Comment on Lacout et al, page 1652
A low incidence and phenotypic mimicry have been impediments to investigating the chronic MPDs: PV, IMF, and ET. The discovery of JAK2V617F, however, provided an anodyne, since it offered the possibility of developing animal models for the MPDs and 2 groups have now done this.
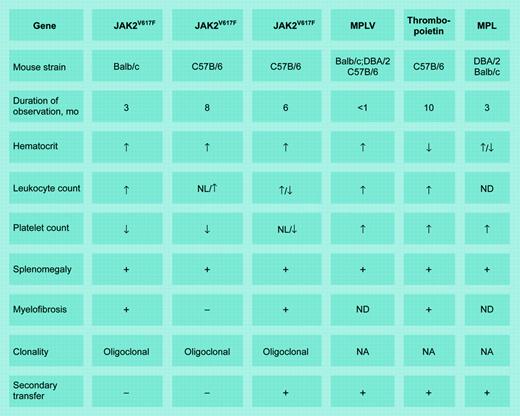
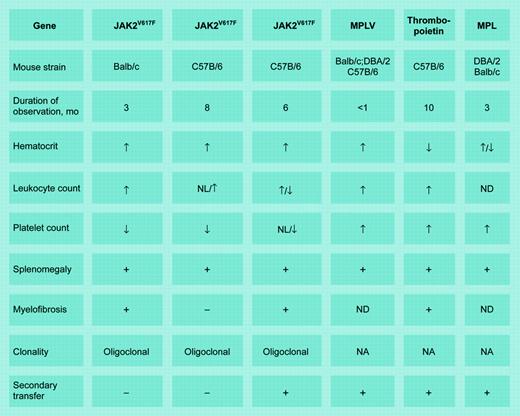
In this issue of Blood, Lacout and colleagues describe a murine polycythemia vera (PV) model created by adoptive transfer of C57B/6 marrow cells expressing JAK2V671F. The myeloproliferative disorder (MPD) phenotype varied according to the extent of JAK2V617F expression; high expression was associated with erythrocytosis, leukocytosis, splenomegaly, and myelofibrosis but not thrombocytosis, followed by anemia and thrombocytopenia. Secondary transfer recapitulated these results, except that transient thrombocytosis was observed with low JAK2V617F expression. By contrast, Wernig et al1 observed persistent erythrocytosis, leukocytosis, splenomegaly, and myelofibrosis in Balb/c mice but no myelofibrosis, inconsistent leukocytosis, and longer survival in C57B/6 mice. There was no thrombocytosis in either strain, defective megakaryocyte maturation was detected, and secondary transfer was unsuccessful.
How are we to reconcile these disparate results? Technically, different retroviral vectors were employed (MESV versus MSCV), there was mouse strain variability, the observation periods differed, and JAK2V617F expression efficiency in Wernig et al's animals is unknown.1 The association of low JAK2V617F expression with thrombocytosis fits an essential thrombocytosis (ET) clinical phenotype better than PV and might be explained by competition between wild-type and mutant JAK2. We also do not know which hematopoietic stem cells were transduced, but the different timing of retroviral infection after 5-FU exposure in these studies suggests that the stem cell populations differed. Oligoclonality also indicates target cell heterogeneity, whereas lack of secondary transfer suggests JAK2V617F expression in stem cells incapable of significant self-replication. The short observation periods also cannot be ignored, since primitive, self-replicating stem cells require time for expansion.2
Murine models of chronic MPDs. MPLV indicates myeloproliferative leukemia virus; MPL, the thrombopoietin receptor gene; NL, normal; ND, not done; and NA, not applicable. Illustration by Frank Forney.
Murine models of chronic MPDs. MPLV indicates myeloproliferative leukemia virus; MPL, the thrombopoietin receptor gene; NL, normal; ND, not done; and NA, not applicable. Illustration by Frank Forney.
And what are we to make of Lacout et al's claim to have replicated “spent phase” PV? “Spent phase” is an uncritical descriptor adopted over 50 years ago when alkylating agents and irradiation were therapeutic mainstays and primarily applies to that group of patients exposed to those stem cell poisons.3 It must also be remembered that mice are not small humans. Splenic hematopoiesis is a default process in mice where there exists stress-responsive erythroid progenitors not identified in humans.4 Importantly, simple cytokine overexpression can create a mouse model of idiopathic myelofibrosis (IMF; see table), as can overexpression of an activated tyrosine kinase. Thus, elevated blood counts, splenomegaly, and myelofibrosis lose their specificity in this milieu. Importantly, the assumption that JAK2V617F expression alone is sufficient to cause an MPD is questionable. The age-dependent increase in MPD incidence, its rarity in children, and the dissidence between JAK2V617F and clonality suggest that more than one mutation is required. Nevertheless, at a minimum, these animal models could prove useful for identifying JAK2V617F inhibitors.5
Lacout and colleagues and Wernig et al1 have shown JAK2V617F's value for creating murine MPD models but their work is only the end of the beginning with respect to solving the riddle of the MPDs. ▪