Lyn kinase is known to modulate the formation and function of B cells, monocytes, and mast cells. However, Lyn-/- mice also develop erythrosplenomegaly, and cases for both negative and positive erythropoietic actions of Lyn recently have been outlined. In phenylhydrazine-treated Lyn-/- mice, extramedullary splenic erythropoiesis was hyperactivated, but this did not lead to accelerated recovery from anemia. Furthermore, ex vivo analyses of the development of bone marrow-derived Lyn-/- erythroblasts in unique primary culture systems indicated positive roles for Lyn at 2 stages. Late-stage Lyn-/- erythroblasts exhibited deficit Ter119pos cell formation, and this was paralleled by increased apoptosis (and decreased Bcl-xL expression). During early development, Lyn-/- erythroblasts accumulated at a KitposCD71high stage, possessed decreased proliferative capacity, and were attenuated in entering an apparent G1/S cell-cycle phase. In proposed compensatory responses, Lyn-/- erythroblasts expressed increased levels of activated Akt and p60-Src and decreased levels of death-associated protein kinase-2. Stat5 activation and Bcl-xL expression, in contrast, were significantly decreased in keeping with decreased survival and developmental potentials. Lyn, therefore, is proposed to function via erythroid cell-intrinsic mechanisms to promote progenitor cell expansion beyond a KitposCD71high stage and to support subsequent late-stage development.
Introduction
Src family kinases (SFKs) modulate a broad spectrum of bioresponse pathways including antigen and immune complex signaling, cytokine responses, integrin actions, G-protein-coupled pathways, cytoskeletal organization, and ion channels.1-6 Within hematopoietic tissues, SFKs can function as key regulators as illustrated by original p60-Src gene disruption experiments that identified roles for this SFK during osteoclast development.7 Studies have since extended to include investigations of SFK actions in B-cell, myeloid, and mast cell lineages with one major focus on Lyn as a hematopoietic SFK that is expressed in all blood cell lineages (with the exception of T cells).8 In part, this focus on Lyn derives from negative roles exerted on monocyte production and plasma cell function as revealed in Lyn-/- mice by M-phi tumorigenesis9 and IgM hyperglobulinemia.10 In B cells, negative actions involve Lyn-mediated phosphorylation of immunoreceptor tyrosine-based inhibitory motifs (ITIM) within B-cell receptor-associated CD22 and FcγRII chains.11,12 In monocytes (and B cells), the phosphorylation of related motifs within inhibitory paired Ig-like receptor B (PIR-B) and/or signal recognition peptide receptor-alpha subunit (SIRP-1α) accessory receptors also is Lyn mediated.13 In each system, these phosphorylated motifs can recruit Src homology region 2 domain-containing phosphatase-1 (SHP-1) as a negatively acting tyrosine phosphatase.14,15 Lyn also can stimulate p62 downstream of tyrosine kinase (Dok),16 an adaptor protein that can repress B-cell proliferation.17
Lyn, nonetheless, also can exert important positive hematopoietic effects. During B-cell development, this is illustrated by a requirement for Lyn within marginal and follicular zones10,18,19 ; within recirculating B cells; and for pre-B-cell development in the absence of Fyn and Blk.20 These positive effects involve Lyn-mediated phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAM) within B-cell-receptor (BCR) Ig-α/β chains, as well as Lyn activation of spleen tyrosine kinase (Syk) and positively acting molecular adaptors (including B-cell linker protein [BLNK])21,22 and up-modulation of calcium flux, phospholipase C-gamma (PLC-γ), Ras/extracellular signal regulated protein kinase (ERK), and phosphoinositide-3 (PI-3) kinase pathways.23,24 In mast cells, ITAM motifs (within Fc-epsilon receptor RI) also are targets for Lyn25 and can similarly recruit Syk.26 Finally, and in leukemogenic contexts, contributing roles for Lyn in chronic myeloid leukemia (CML),27 B-cell acute lymphoblastic leukemia (B-ALL),28 and B-cell chronic lymphocytic leukemia (B-CLL)29 recently have been outlined.
Although less well studied, SFKs also have been implicated as important modulators of erythropoiesis. Avian Src (as a Rous virus-transduced proto-oncogene) was originally discovered to promote sarcomas and erythroleukemia.30,31 Among mice with disrupted SFK genes, however, Lyn-/- mice interestingly exhibit age-dependent increases in the production of splenic erythroblasts.32 As framed by Lyn's functional duality during B-cell development, the extent to which such extramedullary erythropoiesis might reflect a compensatory response due to Lyn deficiency, or possibly deregulated erythropoiesis due to a loss of inhibitory effects, is unclear (as is the nature of underlying mechanisms). During the course of the present investigations, 2 published studies have advanced this issue. In analyzing erythroid splenocytes from Lyn-/- mice, Ingley et al33 characterized decreased expression of GATA-1, Stat5, and EKLF (together with escalated erythrophagocytosis by bone marrow macrophages). Lyn, therefore, was indicated to perhaps support late erythroblast formation. In parallel, Harder et al34 observed heightened anemia and decreased colony-forming unit-erythroid (CFUe) levels in Lyn-/- mice in response to 5-fluorouracil (as well as elevated production of splenic Lyn-/- erythroid progenitor cells upon transplantation into B-cell-deficient recipients). Here, Lyn was suggested to negatively regulate erythroid progenitor cell proliferation (but Lyn-/- erythroid progenitor cells did not exhibit increased rates of BrdU incorporation).34
Presently, we have used Lyn-/- mice18 (together with unique primary erythroblast expansion systems) to define subpopulations of erythroid progenitor cells that are affected by Lyn and to advance insight into action mechanisms. In late-stage adult bone marrow erythroblasts, Lyn is shown to support survival and Ter119pos cell formation. In earlier progenitors, Lyn is revealed to be important for expansion beyond a KitposCD71high stage. In proposed compensatory responses, Lyn-deficient erythroblasts exhibit increased Akt and p60-Src activation, and decreased death-associated protein kinase-2 (DAPK2) expression. In keeping with overall defects in Lyn-/- erythroblast formation, however, Stat5 activation and Bcl-xL expression both were deficient. Lyn is therefore suggested to act via erythroid cell-intrinsic mechanisms to reinforce erythropoiesis at 2 separable stages. Findings also bring attention to possible untoward effects of recently described antileukemogenic Lyn inhibitors28,35 on red cell production.
Materials and methods
Mice, anemia models, blood analyses, and colony-forming assays
Lyn-/- mice (analyzed at age 15-20 weeks) were as described by Chan et al.18 Age-matched congenic C57BL/6J controls were from Jackson Laboratories (Bar Harbor, ME). EpoR-H mice were as characterized previously.36 Phenylhydrazine (no. P6926; Sigma-Aldrich, St Louis, MO) was administered subcutaneously (60 mg/kg) at 1 and 24 hours. Hematocrits were determined at 4-day intervals by microcapillary centrifugation (Autocrit Ultra-3; Clay Adams, Parsippany, NJ). Splenocytes were prepared as detailed by Miller et al.37 In CFUe and burst-forming unit-erythroid (BFUe) assays, cells were plated in triplicate (at 9.0 × 104 and 1.5 × 105 cells/mL) with Epo (Epoetin alfa; Amgen, Thousand Oaks, CA) at 5 U/mL and murine stem cell factor (m-SCF, no. 250-03; Peprotech, Rocky Hill, NJ) at 100 ng/mL. Hemoglobinized colonies were stained with benzidine (0.15% in 8% acetic acid, 5% H2O2) and scored.
Bone marrow cell isolation and erythroid cell expansion systems
Marrow cells were flushed from femurs and tibia and resuspended (via stepwise passages through 21-ga and 23-ga needles) in IMDM (no. 12 440-053; Invitrogen, Grand Island, NY), 1% fetal bovine serum (FBS, no. F4135; Sigma-Aldrich), 0.1 U Epo/mL plus penicillin (100 U/mL), streptomycin (100 μg/mL), and amphotericin B (0.25 μg/mL, 1 × PSF, no. A5955; Sigma-Aldrich). Washed cells then were resuspended in 1 mL PBS (no. 14 190-144; Invitrogen) and exposed for 2 minutes to 9 volumes potassium bicarbonate-buffered 0.8% ammonium chloride, 0.1 mM Na2 EDTA (ethylenediaminetetraacetic acid) solution, pH 7.5 (red cell lysis). One-tenth volume of 10 × PBS was then added, and cells were collected through 50% FBS in PBS. Short-term expansion cultures (2.5 days) were initiated at 7.5 × 105 cells/mL in SP34-EX media as StemPro-34 medium (no. 10 640-019; Invitrogen) supplemented with 1 × Stempro-34 Nutrient (no. 10 641-025; Invitrogen), 2 mM l-glutamine (no. 25 030-81; Invitrogen), 2.5 U/mL Epo, 100 ng/mL mSCF, 1 μM dexamethasone (no. D4902; Sigma-Aldrich), 1 μM beta-estradiol (no. E2758; Sigma-Aldrich), 40 ng/mL murine insulin-like growth factor-1 (mIGF-1, no. 250-19; Peprotech), 75 μg/mL h-transferrin (no. T0665; Sigma-Aldrich), 0.1 mM 2-mercaptoethanol, 0.5% BSA (no. 09 300; Stem Cell Technologies, Vancouver, BC, Canada) plus 1 × PSF. At 20 and 40 hours, cultures were supplemented with 0.5 volumes SP34-EX. In the culture of earlier stage erythroid progenitor cells, a Kitpos subpopulation was isolated from marrow preparations via magnetic-activated cell sorting (MACS; Miltenyi, Auburn, CA) prior to expansion. On days 1, 3, and 5, cultures were supplemented with 0.5 volumes SP34-EX. On days 2, 4, and 6, cells were collected and replated at 7.5 × 105 cells/mL.
Flow cytometry and cell survival assays
In flow cytometry, cells (1 × 106) were washed, resuspended in 0.2 mL PBS plus 0.1% BSA, incubated for 10 minutes at 4°C with rat IgG (1 μg, no. 012-000-003; Jackson Immunoresearch, Westgrove, PA), and stained with PE-Ter119 (1 μg), FITC-CD71 (1 μg), APC-CD117 (1 μg), or PE-CD117 (1 μg) (no. 553 673, no. 553 266, no. 553 356, and no. 553 355, respectively; BD Biosciences, San Jose, CA) in darkness at 4°C for 30 minutes. Cells then were washed, resuspended, and analyzed (BD FACS Calibur; Becton Dickinson, Franklin Lakes, NJ). Annexin V binding was performed in 0.1 mL annexin V buffer (140 mM NaCl, 2.5 mM CaCl2, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/NaOH, pH 7.4]) with 5 μL either FITC- or PE-annexin V (no. 556 420 and no. 556 422, respectively; BD Biosciences) (room temperature, 15 minutes). Flow analyses were performed following the addition of 0.4 mL annexin V buffer. In cell-cycle analyses, 1 × 106 cells were stained in 0.1 mL PBS containing 5 μM DRAQ5 (no. BOS-889-001-R050; Alexis USA, San Diego, CA) (2 minutes at 37°C in darkness). Flow analyses were performed following the addition of 0.9 mL 4°C PBS. Cell-cycle profiles were analyzed using ModFit LT software (Verity Software House, Topsham, ME). Cytospin analyses (1|×|105 cells) involved slide centrifugation for 15 minutes at 150g (Hettich Universal-16A cytocentrifuge; GMI, Ramsey, MN) and Dip-Stain reagent staining (Volu-Sol VSS016; Volu-Sol, Salt Lake City, UT). Photomicrographs were obtained using a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Thornwood, NY) equipped with a 100 ×/1.3 numeric aperture oil-immersion objective. Images were acquired using a Spot FLEX camera and Spot Advanced software version 4.5 (Diagnostic Instruments, Sterling Heights, MI).
Western blotting and proliferation assays
In Western blot analyses, both KitposCD71high and KitnegCD71high erythroblasts were isolated (via MACS) from expansion cultures. Washed cells (at 8 × 105 cell/mL) then were incubated for 6 hours in 0.25% BSA, 10 μg/mL transferrin, 0.1 mM 2-mercaptoethanol, and 10 ng/mL insulin (no. 41 400-045; Invitrogen) in IMDM. Cells then were exposed to Epo (5 U/mL) plus m-SCF (100 ng/mL). At defined intervals, samples were rapidly chilled, washed in 2°C PBS, and used to prepare lysates. Cells (5 × 106) were lysed initially by gently vortexing in 0.075 mL 1.0% Igepal, 7.5% glycerol, 100 mM NaCl, 1.5 mM Na2EDTA, 30 mM NaF, 10 mM sodium pyruvate, 1 mM dithiothreitol, 25 mM β-glycerol phosphate, 30 mM HEPES (pH 7.5), 0.25 mg/mL phenylmethylsulfonyl fluoride, 1 × protease inhibitor cocktail (no. P8340; Sigma-Aldrich), and 1 × phosphatase inhibitor cocktail (no. P5726; Sigma-Aldrich). At 20 minutes, 0.075 mL 0.5% Triton X-100, 0.25% sodium deoxycholate, 0.1% SDS, 150 mM NaCl and 50 mM Tris-HCL (pH 7.5) were added, and samples were vortexed 5 times over a 5-minute interval. Cleared lysates were assayed for protein concentration; denatured in 5% glycerol, 1% SDS, 100 mM dithiothreitol, 0.15 mM bromophenol blue, 60 mM Tris-HCL (pH 6.8); electrophoresed (35 μg); blotted (PVDF membranes); and blocked using 5% nonfat dry milk, 1% BSA (no. 15 260-037; Invitrogen), 0.05% Tween-20 (polyoxyethylenesorbitanmonolaurate, no. P-1379; Sigma-Aldrich), 150 mM NaCl, 20 mM Tris-HCL (pH 7.4). Antibodies to Stat5 (no. 9351), p-Stat5 (Tyr-464, no. 9352), Akt (no. 9272), p-Akt (Ser-473, no. 9271), Erk (no. 9102), and p-Erk (Thr202/Tyr204, no. 9101) were from Cell Signaling (Beverly, MA). Additional antibodies to Bcl-x (no. 61 011; BD Transduction Laboratories), DAPK2 (no. AP 7033A; Abgent, San Diego, CA), GATA-1 (no. sc265; Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH (no. ab9485; Novus Biologicals, Littleton, CO), and anti-EKLF (see “Acknowledgments”) were used. Enhanced chemiluminescence used HRP-conjugated secondary antibodies (no. 111-035-045, no. 115-035-062; Jackson Immunoresearch) and Super-Signal West Dura reagent (no. 34 076; Pierce Biotechnology, Rockford, IL). Band signal intensities were analyzed using Image J software (National Institutes of Health, http://rsb.info.nih.gov/ij/). In 3HdT-incorporation assays, Kitpos erythroblasts were isolated from day-5 expansion cultures (via lineage depletion and MACS) and following lineage depletion from day-5 expansion cultures, and were incubated (at 1 × 105 cells/mL) in triplicate in SP34-EX medium with Epo and/or m-SCF as indicated. At 24 hours, 3HdT was added (1 μCi [0.037 MBq]/assay, NET-027A, 2 Ci [0.074 MBq]/mol; Perkin Elmer, Boston, MA) and incorporation rates (at 6 hours) were determined.
Results
Compromised formation of Lyn-/- late-stage erythroblasts
In original characterizations of altered erythropoiesis in Lyn-/- mice, age-dependent splenomegaly was described that included elevated levels of B cells,18 monocytes, and Ter119pos erythroblasts.32 The present studies used a distinct knock-out line, and erythroid splenomegaly therefore was first assessed. In Lyn-/- mice, aged 8 to 14 weeks, no spontaneous splenomegaly was detected. Upon phenylhydrazine induction of hemolytic anemia, however, frequencies of splenic Ter119pos and CD71high erythroblasts in Lyn-/- mice were increased approximately 3-fold above age-matched congenic controls (Figure 1A; Table 1). This elevated erythroid response also was paralleled by significant increases in spleen size (Figure 1C). In addition, frequencies of splenic CFUe's and BFUe's (as analyzed at day 2.5) increased 2.7- and 2.6-fold, respectively, over phenyhydrazine-treated wild-type controls (Figure 1B). Despite this enhancement of phenylhydrazine-induced splenic erythropoiesis in Lyn-/- mice, no correspondingly significant increases in hematocrits were realized over age-matched controls as monitored over a 16-day time course (Figure 1D). For comparison, mice expressing a truncated Epo receptor allele with somewhat heightened activity (EpoR-H)36 also were analyzed. EpoR-H mice responded similarly to phenylhydrazine with moderately enhanced splenic erythropoiesis (data not shown) but, unlike Lyn-/- mice, went on to produce elevated red cell levels (Figure 1D).
Phenylhydrazine-induced extramedullary erythropoiesis in Lyn-/- mice is enhanced but does not assist recovery from anemia. (A) Lyn+/+ and Lyn-/- mice (8-12 weeks) were treated with phenylhydrazine (PHZ). At day 3.5, splenocyte preparations were assayed for frequencies of Ter119pos erythroblasts. Data shown (including several-fold increases in CD71pos and Ter119posLyn-/- erythroblasts) are representative of 3 independent experiments (Table 1). (B-C) Frequencies of splenic CFUe's and BFUe's also were determined for phenyhydrazine-treated Lyn-/- and control mice (means ± SD, n = 3) together with increases in spleen weight (means ± SE, n = 3 per group). (D) Possible effects of enhanced splenic erythropoiesis in phenylhydrazine-treated Lyn-/- mice on red cell mass were assessed based on mean hematocrits (± SE, n = 6). For comparison, mice expressing a knocked-in EpoR-H allele also were analyzed. For EpoR-H mice (but not Lyn-/- mice), mean hematocrits at day 15 differed significantly from wild-type controls. *P < .01.
Phenylhydrazine-induced extramedullary erythropoiesis in Lyn-/- mice is enhanced but does not assist recovery from anemia. (A) Lyn+/+ and Lyn-/- mice (8-12 weeks) were treated with phenylhydrazine (PHZ). At day 3.5, splenocyte preparations were assayed for frequencies of Ter119pos erythroblasts. Data shown (including several-fold increases in CD71pos and Ter119posLyn-/- erythroblasts) are representative of 3 independent experiments (Table 1). (B-C) Frequencies of splenic CFUe's and BFUe's also were determined for phenyhydrazine-treated Lyn-/- and control mice (means ± SD, n = 3) together with increases in spleen weight (means ± SE, n = 3 per group). (D) Possible effects of enhanced splenic erythropoiesis in phenylhydrazine-treated Lyn-/- mice on red cell mass were assessed based on mean hematocrits (± SE, n = 6). For comparison, mice expressing a knocked-in EpoR-H allele also were analyzed. For EpoR-H mice (but not Lyn-/- mice), mean hematocrits at day 15 differed significantly from wild-type controls. *P < .01.
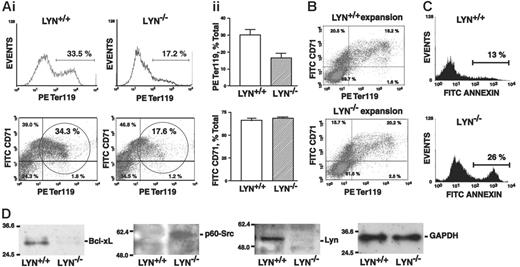
To further assess developmental potentials first of late-stage Lyn-/- erythroblasts, adult bone marrow progenitor cells were isolated from knock-out and control mice, and were expanded for 2.5 days in SCF- and Epo-containing SP34-EX media to generate populations of 40% or more CD71high erythroblasts. These progenitor cell populations were then shifted to an Epo, insulin, and transferrin-containing medium to induce late-stage differentiation. Erythroblasts generated from Lyn-/- mice reproducibly exhibited a 2-fold or higher defect in the formation of Ter119pos erythroblasts compared directly with wild-type controls (Figure 2A), and this was observed in 3 independent experiments. In initial short-term expansions, no such differences in CD71 or Ter119 marker expression were observed between Lyn-/- and control cell preparations (Figure 2B). These findings prompted analyses of apoptosis within differentiating cultures. Among Lyn-/- cells, frequencies of annexin V-positive erythroblasts reproducibly were increased 2-fold at a Ter119pos stage (Figure 2C). Levels of Bcl-xL and PY416-p60Src expression also were analyzed (Figure 2D). In late-stage Lyn-/- erythroblasts, Bcl-xL levels were decreased 2- to 3-fold, while levels of activated PY416 p60Src were elevated approximately 2-fold. Lyn-deficient late-stage erythroblasts therefore exhibit erythroid cell-intrinsic defects in survival (and possibly late-stage maturation).
Early-stage Lyn-/- erythroblasts accumulate as KitposCD71high progenitors and falter in apparent cell-cycle entry at a KitnegCD71high stage
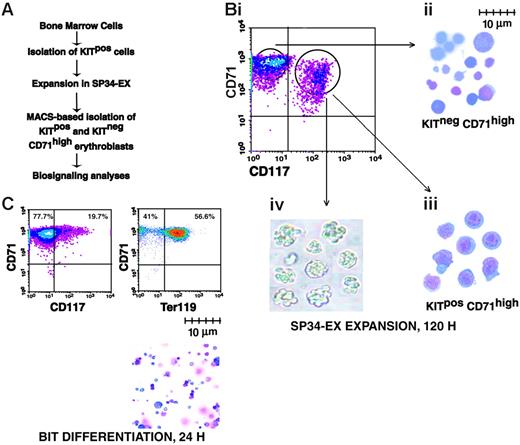
To assess possible effects of Lyn deficiency on early-stage erythroblast development, a modified ex vivo expansion system was implemented. Specifically, this involved MACS-based isolation of Kitpos progenitors from adult bone marrow cell preparations and their subsequent culture in SP34-EX medium (Figure 3A). In this system, KitposCD71high and KitnegCD71high progenitors developed over a 3- to 5-day period at high frequencies (up to 95% of total cells). As analyzed in cytospin preparations, KitposCD71high cells were larger (8-10 μm diameter) blastlike cells, while KitnegCD71high cells were primarily smaller (2-5 μm diameter) more mature cells (Figure 3B). In colony-forming assays, KitposCD71high cells gave rise to 60% to 70% CFUe-like colonies (Figure 3Biv). In differentiation assays, KitposCD71high cells (as well as KitnegCD71high cells; data not shown) efficiently gave rise to late-stage Ter119pos erythroblasts (Figure 3C).
Bone marrow-derived Lyn-/-erythroblasts falter in their development to Ter119pos erythroblasts. (A) Defects in Lyn-/- bone marrow-derived erythroblast development at a Ter119pos stage. Following 2.5 days of culture in SP34-EX medium, expanded Lyn+/+ and Lyn-/- erythroid progenitor cells were shifted to a transferrin, insulin, and Epo-containing differentiation medium. At day 1.5, frequencies of maturing erythroblasts were assayed based on Ter119 marker expression (i). Frequencies of CD71highTer119pos copositive cells also were assayed (bottom panels, circled populations). Overall frequencies of Ter119-positive and CD71+ erythroblasts from 3 independent experiments (n = 3 mice per experiment) are also graphed (i; mean ± SE). (B) CD71 and Ter119 marker expression among expanded erythroblasts also was assessed prior to differentiation. During this short-term expansion, marker profiles for Lyn+/+ and Lyn-/- populations were essentially equivalent. (C) Lyn-/- erythroblasts exhibit decreased survival at a Ter119pos stage. Bone marrow-derived erythroblasts from Lyn-/- and Lyn+/+ mice were expanded and shifted to differentiation conditions. At 24 hours of culture, frequencies of annexin V-positive cells among the maturing erythroblasts were assayed. Increased death among Lyn-/- erythroblasts also was confirmed by propidium iodide staining (data not shown). (D) In these cell populations (and at 24 hours of differentiation), levels of Bcl-xL and of activated PY416-p60-Src (p-Src) expression also were analyzed (by Western blotting) together with Lyn and GAPDH.
Bone marrow-derived Lyn-/-erythroblasts falter in their development to Ter119pos erythroblasts. (A) Defects in Lyn-/- bone marrow-derived erythroblast development at a Ter119pos stage. Following 2.5 days of culture in SP34-EX medium, expanded Lyn+/+ and Lyn-/- erythroid progenitor cells were shifted to a transferrin, insulin, and Epo-containing differentiation medium. At day 1.5, frequencies of maturing erythroblasts were assayed based on Ter119 marker expression (i). Frequencies of CD71highTer119pos copositive cells also were assayed (bottom panels, circled populations). Overall frequencies of Ter119-positive and CD71+ erythroblasts from 3 independent experiments (n = 3 mice per experiment) are also graphed (i; mean ± SE). (B) CD71 and Ter119 marker expression among expanded erythroblasts also was assessed prior to differentiation. During this short-term expansion, marker profiles for Lyn+/+ and Lyn-/- populations were essentially equivalent. (C) Lyn-/- erythroblasts exhibit decreased survival at a Ter119pos stage. Bone marrow-derived erythroblasts from Lyn-/- and Lyn+/+ mice were expanded and shifted to differentiation conditions. At 24 hours of culture, frequencies of annexin V-positive cells among the maturing erythroblasts were assayed. Increased death among Lyn-/- erythroblasts also was confirmed by propidium iodide staining (data not shown). (D) In these cell populations (and at 24 hours of differentiation), levels of Bcl-xL and of activated PY416-p60-Src (p-Src) expression also were analyzed (by Western blotting) together with Lyn and GAPDH.
In the ex vivo culture system (see Figures 2, 3), wild-type progenitors developed stepwise over a 5-day period from KitposCD71low (cohort I) to KitposCD71high (cohort II) cells, and further to KitnegCD71high erythroblasts (Figure 4A, left panels). On subsequent days (ie, days 6 and 7), cell numbers increased approximately 2- to 4-fold further (Figure 4C). By comparison, Lyn-/- progenitor cells faltered in their ex vivo development, accumulated (especially at 72 hours of culture) as 2 discernible Kitpos subpopulations, and did not efficiently give rise to KitnegCD71high erythroblasts (Figures 4A, right panels, and 4B). In repeated independent analyses, these apparent stage-specific defects in Lyn-/- (pro)erythroblast development were reproducibly observed (Figure S1).
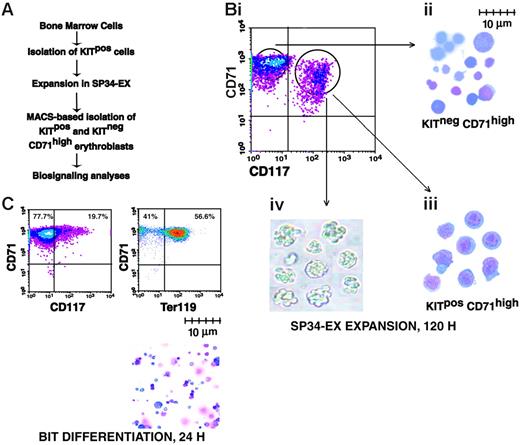
System for the ex vivo development of adult bone marrow-derived erythroblasts. (A) Outlined are steps used in the serum-free expansion and MACS-based isolation of marrow-derived erythroid progenitor cells. (B) Flow cytometric, cytospins, and colony-forming features of isolated erythroid progenitor cells. (i) Upon expansion for 4.5 days, cultures routinely were composed of approximately 50% to 60% KitposCD71high and approximately 40% to 50% KitnegCD71high erythroblasts. (ii-iii) Wright-Giemsa staining of isolated KitnegCD71high and KitposCD71high erythroblasts. (iv) Colony morphologies of KitposCD71high cells cultured for 2.5 days in 0.9% methylcellulose with Epo at 5 U/mL. (C) Differentiation capacities of expanded erythroblasts. KitposCD71high cells were isolated (by MACS) from expansion cultures and transferred to BSA, insulin, and transferrin (BIT)-containing medium. At 24 hours of culture, few cells continued to express Kit (CD117) while more than 50% routinely differentiated to Ter119pos erythroblasts (lower right panel, cytospin preparation).
System for the ex vivo development of adult bone marrow-derived erythroblasts. (A) Outlined are steps used in the serum-free expansion and MACS-based isolation of marrow-derived erythroid progenitor cells. (B) Flow cytometric, cytospins, and colony-forming features of isolated erythroid progenitor cells. (i) Upon expansion for 4.5 days, cultures routinely were composed of approximately 50% to 60% KitposCD71high and approximately 40% to 50% KitnegCD71high erythroblasts. (ii-iii) Wright-Giemsa staining of isolated KitnegCD71high and KitposCD71high erythroblasts. (iv) Colony morphologies of KitposCD71high cells cultured for 2.5 days in 0.9% methylcellulose with Epo at 5 U/mL. (C) Differentiation capacities of expanded erythroblasts. KitposCD71high cells were isolated (by MACS) from expansion cultures and transferred to BSA, insulin, and transferrin (BIT)-containing medium. At 24 hours of culture, few cells continued to express Kit (CD117) while more than 50% routinely differentiated to Ter119pos erythroblasts (lower right panel, cytospin preparation).
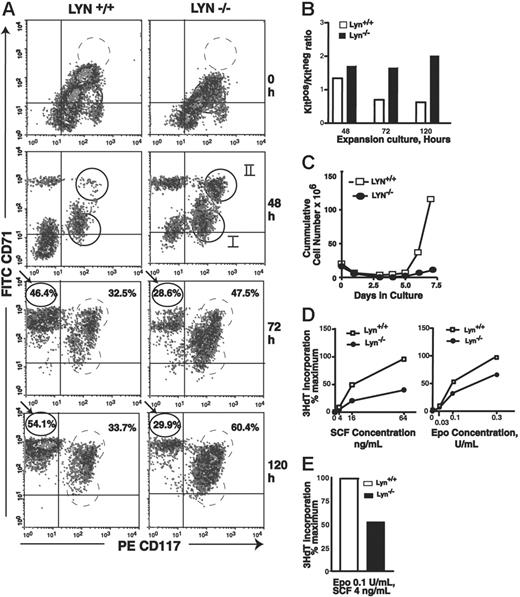
Early-stage Lyn-/-erythroid progenitor cells accumulate at a KitposCD71high stage of development and are deficient in Epo and SCF proliferative responsiveness. (A) At the indicated time points, KitposCD71low, KitposCD71high, and KitnegCD71high erythroblast formation was assayed. At 48 hours, 2 subpopulations of Kitpos cells reproducibly were detected (and are designated cohorts I and II). For Lyn-/- erythroblasts, note accumulations at a KitposCD71high stage (especially at 72 and 120 hours) and corresponding deficits in KitnegCD71high cell formation. Data illustrated are representative of 3 independent experiments (and additional representative experiments are illustrated in Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). (B) Also graphed are ratios of Kitpos/Kitneg erythroblast formation for wild-type versus Lyn-/- progenitors at 48, 72, and 120 hours of culture. (C) Over an extended period of culture, expansion capacities of Lyn-/- and control Lyn+/+ cultures also were assayed by direct cell counts. Note the deficient expansion of Lyn-/- erythroblasts (observed in 3 independent experiments). (D) Levels of Epo- and SCF-induced 3HdT incorporation in expanded Lyn-/- and control erythroblasts. Expanded KitposCD71high erythroblasts were isolated by lineage depletion and MACS, and cultured for 24 hours in SP34-EX medium in the presence of Epo and/or SCF as indicated. Cultures then were pulsed with 3HdT, and mean incorporation rates (± SE) were determined.
Early-stage Lyn-/-erythroid progenitor cells accumulate at a KitposCD71high stage of development and are deficient in Epo and SCF proliferative responsiveness. (A) At the indicated time points, KitposCD71low, KitposCD71high, and KitnegCD71high erythroblast formation was assayed. At 48 hours, 2 subpopulations of Kitpos cells reproducibly were detected (and are designated cohorts I and II). For Lyn-/- erythroblasts, note accumulations at a KitposCD71high stage (especially at 72 and 120 hours) and corresponding deficits in KitnegCD71high cell formation. Data illustrated are representative of 3 independent experiments (and additional representative experiments are illustrated in Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). (B) Also graphed are ratios of Kitpos/Kitneg erythroblast formation for wild-type versus Lyn-/- progenitors at 48, 72, and 120 hours of culture. (C) Over an extended period of culture, expansion capacities of Lyn-/- and control Lyn+/+ cultures also were assayed by direct cell counts. Note the deficient expansion of Lyn-/- erythroblasts (observed in 3 independent experiments). (D) Levels of Epo- and SCF-induced 3HdT incorporation in expanded Lyn-/- and control erythroblasts. Expanded KitposCD71high erythroblasts were isolated by lineage depletion and MACS, and cultured for 24 hours in SP34-EX medium in the presence of Epo and/or SCF as indicated. Cultures then were pulsed with 3HdT, and mean incorporation rates (± SE) were determined.
Lyn-/- erythroblasts, furthermore, failed to efficiently expand upon extended culture, with an approximate 6-fold deficiency realized at days 6 and 7 (Figure 4C). This proliferative disadvantage was investigated further first by isolating KitposCD71high erythroblasts from expansion cultures and assaying rates of SCF- and Epo-induced 3HdT incorporation. Specifically, erythroid progenitors were expanded from wild-type and Lyn-deficient mice, and KitposCD71high cells were isolated from each (via stepwise lineage depletion and MACS selection). Rates of Epo- and/or SCF-dependent 3HdT incorporation then were assayed. Lyn-deficient erythroblasts exhibited substantially attenuated responses to SCF and Epo. This effect also was observed at combined doses of SCF plus Epo that best supported more-than-additive effects (Figure 4D, right panel). These outcomes were observed consistently in independent marrow preparations and repeated experiments (Figure S2). As observed by Ingley et al33 and Harder et al,34 phenotypes for Lyn-/- erythroid cells interestingly also were age dependent and were less than uniformly observed in younger mice.
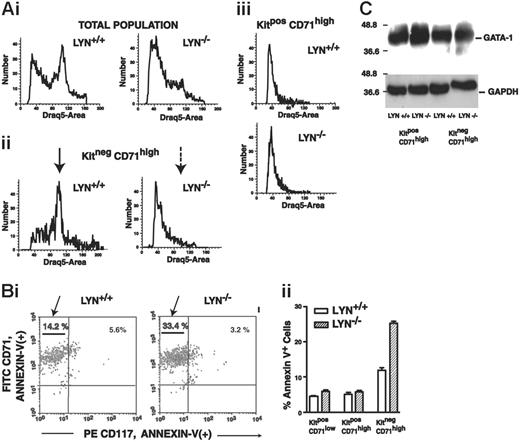
Extended analyses involved DRAQ5 and annexin V staining of both KitposCD71high and KitnegCD71high subpopulations within expanding Lyn-/- and Lyn+/+ erythroblast populations (Figure 5). In DRAQ5 studies, Lyn+/+ erythroblasts exhibited an apparent S-phase peak within a KitnegCD71high pool (which interestingly was not obvious among KitposCD71high cells) (Figure 5A, left panels). For Lyn-/- erythroblasts, however, DRAQ5 staining profiles revealed poor representation of a corresponding S-phase peak specifically among KitnegCD71high erythroblasts (Figure 5A, right panels), and this result is consistent with a limited capacity for cell-cycle entry. Annexin V staining experiments also revealed 2-fold increased frequencies of apoptotic KitnegCD71high erythroblasts in Lyn-/- mice over Lyn+/+ controls (Figure 5B). By comparison, comparable frequencies of annexin V positivity were observed between Lyn-/- and Lyn+/+ erythroblasts at a KitposCD71high stage. Lyn-/- KitnegCD71high erythroblasts therefore appear to falter in apparent cell-cycle entry and commit at increased rates to programmed cell death. In these erythroblast populations, GATA-1 expression also was analyzed based (in part) on the nature of this essential erythroid transcription factor as a caspase target.38 However, no significant differences in GATA-1 expression levels (or cleavage; data not shown) were observed between primary Lyn-/- versus Lyn+/+ erythroblast cohorts (Figure 5C). EKLF expression likewise was not significantly altered due to Lyn disruption (data not shown).
Altered signal transduction events and survival factor expression in Lyn-/- erythroblasts
The present development of a marrow-derived erythroblast expansion system also provided opportunities to investigate possible skewing in signal transduction events and/or survival factor expression that might parallel altered erythroblast development due to Lyn deficiency. When expanded under optimized conditions in SP34-EX medium, bone marrow-derived Lyn+/+ and Lyn-/- Kitpos progenitor cell preparations gave rise at day 5 to 90% or more CD71high cells. MACS then was used to isolate KitposCD71high and KitnegCD71high cell subpopulations. Signaling in these Lyn+/+ and Lyn-/- erythroblast populations then was studied by withdrawing cytokines for 6 hours and restimulating cells with Epo plus SCF (due to limiting Lyn-/- cells, SCF and Epo actions were not studied separately). Factors analyzed included DAPK-2,Akt, Stat5, and Bcl-xL.
At a KitnegCD71high stage of development, Lyn-/- erythroid progenitor cells fail to efficiently enter an apparent G1/S cell cycle phase and undergo increased apoptosis. (A) Cultures of expanded Lyn+/+ and Lyn-/- erythroblasts were analyzed for Kit and CD71 marker expression, and were costained for DNA content with DRAQ5. DRAQ5 staining distributions are shown for total cell populations (i), for KitnegCD71high cells (ii), and for KitposCD71high cells (iii). Note the limited frequencies of KitnegCD71highLyn-/- erythroblasts in the S-phase peak compared directly with Lyn+/+ controls (arrows, ii). (B) Increased apoptosis of Lyn-/- erythroblasts at a KitnegCD71high stage. Bone marrow-derived Lyn-/- and wild-type control erythroblasts were cultured in SP34-EX (with SCF and Epo at nonlimiting concentrations). At 120 hours of culture, cells were coanalyzed for Kit (CD117) and CD71 expression, and for annexin V positivity. Selectively within a KitnegCD71high population, Lyn-/- cells exhibited significantly increased levels of annexin V staining (33.4% vs 14.2%, or 220% over Lyn+/+ controls). This is illustrated in a representative flow cytometric profile (i) and in histograms as mean values (± SE) for triplicate analyses (ii) (results shown are representative of 2 independent experiments). (C) Also analyzed (by Western blotting) were levels of GATA-1 expression (and GAPDH) in KitposCD71high as well as KitnegCD71highLyn-/- and Lyn+/+ erythroblasts (as isolated by MACS).
At a KitnegCD71high stage of development, Lyn-/- erythroid progenitor cells fail to efficiently enter an apparent G1/S cell cycle phase and undergo increased apoptosis. (A) Cultures of expanded Lyn+/+ and Lyn-/- erythroblasts were analyzed for Kit and CD71 marker expression, and were costained for DNA content with DRAQ5. DRAQ5 staining distributions are shown for total cell populations (i), for KitnegCD71high cells (ii), and for KitposCD71high cells (iii). Note the limited frequencies of KitnegCD71highLyn-/- erythroblasts in the S-phase peak compared directly with Lyn+/+ controls (arrows, ii). (B) Increased apoptosis of Lyn-/- erythroblasts at a KitnegCD71high stage. Bone marrow-derived Lyn-/- and wild-type control erythroblasts were cultured in SP34-EX (with SCF and Epo at nonlimiting concentrations). At 120 hours of culture, cells were coanalyzed for Kit (CD117) and CD71 expression, and for annexin V positivity. Selectively within a KitnegCD71high population, Lyn-/- cells exhibited significantly increased levels of annexin V staining (33.4% vs 14.2%, or 220% over Lyn+/+ controls). This is illustrated in a representative flow cytometric profile (i) and in histograms as mean values (± SE) for triplicate analyses (ii) (results shown are representative of 2 independent experiments). (C) Also analyzed (by Western blotting) were levels of GATA-1 expression (and GAPDH) in KitposCD71high as well as KitnegCD71highLyn-/- and Lyn+/+ erythroblasts (as isolated by MACS).
ERK1/2 activation profiles also were assayed but did not differ significantly between Lyn-/- and Lyn+/+ controls (Figure S3).
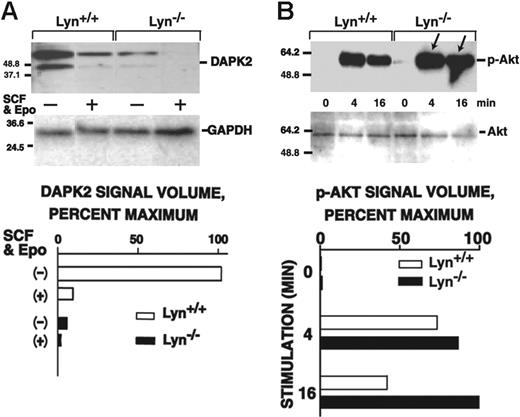
DAPK2 expression and Akt activation proved to be altered in Lyn-/- erythroblasts but in somewhat unexpected ways. Specifically, DAPK2 levels in KitposCD71high progenitors were decreased as much as 10-fold below control levels (Figure 6A). (In Lyn+/+ and Lyn-/- erythroblasts, however, Epo plus SCF signaling appeared to rapidly down-modulate DAPK2.) In KitnegCD71high erythroblast populations, DAPK2 expression also was observed but was expressed at similar levels in Lyn-/- and Lyn+/+ cells (and was substantially less subject to cytokine modulation; data not shown). Akt activation in Lyn-/- erythroblasts, in contrast, proved to be up-modulated, and this also was characterized by sustained Akt phosphorylation in Lyn-/- cells following cytokine withdrawal (0-minute time points). Increased Akt activation also was observed in KitnegCD71high erythroblasts (Figure S4). Decreased DAPK2 expression and increased Akt activation are incongruous with observed disadvantages in Lyn-/- erythroblast growth and survival, and therefore are proposed to comprise compensatory (but not fully compensating) responses.
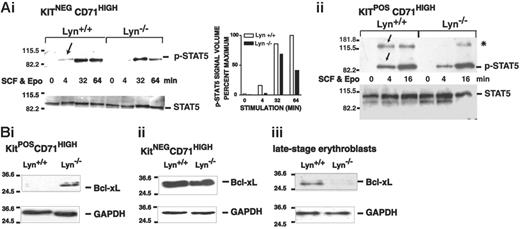
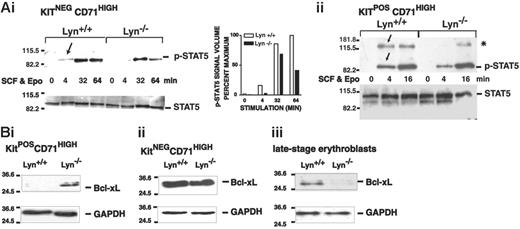
For Stat5 and Bcl-xL, Lyn deficiency resulted in decreased activation and expression (Figure 7). Specifically, in KitnegCD71highLyn-/- erythroblasts, Stat5 activation was somewhat delayed and was also diminished compared with Lyn+/+ controls (Figure 7A). In KitposCD71highLyn-/- erythroblasts, Stat5 activation was likewise delayed (as was the induced phosphorylation of an Mr 130 000 factor that reacted with the phospho-specific antibody used in these analyses). For Bcl-xL, expression was initially up-modulated in early-stage KitposCD71high erythroblasts but was decreased to below control levels in maturing KitnegCD71high erythroblasts (Figure 7B). These defects in Stat5 signaling and Bcl-xL expression are consistent with the overall defects in expansion and survival observed for Lyn-/- erythroblasts and therefore are suggested to comprise underlying dysregulated molecular events.
Altered DAPK2 expression and Akt activation in Lyn-/-erythroblasts. (A) Lyn-/- and wild-type erythroblasts were expanded from bone marrow preparations in SP34-EX media to yield 90% or more CD71high populations. Kitpos erythroblasts were isolated via MACS, washed, deprived of cytokines for 6 hours, and then exposed to SCF plus Epo (150 ng/mL, 5 U/mL) for the indicated intervals. Levels of DAPK2 in KitposCD71high cells then were assayed by Western blotting and quantitatively by densitometry (bottom panels). (B) Lyn-/- and wild-type erythroblasts were expanded from bone marrow preparations in SP34-EX media, and KitposCD71high erythroblasts were isolated. Cells were then washed and cultured in the absence of SCF and Epo. At the indicated intervals of subsequent exposure to SCF plus Epo (150 ng/mL, 5 U/mL), levels of phospho-Akt were determined. In repeated independent experiments, KitnegCD71high erythroblasts also were analyzed (together with KitposCD71high cells) (Figure S4).
Altered DAPK2 expression and Akt activation in Lyn-/-erythroblasts. (A) Lyn-/- and wild-type erythroblasts were expanded from bone marrow preparations in SP34-EX media to yield 90% or more CD71high populations. Kitpos erythroblasts were isolated via MACS, washed, deprived of cytokines for 6 hours, and then exposed to SCF plus Epo (150 ng/mL, 5 U/mL) for the indicated intervals. Levels of DAPK2 in KitposCD71high cells then were assayed by Western blotting and quantitatively by densitometry (bottom panels). (B) Lyn-/- and wild-type erythroblasts were expanded from bone marrow preparations in SP34-EX media, and KitposCD71high erythroblasts were isolated. Cells were then washed and cultured in the absence of SCF and Epo. At the indicated intervals of subsequent exposure to SCF plus Epo (150 ng/mL, 5 U/mL), levels of phospho-Akt were determined. In repeated independent experiments, KitnegCD71high erythroblasts also were analyzed (together with KitposCD71high cells) (Figure S4).
Attenuated Stat5 activation and late-stage-specific decreases in Bcl-xL expression in Lyn-/-erythroblasts. (A) Stat5 activation in Lyn-/- erythroblasts is attenuated. Bone marrow-derived progenitor cells from Lyn-/- and Lyn+/+ mice were expanded in SP34-EX medium. KitnegCD71high and KitposCD71high subpopulations of erythroblasts were isolated (by MACS), and washed cells were incubated in the absence of cytokines. Cells then were exposed to SCF plus Epo (150 ng/mL, 5 U/mL) and lysed at the indicated intervals. Levels of phospho- and total Stat5 then were assayed by Western blotting. In Lyn-/- erythroblasts, levels of phospho-Stat5 were significantly decreased. In addition, levels of an approximately Mr 130 000 factor (*), which crossreacted with the phospho-Stat5-specific antibody used in the present studies, also were diminished. (B) Bcl-xL expression is up-modulated in KitposCD71high cells but decreased in KitnegCD71high and later-stage erythroblasts. In these erythroblast populations (i-ii), and in expanded Lyn-/- and Lyn+/+ erythroblasts that were differentiated for 1.5 days (iii), Bcl-xL levels also were analyzed (together with GAPDH).
Attenuated Stat5 activation and late-stage-specific decreases in Bcl-xL expression in Lyn-/-erythroblasts. (A) Stat5 activation in Lyn-/- erythroblasts is attenuated. Bone marrow-derived progenitor cells from Lyn-/- and Lyn+/+ mice were expanded in SP34-EX medium. KitnegCD71high and KitposCD71high subpopulations of erythroblasts were isolated (by MACS), and washed cells were incubated in the absence of cytokines. Cells then were exposed to SCF plus Epo (150 ng/mL, 5 U/mL) and lysed at the indicated intervals. Levels of phospho- and total Stat5 then were assayed by Western blotting. In Lyn-/- erythroblasts, levels of phospho-Stat5 were significantly decreased. In addition, levels of an approximately Mr 130 000 factor (*), which crossreacted with the phospho-Stat5-specific antibody used in the present studies, also were diminished. (B) Bcl-xL expression is up-modulated in KitposCD71high cells but decreased in KitnegCD71high and later-stage erythroblasts. In these erythroblast populations (i-ii), and in expanded Lyn-/- and Lyn+/+ erythroblasts that were differentiated for 1.5 days (iii), Bcl-xL levels also were analyzed (together with GAPDH).
Discussion
As a major Src family kinase of hematopoietic cells, Lyn previously has been shown to exert both negative and positive effects on B-cell, monocyte, and mast-cell development and function. Examples of Lyn's lineage- and context-specific duality include the following: repression of IgM hyperresponsiveness and autoimmune disease10,18 as contrasted with positive roles for Lyn in peripheral B-cell development10,18,19 ; repression of degranulation39 in parallel with enhancement of mast cell migration and proliferation40-42 ; and inhibition of M-phi leukemogenesis9 versus reported positive contributions to CML.27 With Lyn's pleiotropic properties in mind, the present investigations sought to advance an understanding of the effects of this SFK on erythropoiesis. Studies of adult bone marrow-derived Lyn-/- erythroblasts (as investigated using novel primary cell expansion systems) suggest that Lyn positively modulates erythroid progenitor cell expansion beyond a KitposCD71high stage and also acts subsequently to promote survival and/or development at a late Ter119pos stage. In addition, this model system enabled analyses of signal transduction events that might be altered due to Lyn deficiency.
In Lyn-/- mice, initial analyses suggested a negative role for Lyn based on observed increases in splenic BFUe's, CFUe's, and erythroblasts during phenylhydrazine-induced erythropoiesis. However, this failed to give rise to corresponding increases in hematocrits. Phenylhydrazine markedly promotes erythropoiesis, and production of red cells beyond wild-type levels might be challenging to achieve. Heightened phenylhydrazine-induced erythropoiesis beyond control levels nonetheless has been observed in mice lacking Lnk (a negatively acting adaptor protein),43 and presently in mice expressing an Epo-H allele that lacks an inhibitory distal cytoplasmic domain.36 Multilineage effects precipitated by Lyn deficiency also might complicate interpretations of erythrosplenomegaly. In SHP-1 phosphatase-deficient mice, for example, monocytes have been shown to perturb red cell oxidation and fragility, and to lead to regenerative anemia.44 Finally, erythrosplenomegaly can be induced in compensatory modes upon the disruption of positively acting effectors as illustrated by spontaneous splenomegaly in Stat5a, b-/- mice.45 To better focus on erythroid lineage-intrinsic events, and to avoid factors associated specifically with splenic stress erythropoiesis,46 the balance of the present studies sought to analyze the growth, survival, and differentiation potentials of primary Lyn-deficient erythroblasts as isolated from adult bone marrow.
In marrow-derived cultures, early-stage Lyn-/- erythroblasts were substantially compromised in their expansion capacities, and in their ability to develop beyond a KitposCD71high stage. While specific molecular mechanisms underlying this defect are unclear, one basic observation (suggested by DRAQ5-staining analyses) is that cell-cycle entry at this stage may falter in the absence of Lyn. SFKs are broadly established cell-cycle regulators,47 and positive roles for Lyn in G1 phase progression previously have been described during SCF-48 and prolactin-induced proliferation.49 The presently observed acquisition of mitogenic potential by KitnegCD71high cells compared with their KitposCD71high precursors also is of basic interest. Specific factors that promote such rapid proliferation presently are unknown, but this transition does appear to be affected significantly by Lyn. Consistent with possible cell-cycle defects, Lyn-/- erythroblasts also were deficient in Epo- and SCF-dependent 3HdT incorporation.
With regard to effects of Lyn deficiency on proliferation, our findings are interesting to compare first with studies by Harder et al.34 The latter pointed to an apparent proliferative advantage for Lyn-deficient erythroid progenitor cells based (in part) on elevated CFUe levels in spleen and bone marrow, and an increased sensitivity to 5-fluorouracil was heightened as analyzed within a bone marrow CFUe compartment. In keeping with these observations, skewing toward increased proliferation in the absence of Lyn was proposed. In assessing differential 5-fluorouracil effects, however, elevated CFUe levels in untreated Lyn-/- mice could complicate interpretations (and 5-fluorouracil can also significantly compromise cell survival).50,51 In addition, in BrdU incorporation assays, no significant differences between Lyn-/- and Lyn+/+ CD71pos cell preparations were detected.34 Based on these considerations, on an apparent lack of detectable effects of Lyn deficiency on Epo activation of SHP-1 and SHIP-1,34 and on our present observations, it is suggested that frequencies of splenic and bone marrow Lyn-/- erythroid progenitor cells may increase through age-dependent (yet presently undefined) compensatory responses, especially during stress erythropoiesis. These interpretations more closely parallel those outlined by Ingley et al33 who observed enhanced extramedullary erythropoiesis in Lyn-/- mice and also characterized an accelerated phagocytosis of Lyn-/- erythroblasts. Such interpretations also are consistent with a series of studies that characterized positive roles for Lyn during erythroid J2E cell differentiation.52,53
The presently developed erythroblast expansion system also provided opportunities to examine signal transduction factors whose expression and/or activation might be affected by Lyn deficiency. Two factors that were affected in ways consistent with observed defects in Lyn-/- erythroblast development were Stat5 and Bcl-xL. Specifically, Stat5 activation was attenuated, and Bcl-xL levels in Lyn-/- erythroblasts were deficient (especially at a late Ter119pos stage). For Stat5, positive regulation by SFKs has been described in megakaryocytic progenitor cells within an SCF/Kit signaling context,54 and the nature of Stats as common downstream transducers of SFKs recently has been reviewed.55 Notably, Ingley et al33 likewise described attenuated Epo-induced Stat5 activation in splenocytes from phenylhydrazine-treated Lyn-/- mice. The extent to which Lyn's presently observed effects on Stat5 depend upon Kit56 and/or Epo receptor57 modulation of Lyn is unknown (but might comprise one mechanism for SCF and Epo synergy). Finally, no decreases in overall expression levels of Stat5 (or for EKLF or GATA1) were observed in Lyn-/- erythroblasts at either KitposCD71high or KitnegCD71high stages. This contrasts with analyses of unfractionated splenocytes from phenylhydrazinetreated Lyn-/- mice33 and might reflect differences in the developmental stage, purity, and/or properties of spleen versus bone marrow erythroblasts.
With regard to decreases in Bcl-xL expression in Lyn-/- erythroblasts, Lyn and SFKs have been linked in several systems to Bcl-xL and/or Bcl-2 expression.58,59 In addition, in Bcr/Abl models several forms of STI-571 resistance appear to use Lyn to enforce Bcl-2 expression60 ; in pre-T Nb2 cells, prolactin induction of Bcl-xL occurs via SFK kinase-dependent (but Jak2-independent) routes58 ; in T1 stage B cells, failed survival (and selection) in the absence of Lyn can be rescued by Bcl-259 ; and in eosinophils, Lyn (together with Raf1) supports IL5-dependent survival effects.61 Specific mechanisms of Lyn-mediated Bcl-xL (and Bcl-2) up-modulation, however, are unresolved. Based on Stat5′s apparent ability to promote Bcl-xL expression via a consensus Stat5 element in intron-1,62 one mechanism for Bcl-xL up-modulation might involve Stat5′s activation of Lyn. Presently defined survival defects in Lyn-/- erythroblasts therefore might involve a disruption of this proposed pathway.
In Lyn-/- erythroblasts, levels of activated Akt were increased at both KitposCD71high and KitnegCD71high stages. Akt can link to several survival pathways (eg, via ASK1 and forkhead FKHRL1 inhibition).63,64 Observed increases in activated Akt expression in Lyn-/- erythroblasts might therefore occur as a compensatory response to growth and survival defects. However, Lyn also has been shown to negatively regulate Akt in primary mast cells65 (and to consequently affect NF-kappaB, NF-AT, and AP-1 expression66 ). Whether related circuits are operable in developing erythroblasts is under investigation. Finally, DAPK2 expression in Lyn-/- erythroblasts was decreased several fold. DAPK2 is a DAPK1-related S/T CAM-regulated kinase,67 which recently has been shown to be expressed at high levels in developing erythroblasts.68 Ectopic DAPK2 expression furthermore induces membrane blebbing and apoptosis.67 Decreases observed in DAPK2 levels in Lyn-deficient erythroblasts therefore might reflect a compensatory survival effect (or possibly, the attenuation of Lyn-/- erythroblast development). Despite these suggested compensatory responses, overall consequences of Lyn deficiency in bone marrow-derived erythroblasts appear to involve not only deficiency in KitposCD71high cell expansion, but also subsequent survival defects within maturing erythroblasts.
Prepublished online as Blood First Edition Paper, May 16, 2006; DOI 10.1182/blood-2005-09-008243.
Supported by National Institutes of Health (NIH) grants HL044491 and P20RR18789.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Jane Mitchell (MMCRI Progenitor cell Isolation Core) for expert assistance with flow cytometry and Dr James Bieker (Mt Sinai School of Medicine, New York, NY) for provision of 4B9 anti-EKLF mouse monoclonal antibody.