and immunoblotted for phosphotyrosine and SHP2, respectively. (E) Direct interaction of pY599 with SHP2-SH2 was demonstrated by incubation of purified GST fusion protein (1 μg) with immobilized Y599 peptide (lane A) or pY599 (lanes B-D) and 0.5 mg/mL of competing soluble pY599 (lane C) or Y599 (lane D) peptide, respectively. After 2-hour incubation end-over-end, immobilized peptide beads were boiled in Laemmli buffer and subjected to a Western blot probing for GST. One representative experiment is shown of at least 2 performed with consistent results.
SHP2 rather than Src family kinases is involved in Erk activation and proliferation/survival upon FL stimulation in 32D cells. (A) Ten micromolar PP1, 15 μM PP2, or DMSO was added to Flt3-32D cells 15 minutes before ligand stimulation. FL (100 ng/mL) was added for the indicated periods of time before cells were lysed and cell lysates were subjected to a Western blot analysis for pErk and total Erk2. (B) SHP2 siRNA was introduced into WT- and Y599F-Flt3-32D cells by electroporation 72 hours before the experiment was performed. SHP2 knockdown (KD) was confirmed by probing total cell lysates for SHP2 and Erk2, respectively. Bands were densitometrically quantified and relative SHP2/Erk2 ratios are depicted under each lane (i). Parental and SHP2-KD cells were starved and stimulated with FL (100 ng/mL) as indicated before lysates were subjected to Western blot analysis for pErk and Erk2, respectively (ii). Densitometric values for the relative pErk/Erk ratios are depicted in 5Biii. (C) WT-Flt3-32D cells, Y599F-32D-Flt3, and KD counterparts were assessed for FL-dependent proliferation/survival by an MTT assay. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001.
SHP2 rather than Src family kinases is involved in Erk activation and proliferation/survival upon FL stimulation in 32D cells. (A) Ten micromolar PP1, 15 μM PP2, or DMSO was added to Flt3-32D cells 15 minutes before ligand stimulation. FL (100 ng/mL) was added for the indicated periods of time before cells were lysed and cell lysates were subjected to a Western blot analysis for pErk and total Erk2. (B) SHP2 siRNA was introduced into WT- and Y599F-Flt3-32D cells by electroporation 72 hours before the experiment was performed. SHP2 knockdown (KD) was confirmed by probing total cell lysates for SHP2 and Erk2, respectively. Bands were densitometrically quantified and relative SHP2/Erk2 ratios are depicted under each lane (i). Parental and SHP2-KD cells were starved and stimulated with FL (100 ng/mL) as indicated before lysates were subjected to Western blot analysis for pErk and Erk2, respectively (ii). Densitometric values for the relative pErk/Erk ratios are depicted in 5Biii. (C) WT-Flt3-32D cells, Y599F-32D-Flt3, and KD counterparts were assessed for FL-dependent proliferation/survival by an MTT assay. Data from 3 individual experiments were subjected to ANOVA statistical analysis. Error bars indicate standard deviation. *P < .05; **P < .01; ***P < .001.
Therefore, we conclude that lack of SHP2 binding, rather than Src binding, accounts for the observed Y599F-Flt3 phenotype in 32D cells and that SHP2 is a major player in FL-induced Erk activation.
Discussion
We show here for the first time that Y572, Y589, Y591, and Y599 in the juxtamembrane region of Flt3 are phosphorylated in vivo, which could provide valuable insights in how Flt3 mediates its very early signaling steps. The more AML-related ITD mutations of Flt3 often contain at least duplication of one of the juxtamembranous tyrosines. We further demonstrate that Y589 and Y599 are binding sites for downstream signaling molecules including SFKs and the protein tyrosine phosphatase SHP2.
Tyrosine 589 appeared to be involved mainly in the negative regulation of Flt3 signaling. Like its homologue in c-Kit, it was found to interact with SFKs, which constitute a family of nonreceptor tyrosine kinases and share a common modular structure consisting of the catalytic SH1, an SH2, an SH3, and a C-terminal regulatory domain that renders SFKs ideal for transducing signals from autophosphorylated receptors to downstream targets.45 Depending on which molecules are recruited to or activated by SFKs, they can have positive effects via Erk activation or a negative impact via Cbl and ubiquitination/receptor degradation on receptor signaling.46,47 Mutation of Y589 in Flt3 resulted in sustained ligand-mediated Erk activation and elevated proliferative/survival response consistent with the findings in the Src binding mutant of the CSF-1 receptor.48 A recent report stresses the importance of Lyn for positive FL-mediated signaling in Ba/F3 cells expressing WT-Flt3 but denies its involvement in receptor degradation.24 This apparently contradicts our observations of a stabilization of the Flt3 signal. However, the use of different cell systems and experimental approaches may explain the discrepancies. Moreover, one can think of specific functions of individual Src family members. The latter effects are easily missed by general Src inhibition but may become apparent by specifically mutating single recruitment sites as in Y589F-Flt3. Still, one cannot exclude contributions of other signaling molecules besides SFK to the observed Y589F-Flt3 phenotype in 32D cells. The observed sustained receptor phosphorylation could be due to defective Cbl phosphorylation (Figure 3F) and ubiquitination in the Y589F mutant or deficient protein tyrosine phosphatase recruitment.
Mutation of Y599 to phenylalanine impairs FL-triggered Erk activation, which may imply a role of SFKs in positive Flt3 signaling as suggested in Robinson et al.24 However, inhibition of SFKs by PP1 in Flt3-32D cells did not have any negative impact on FL-induced Erk activation in our hands. Instead, we revealed binding of SHP2 to wild-type Flt3 and strongly reduced SHP2 binding and phosphorylation in Y599F-Flt3-32D cells. Silencing of SHP2 by siRNA impaired Erk activation in ligand-stimulated 32D-Flt3 cells to a similar extent as seen in Y599F-Flt3 cells. We therefore suggest that SHP2 and its binding to pY599 are major prerequisites for FL-triggered positive downstream signaling. SHP2 is a nontransmembrane protein tyrosine phosphatase containing 2 SH2 domains and constitutes a key positive component of RTK signaling. Interestingly, SHP2 was among the first molecules to be identified as a target of Flt3.19 Growth factor-mediated activation of SHP2 has in many but not all cases been associated with phosphorylation of SHP2 on tyrosine residues.49 Association of the 2 SH2 domains of SHP2 with phosphorylated tyrosine residues is an important mechanism in its activation. Early studies using synthetic phosphopeptides demonstrated that doubly phosphorylated peptides were significantly more effective in activating SHP2 compared with singly phosphorylated peptides.50 By requiring both SH2 domains to associate with phosphotyrosine residues for activation, a very high degree of selectivity is achieved.51 In many proteins that associate with and activate SHP2, 2 phosphotyrosine residues are present and required for full activation.43,52 Therefore, it is likely that an additional phosphorylated tyrosine residue(s) participates in recruitment of SHP2 to Flt3, which could explain the additional reduction in growth and Erk phosphorylation by SHP2 knockdown seen in the Y599F-Flt3 mutant (Figure 5B-C). Furthermore, it has been shown that SHP2 can indirectly interact with activated Flt3 via Grb2 or Gab2.22 Dominant-negative mutants of SHP2 inhibit RTK-stimulated activation of the Erk pathway, immediate-early gene activation, cell spreading, and/or cell proliferation in mammalian tissue-culture cells,28 whereas activated mutants of human SHP2 were found to lead to leukemic disorders.29-31,53 Various modes of Erk activation by SHP2 have been proposed, ranging from SHP2 being an adapter protein for Grb254 to SHP2 dephosphorylating and thus inactivating negative regulators of Erk signaling such as Sprouty.55 Moreover, SHP2 has been reported to dephosphorylate Cbp/Pag or paxillin and thereby abolish the binding site for the C-terminal Src kinase (Csk), a negative regulator of SFKs.56,57 Thus, SHP2 is also capable of positively affecting Src kinase activity. For the EGF and PDGF receptors, it has been reported that SHP2 dephosphorylates the receptor binding site for Ras-GAP and thus preserves the activated status of Ras.58,59 The following are interesting questions that require further investigation. Which of the mechanisms of SHP2 action applies primarily to Flt3 signaling? Do SFKs, after all, play a role in positive Flt3 signaling as downstream effectors of SHP2? What is the role of SHP2 and Y599 in FL-mediated Akt activation,60 and what is the impact of SHP2 on ITD signaling?
Overall, our work presents novel insights into Flt3-mediated signal transduction. We identified the in vivo autophosphorylation sites of the juxtamembrane region of Flt3 and revealed SFKs and SHP2 as binding partners of pY589 and/or pY599, respectively, as well as their impact on FL-mediated signaling in Flt3-32D cells. Future work will now focus on elucidation of additional and possibly novel interaction partners of the found phosphorylation sites by employing an unbiased proteomics approach. The potential role of SFKs in regulating ubiquitination and internalization/degradation of FLt3 will be investigated in future studies as well as potential functional selectivities within members of the SFK family. With this gained knowledge it will be of interest to see whether ITDs differing in the nature of the duplicated tyrosines also confer distinct signaling behavior. If so, these tyrosines might serve as a diagnostic marker and point toward a successful combinatorial therapy consisting of an RTK inhibitor and an inhibitor for the specifically affected signal transduction pathway.61
Prepublished online as Blood First Edition Paper, May 9, 2006; DOI 10.1182/blood-2005-07-008896.
Supported by grants from the Swedish Research Council (L.R.), Children's Cancer Foundation Sweden (L.R.), the research funds at Malmö University Hospital (E.H., C.S.), Siv-Inger and Per-Erik Andersson Foundation (C.S.), and the Swedish Society of Medicine (E.H.). E.H. is supported by a postdoctoral fellowship from the Wenner-Gren Foundation, and the salary of L.R. is partially funded by the Swedish Research Council.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs G. Gilliland and T. Pawson for providing Flt3-cDNA and GST fusion proteins, respectively, and I. Dahlquist and P. Hansvik for expert assistance with the Edman-based radiosequencing and peptide syntheses.

![Figure 1. Mapping of autophosphorylation sites. (A) Identification of Y572, Y589, and Y591 as sites of in vivo autophosphorylation: PAE cells expressing WT or the indicated Y→F mutant of Flt3 were labeled with 2 to 6 mCi (74-222 MBq) [32P]-orthophosphate for 6 hours before they were stimulated with FL for 10 minutes, and labeled Flt3 was immunoprecipitated (IP) and subsequently digested with trypsin. Tryptic fragments of Flt3 containing aa's 572-595 were precipitated with 1 μg of a peptide-specific antibody described in “In vivo 32P- or- thophosphate labeling of cells” and subjected to phosphoamino acid analysis and Edman-based degradation (after an additional digest with AspN when indicated). The amount of radioactivity released in each cycle of Edman degradation was quantitated using a PhosphorImager and MultiGauge software (Fujifilm, Tokyo, Japan). (B) Identification of Y599 as in vivo autophosphorylation site: PAE cells expressing WT or Y599F-Flt3 were treated as described for panel A. The tryptic peptide containing aa's 596-602 of Flt3 was immunoprecipitated and processed as mentioned for panel A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2005-07-008896/2/m_zh80170600420001.jpeg?Expires=1769159550&Signature=cmPLeXk9hnFzwOzXVvUAamwqVyGfwYuszzQL7xeDP11y47cTvIuHb1m2GWgVl4xceTJmfQbA88A6ncwW3gHx5suEbdCMH6XspOBjB3RdiaM8Tbxmbz0JwzbKYrFtWZQg4~2nPlUR7WcVAaVhTqELdKZl29PMONOM0VMvFFYeFrEbp-DvaWRuQsQQV0Kp~gHKGrjNR3zNV-RycyMnf9VXqRDp9d21R5BNqSrfMAa30RJ7pA65pjnNDTjMB3p45nWyT7ZFmnqSD1E9E2KnYjfHjnMM7xIlBRNOKFs6l-gKQvsE3E5KBAabi97Ue02mAi0HZyH4lYie0q-djKmX5HYyDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. pY589 and pY599 are binding sites for Src family kinases and are involved in activation of Lyn. (A) The 32D cells were metabolically labeled with [35S] methionine/[35S] cysteine for 4 hours. Lysates were subjected to an affinity chromatography with the indicated immobilized peptides as described in “Affinity fishing of proteins with immobilized peptides.” Bound proteins were eluted, separated by SDS-PAGE, and detected by exposure on a PhosphorImager. The letters a, b, and c highlight proteins of the sizes 55, 52, and 45 kDa, respectively, showing phosphospecific interaction with pY589, whereas d, e, f, and g point to proteins of the sizes 70, 55, 52, and 45 kDa, respectively, that specifically interact with pY599. (B) Total 32D cell lysate was incubated with the following immobilized synthetic peptides: Y589 (lane A), pY589 (lanes B-D), Y599 (lane E), or pY599 (lanes F-H). To prove phosphoselectivity of found interactions, soluble phosphorylated or unphosphorylated peptides (0.5 mg/mL, Y589, lane C; pY589, lane D; Y599, lane G; pY599, lane H) were included in the reactions to compete for binding proteins. Bound proteins were eluted, separated on a 10% SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, and Hck. Each lane was densitometrically evaluated and relative intensities (RIs) are depicted under each lane. Data of 1 of 3 performed experiments with consistent results are displayed. (C) The 32D cells were incubated in the presence or absence of FL, followed by lysis and pulldown (PD) with a GST fusion protein containing the SH2 domain of Src. Samples were separated by SDS-PAGE, electrotransferred to Immobilon P, and probed with an antibody against GST. In parallel, an aliquot of each cell lysate was immunoprecipitated with an antibody against Flt3, separated by SDS gel electrophoresis, electrotransferred to Immobilon P, and probed with an antibody against Flt3 to verify equal loading. (D) Lysates from 32D cells expressing either wild-type, Y589F, or Y599F mutant Flt3 were stimulated with FL for 10 minutes, lysed, and subjected to immunoprecipitation with an antibody against Flt3. After washing of immunoprecipitates, proteins were separated by SDS-PAGE, immunoblotted, and probed for Lyn, Fgr, or Hck, respectively. The filter was consecutively stripped and reprobed with an Flt3 antibody to ensure equal loading and a phosphotyrosine antibody to ensure equal ligand stimulation. (E) WT-Flt3, Y589F-Flt3, and Y599F-Flt3-32D cells were starved followed by stimulation for 7 minutes with FL (100 ng/mL) and subsequent lysis. Lyn was immunoprecipitated from the cell lysates and subjected to an in vitro kinase assay with enolase as exogenous substrate. Phosphorylated enolase was run out on a 10% gel, blotted, and visualized on a PhosphorImager as well as quantified densitometrically. Results are means of 3 independent experiments and depicted as fold Lyn activation upon ligand binding (▪) compared with unstimulated cells (□). Error bars indicate SEM of 3 different experiments. (F) Phosphorylation of Cbl is impaired in Y589F-Flt3 and Y599F-Flt3 32D cells. The 32D infectants were starved and stimulated with FL (100 ng/mL) for the indicated periods of time. After lysis, Cbl was immunoprecipitated, run on a gel, and probed against phosphotyrosine. To ensure equal protein loading, the membrane was stripped and reprobed for Cbl.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2005-07-008896/2/m_zh80170600420003.jpeg?Expires=1769159550&Signature=3IGiYAETHEFP1tLnap0Wuxg7RTIfqgFtODtRdFn80cOubXFEK45ygBTr7xtpS39NXD7GxFFBevjP7Z9jyVXDYT3yfj0le9P3tX5xLgBKEYiVDD~iiTGWQmFvRizcRAz~aXF4FQ~KsqaRrKCFn0ahFY9oxcrcxvThvTP5S29Vac-By7Z3Sj6yAOBAvq5pXwCyqenacUgOpeF2lxUolo-tvQtCzFk-7ph1V5~xxItlaB6fYPqARIbxS8sgVqZYNF0EYzps0Mc96oVtqXa4uPSEAaVdgacehtbcDZfOaasir6CdCU0Z7G2xrxtYlkZYevjTQF6slPNxxEPJD2qJj8j82Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)