Abstract
Lymphoma cells are malignant cells of the T- or B-cell lineage that often express many surface markers inappropriately, yet are not recognized as abnormal by the immune system. We modeled this situation by inoculating ovalbumin-expressing E.G7-OVA lymphoma cells into mice that expressed ovalbumin as a self antigen in pancreatic islets, and investigated the efficacy of dendritic cell (DC) vaccination in these mice. Although vaccination with DC-expressing ovalbumin induced strong cytotoxic T-cell immunity, which led to clearance of E.G7-OVA lymphoma cells in naive C57BL/6 mice, DC vaccination was ineffective in mice expressing ovalbumin as a self antigen. Antigen modification to increase its processing via the endosomal processing pathway dramatically increased CD4 T-cell activation but paradoxically, impaired the protective effect of DC vaccination even in naive mice. Depletion of CD25+ T cells (regulatory T cells [Tregs]) prior to vaccination restored the efficacy of DC vaccination and allowed eradication of lymphoma also in mice expressing ovalbumin as a self antigen. We conclude that lymphoma cells may be eradicated using DC vaccination if activation of CD25+ Tregs is simultaneously inhibited, and that intentionally enhanced endosomal antigen processing in DC vaccines may shift the balance from CD4 T-cell help toward stimulation of Tregs.
Introduction
Immunotherapy has become clinically important in the treatment of malignant lymphomas, as exemplified by anti-CD20 antibody therapy in non-Hodgkin B-cell lymphomas.1 Yet, treatment with antibodies represents a form of passive immunization whose effect is always limited in its duration, and relapses after treatment are not uncommon. A cell-mediated antitumor immune response generated by active immunization could prevent such relapses, but this would require activation of a long-lasting and antigen-specific cytotoxic T-cell (CTL) immunity against a lymphoma antigen. Lymphoma cells often express differentiation markers inappropriately2,3 and similarly to all nucleated cells, present processed peptides on their plasma membrane. Thus, tumor-associated antigens (TAAs) are available for recognition by CTLs, but their activation against these antigens is difficult to achieve. This could be due not only to a degree of paralysis of the immune system induced by the disease or its treatment, but also to mechanisms of self-tolerance that inhibit effective activation.
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) of the immune system. They are able to induce strong T-cell responses, and their exploitation in vaccination against tumor cells is intensely studied.4,5 Activation of CTLs has been the main goal of antitumor immune therapies, but recently it has become obvious that for optimal long-lasting CTL responses,6-9 and in order for CD8 T cells to infiltrate tumors,10 CD4 T cells must also become activated. CD4 T cells can also promote antitumor immune protection independently or even in the absence of CD8 T cells.11-14 Therefore, antitumor vaccines capable of activating both CD8 and CD4 T cells are being developed.
DCs can be made to express TAAs in several different ways (reviewed in Schuler et al5 ), but selection of the method affects the pathway of antigen presentation. Antigens endocytosed from tumor lysates are efficiently presented only to CD4 T cells via MHC class II molecules, whereas intracellular antigens (encoded by viral vectors, cDNA plasmids, or mRNA) are presented preferentially to CD8 T cells via MHC class I molecules. However, antigens encoded by mRNA or plasmids can be genetically engineered to be efficiently processed and presented also by MHC class II molecules (reviewed in Bonehill et al15 ). TAAs have been directed to the MHC class II pathway by combining them with molecules participating in the antigen presentation such as Ii,16,17 MHC class II beta chain,18 or with signal peptides of molecules locating in endosomal or lysosomal compartments as DC-LAMP,19 LAMP-1,20 and LIMP-2.21 The ability of a DC vaccine to activate both CD4 and CD8 T cells is supposedly a desirable characteristic, but it raises the concern of whether CD25+CD4+ regulatory T cells (Tregs) also become activated.
In this study, we investigated the possibility of inducing active CTL immunity against lymphoma cells in mice by DC vaccination. Ex vivo–propagated DCs were activated and made to express ovalbumin (OVA) by nucleofection with OVA-encoding plasmid in vitro. To model the situation in which lymphoma cells express a self antigen, we used mice that express OVA as a model self antigen in their pancreatic islets and EG.7-OVA22 lymphoma cells.
Our results indicate that efficient eradication of lymphoma cells was not possible in mice expressing the TAAs as a self antigen, unless preexisting CD25+ T cells were first eliminated. Eradication of lymphoma cells was achieved without autoimmunity after depletion of CD25+ T cells, suggesting that this approach could be applied to enhance the effect of DC vaccination against lymphomas. Our results also suggest that intentionally enhanced endosomal antigen processing in DC vaccines may shift the balance from CD4 T-cell help toward stimulation of Tregs.
Materials and methods
Mice and cell lines
Wild-type C57BL/6 mice were used as tumor-cell and DC vaccine recipients, whereas Rag2–/– mice on the same background were used as bone marrow donors for DC propagation (both from The Jackson Laboratory, Bar Harbor, ME). OT-I and OT-II mice were used as donors of naive, OVA-reactive T cells. In certain experiments, transgenic RIP-OVAlo mice, expressing OVA as a self antigen in islet β-cells, were also used as tumor-cell recipients. All the transgenic lines23 were on C57BL/6 background and were provided by Dr L.C. Harrison (the Walter and Eliza Hall Institute [WEHI], Melbourne, Victoria, Australia). Mice were bred and maintained in the animal facilities of Turku University, and all animal experiments were approved by the local institutional ethics committee.
The murine H-2Kb lymphoma cell line EL-4 (TIB-39; ATCC, Manassas, VA) was used as the source of target cells in CTL assays, and its OVA-expressing subline (E.G7-OVA; ATCC) was used as a lymphoma model. The B16-F10-luc-G5 melanoma cells (Xenogen, Alameda, CA) expressing luciferase were used as a metastatic melanoma model.
Plasmid construction
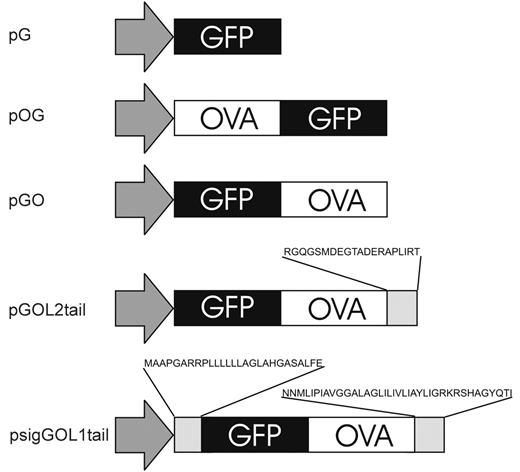
All plasmids were constructed using standard recombinant DNA techniques. The chicken ovalbumin gene was cloned from the purified genomic DNA of the E.G7-OVA cell line using polymerase chain reaction [PCR]. In order to monitor the OVA (antigen) levels in nucleofected cells, OVA was expressed as a fusion protein with enhanced green fluorescent protein (GFP). According to Wolkers et al24 there is a difference in the antigenicity of proteins connected either amino- or carboxyl-terminally to GFP. Therefore, plasmids encoding either GFP-OVA (pGO) or OVA-GFP (pOG) fusion proteins were constructed using pEGFP-C3 or pEGFP-N1 expression vectors (Clontech, Palo Alto, CA), respectively (Figure 1).
The above-mentioned fusion proteins are likely processed in the cytosol and thus, mainly presented by MHC class I molecules. To enhance OVA presentation by MHC class II molecules, we modified the fusion protein using LIMP-221 and LAMP-120 proteins in order to target OVA to the endosomal compartment. In pGOL2tail plasmid, the last 20 amino acids of LIMP-2 protein, comprising an anchor to lysosome membrane, were joined to GFP-OVA fusion protein using PCR. In psigGOL1tail plasmid, both the amino- and carboxyl-terminal signal sequences of LAMP-1 protein were cloned from cDNA prepared from mature DCs using PCR and joined to the GO fragment in pIRES2EGFP plasmid (Clontech) to produce the psigGOL1tail plasmid (Figure 1).
Preparation of DCs and their transfection with plasmids encoding modified antigen
DCs were propagated from bone marrow (BM) cells according to a previously described method25 with slight modifications.26 After 6 days in culture, BM-derived DCs were activated using 10 μg/mL anti-CD40 Ab (clone HM40-3; Pharmingen, San Diego, CA). After overnight incubation, CD11c+ cells were purified using anti–CD11c-MACS beads and MACS-LS columns (Miltenyi Biotech, Bergisch Gladbach, Germany), counted and then used for nucleofection.
Plasmids used for protein expression in DCs. Plasmids were constructed using standard recombinant DNA techniques, and all constructs were sequenced for potential errors. Plasmid pG encodes GFP alone. Plasmids pOG and pGO encode for OVA-GFP and GFP-OVA fusion proteins, respectively. To obtain more efficient CD4 T-cell activation, plasmids pGOL2tail and psigGOL1tail were done: pGOL2 has a carboxyl-terminal sequence of LIMP-2 protein attached to GFP-OVA fusion, whereas psigGOL1tail has both amino- and carboxyl-terminal signal sequences of LAMP-1 protein connected to GFP-OVA fusion.
Plasmids used for protein expression in DCs. Plasmids were constructed using standard recombinant DNA techniques, and all constructs were sequenced for potential errors. Plasmid pG encodes GFP alone. Plasmids pOG and pGO encode for OVA-GFP and GFP-OVA fusion proteins, respectively. To obtain more efficient CD4 T-cell activation, plasmids pGOL2tail and psigGOL1tail were done: pGOL2 has a carboxyl-terminal sequence of LIMP-2 protein attached to GFP-OVA fusion, whereas psigGOL1tail has both amino- and carboxyl-terminal signal sequences of LAMP-1 protein connected to GFP-OVA fusion.
The Amaxa nucleofection protocol for BM DCs was optimized using the Test Nucleofection Solution 5034 (Amaxa, Koeln, Germany) with different programs and amounts of pEGFP-C3 plasmid. After the nucleofection, the cells were cultured overnight in complete medium (RPMI 1640 supplemented with 10% FCS, 20 mm l-glutamine, 5 × 10–5 M 2-mercaptoethanol, penicillin/streptomycin) in the presence of 20 μg/mL recombinant murine granulocyte/macrophage colony-stimulating factor (GM-CSF; Pharmingen) in 5 mL polypropylene tubes (Becton Dickinson, San Jose, CA) (1 × 106 cells/mL per tube). The next day, nucleofection efficiency and viability were analyzed with FACScalibur (Becton Dickinson) and Win-MDI 2.8 software (http://facs.scripps.edu/software.html) using the GFP fluorescence and 7-aminoactinomycin-D (7-AAD; Sigma, St Louis, MO), respectively. The best results were acquired using the T-24 program and 5 μg of plasmid per 2 to 5 million BM DCs, which were used in all subsequent experiments.
The phenotype of the successfully nucleofected DCs was analyzed by staining the cells for flow cytometry using phycoerythin (PE)–conjugated anti-CD11c, anti-CD86, and anti-CD40 (Pharmingen) and biotinylated anti-IAb with streptavidin-PE (Pharmingen), respectively. Control stainings were performed using anti–rat IgG2a (Pharmingen) and biotinylated Hermes-1 (anti–human CD44). IL-12p70 and TNF-α production was measured by DuoSet enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN).
T-cell stimulatory potential of DC expressing modified antigen
To measure the T-cell activation capacity of DCs nucleofected with different antigen-encoding plasmids, naive OT-I or OT-II T cells were cocultured with increasing numbers of nucleofected DCs. Naive OVA-specific CD4 and CD8 T cells were purified from the spleens and lymph nodes (LNs) of OT-II and OT-I mice, respectively, using standard techniques. For the thymidine incorporation and interferon-γ (IFN-γ) production assays, the viable DCs (rested overnight after the nucleofection) and OT cells (0.2 × 106) were plated at the DC/T-cell ratios of 1:200, 1:80, and 1:40 in round-bottom 96-well plates. At day 3, 3H-thymidine (1 μCi [0.037 MBq] per well) was added for the last 6 hours of the culture. Cells were harvested using a semiautomated plate harvester (Tomtech MACH III; Fisher Scientific, Hampton, NH). At days 3 and 6, supernatants were collected for measurement of IFN-γ levels using specific ELISAs (antibodies from Pharmingen).
In some experiments, OT cell proliferation was measured by carboxy-fluorescein diacetate succinimidyl ester (CFSE) dilution assay. After 3 or 6 days of coculture with DCs (at 1:100 ratio), the cells were collected and CFSE dilution was measured using flow cytometry. In other experiments, mice were vaccinated with injections of 1 × 106 nucleofected DCs subcutaneously in the base of the tail and were then given intravenous injections of 4 × 106 CFSE-labeled OT-I or OT-II T cells. Five days later, the mice were killed and CFSE dilution was analyzed by flow cytometry.
CTL priming was measured 7 days after DC vaccination of naive C57Bl/6 mice using a standard in vitro restimulation and a 4-hour 51Cr release assay. Induction of OVA-specific CD8 T cells was measured by tetramer labeling (Class I iTAg MHC tetramer; Beckman Coulter, Fullerton, CA) of lymphocytes prepared from the spleen and peripheral LNs, simultaneously labeled with fluorescein isotiocyanate (FITC)–conjugated anti-CD8 antibody (Pharmingen). The percentage of tetramer-positive CD8 T cells was analyzed using flow cytometry.
Tumor inoculation and DC vaccination
EG.7-OVA lymphoma cells (10 × 106) were injected subcutaneously in the belly area of 6- to 8-week-old recipient mice. DC vaccination (1 × 106 nucleofected DCs) was given subcutaneously above the tail at indicated time points. In some experiments, the CD4+ or CD25+ cells were depleted before DC vaccination using either anti-CD4 (clone GK1.5) or anti-CD25 (clone PC61) antibodies. Purified antibodies (100 μg/injection) were given intraperitoneally on 3 consecutive days starting 7 days before DC vaccination. Depletion efficiency was verified by blood sampling and flow cytometry. In untreated recipients, lymphoma cells formed a solid, subcutaneous tumor around the place of lymphoma cell inoculation. The tumor became visible in 3 to 7 days and within 2 weeks grew to the limit of sacrificing the recipients. The tumor mass was excised and weighed after killing the recipients.
To test for unspecific effects of DC vaccination–induced CD4+CD25+ Tregs on tumor immunity in general, a melanoma model was used as a control tumor. In these experiments, 0.4 × 106 B16-F10-luc-G5 melanoma cells were injected subcutaneously in the left ear of mice and luciferase bioluminescense was measured at days 6, 10, and 13. Mice were immunized against the melanoma by subcutaneous injection of B16-F10-luc-G5 cell lysate (1 × 106 cells/mouse) in complete Freund adjuvant (CFA) 7 days before the melanoma cell inoculation and simultaneous DC vaccination with DCs nucleofected with pGO or psigGOL1tail. For bioluminescense measurements, mice were anesthetized with 2.5% isoflurane (Becton Dickinson), and 150 mg/kg D-luciferin (Synchem, Kassel, Germany) was injected intraperitoneally in mice 10 minutes before imaging. Both black and white image and photon counts were acquired using IVIS Imaging System 50 Series (Xenogen).
Detection, purification, and functional analysis of regulatory T cells
Regulatory T cells were detected using FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 together with the intracellular Foxp3 staining kit (eBioscience, San Diego, CA) according to the manufacturer's instructions. For functional analyses of regulatory T cells, CD4+CD25+ T cells were purified using magnetic separation, according to the manufacturer's protocol (Miltenyi Biotech), from mice vaccinated 6 days earlier with DCs nucleofected with pGO or psigGOL1tail plasmids. Purified CD4+CD25+ T cells were tested for their ability to suppress OVA- or PPD-specific T-cell proliferation in vitro. Briefly, splenocytes from OVA- or PPD-immunized mice were restimulated with antigen (2 mg/mL OVA, grade V, Sigma; or 10 μg/mL PPD, Statens Serum Institute, Copenhagen, Denmark) in the presence of a 1:1 ratio of CD4+CD25+ T cells pooled from 3 vaccinated mice. T-cell proliferation was measured using 3H-thymidine incorporation assay (see “T-cell stimulatory potenital of DC expressing modified antigen”).
Results
DCs expressing plasmid-encoded antigen have a highly activated phenotype
For the induction of a productive immune response, APCs must have a mature (activated) phenotype. Immature APCs are thought to induce anergy or even tolerance toward antigens they present. Therefore, in vaccines against tumors the DCs must both express a tumor-associated antigen and have a highly activated phenotype. In this study, we sought a nonviral transfection method for expression of desired proteins in activated DCs and their presentation to CD4 and CD8 T cells.
The Amaxa nucleofection protocol was optimized using a plasmid encoding GFP (Figure 1, “pG”) as a reporter for transfection efficiency. From the viable cells, almost 80% were positively nucleofected; that is, they appeared green fluorescent when visualized using flow cytometry (Figure 2A). The positively nucleofected DCs (GFP+ DCs) were highly positive for the CD11c surface marker and for the activation markers B7.2 (CD86), MHC class II, and CD40 (Figure 2B). They produced both IL-12p70 and TNF-α after activation (data not shown). Thus, the optimized nucleofection protocol results in a population where successfully nucleofected cells have a highly activated phenotype.
Nucleofected DCs stimulate naive T-cell responses in vitro and in vivo
As the optimized nucleofection protocol resulted in a DC population with a highly activated phenotype, we proceeded to analyze their capability of activating naive CD4 and CD8 T cells. In these experiments we used the transgenic OT-I and OT-II cells, which carry MHC class I– or II–restricted T-cell receptors specific for OVA, respectively.
Nucleofected DCs expressing the GFP-OVA fusion protein were much more green fluorescent than cells expressing the OVA-GFP construct (Figure 2C), suggesting that the amount of OVA antigen is also greater in these cells. Still, these DCs were equally efficient in inducing OT-I cell proliferation (Figure 3A) and IFN-γ production (Figure 3B). Neither of these antigen-constructs was able to induce strong OT-II cell activation (Figure 3D) when measured by thymidine incorporation (Figure 3D) or IFN-γ production (Figure 3E). Due to higher GFP level achieved, the pGO plasmid was chosen for subsequent experiments, in which the antigen-construct was further modified to induce CD4 T-cell activation also.
DCs nucleofected with OVA-encoding plasmids have a highly activated phenotype. At 20 hours after the nucleofection, DCs were collected and analyzed for viability, green fluorescence, and activation markers. (A) Viable (7-AAD–) DCs nucleofected with GFP-encoding plasmid or without any plasmid were analyzed for green fluorescence. (B) The surface expression levels of CD11c, B7.2 (CD86), MHC class II, and CD40 (gray open histograms) were analyzed from viable cells. Appropriate staining controls are shown as black histograms. (C) DCs nucleofected with pGO become more brightly green fluorescent than DCs expressing pOG.
DCs nucleofected with OVA-encoding plasmids have a highly activated phenotype. At 20 hours after the nucleofection, DCs were collected and analyzed for viability, green fluorescence, and activation markers. (A) Viable (7-AAD–) DCs nucleofected with GFP-encoding plasmid or without any plasmid were analyzed for green fluorescence. (B) The surface expression levels of CD11c, B7.2 (CD86), MHC class II, and CD40 (gray open histograms) were analyzed from viable cells. Appropriate staining controls are shown as black histograms. (C) DCs nucleofected with pGO become more brightly green fluorescent than DCs expressing pOG.
DCs nucleofected with OVA-encoding plasmids activate antigen-specific T cells. At 20 hours after the nucleofection, DCs were collected and used to activate either OT-I (CD8) or OT-II (CD4) T cells. OT-I (A-C) and OT-II (D-F) T-cell activation was measured using thymidine incorporation (panels A and D) or IFN-γ production (panels B and E), respectively. n.d. indicates not detectable. Number of DCs used was 1000, 2500, or 5000, from top to bottom in each panel. (C) In vitro proliferation of CFSE-labeled OT-I T cells was analyzed at day 6. DCs (5000) were nucleofected with pGO (black histogram, top panel) or with psigGOL1tail (black histogram, bottom panel) or with pG (gray open histogram, both panels). (F) In vivo proliferation of CFSE-labeled OT-II T cells at day 6 stimulated by 5000 DCs nucleofected with psigGOL1tail (black histogram) or with pG (gray open histogram) was also analyzed using CFSE dilution assay at day 6. (G) In vivo proliferation of adoptively transferred CFSE-labeled OT-I (top panels) and OT-II (bottom panels) cells 5 days after vaccination with 1 × 106 DCs nucleofected with pGO (left panels) or psigGOL1tail (right panels). In panels A, B, D, and E, results are the mean ± standard error of mean of 3 individual experiments.
DCs nucleofected with OVA-encoding plasmids activate antigen-specific T cells. At 20 hours after the nucleofection, DCs were collected and used to activate either OT-I (CD8) or OT-II (CD4) T cells. OT-I (A-C) and OT-II (D-F) T-cell activation was measured using thymidine incorporation (panels A and D) or IFN-γ production (panels B and E), respectively. n.d. indicates not detectable. Number of DCs used was 1000, 2500, or 5000, from top to bottom in each panel. (C) In vitro proliferation of CFSE-labeled OT-I T cells was analyzed at day 6. DCs (5000) were nucleofected with pGO (black histogram, top panel) or with psigGOL1tail (black histogram, bottom panel) or with pG (gray open histogram, both panels). (F) In vivo proliferation of CFSE-labeled OT-II T cells at day 6 stimulated by 5000 DCs nucleofected with psigGOL1tail (black histogram) or with pG (gray open histogram) was also analyzed using CFSE dilution assay at day 6. (G) In vivo proliferation of adoptively transferred CFSE-labeled OT-I (top panels) and OT-II (bottom panels) cells 5 days after vaccination with 1 × 106 DCs nucleofected with pGO (left panels) or psigGOL1tail (right panels). In panels A, B, D, and E, results are the mean ± standard error of mean of 3 individual experiments.
DCs expressing a GFP-OVA fusion protein modified to include the tail of LIMP-2 or the signal peptide and tail of LAMP-1 (GOL2tail or sigGOL1tail) and thus targeted into endosomal compartments were both able to induce CD4 (OT-II) T-cell activation. DCs expressing sigGOL1tail induced the strongest activation, whereas GOL2tail induced only a slightly stronger response than the unmodified GO (Figure 3D-E). Since it was our objective to drive activation of both CD4 and CD8 T cells, we tested the sigGOL1tail construct's ability to activate OT-I cells also. DCs expressing sigGOL1tail were indeed able to induce strong OT-I cell proliferation (Figure 3A), and production of IFN-γ, although IFN-γ production was slightly weaker compared with DCs expressing GFP-OVA or OVA-GFP constructs (Figure 3B). To test the ability of these DCs to drive T-cell proliferation into multiple cell divisions, we used the CFSE-dilution assay. Unmodified GO and sigGOL1tail were equal in their ability to drive OT-I cell proliferation (Figure 3C), whereas proliferation of OT-II cells was detectable only if DCs were expressing the sigGOL1tail fusion protein (Figure 3F). Thus, the amino- and carboxyl-terminal signal sequences of the LAMP-1 protein efficiently directed the GFP-OVA to the endosomal antigen processing pathway. Altogether, the nucleofected DCs were capable of inducing strong CD4 and CD8 T-cell responses in vitro depending on the plasmid construct used. We then analyzed T-cell activation by nucleofected DCs in vivo. First, we followed the activation of adoptively transferred CFSE-labeled OT-I and OT-II cells and then measured the induction of OVA-specific immune response of host T cells in C57BL/6 mice. Five days after the DC vaccination and T-cell transfer, the CFSE dilution was analyzed from cells prepared from the inguinal LN draining the DC injection site. DCs nucleofected with pGO induced strong OT-I cell proliferation but OT-II cells did not show detectable proliferation (Figure 3G, left panels). However, DCs nucleofected with psigGOL1tail induced both OT-II and OT-I cell proliferation (Figure 3G, right panels).
We next measured whether DC vaccination could induce expansion of endogenous OVA-reactive CD8 T cells in C57BL/6 mice. The recipient mice were vaccinated subcutaneously using DCs nucleofected with pG or pGO plasmids and 5 days after vaccination, reactivity of CD8 T cells in the spleen with the OVA-tetramer was measured. Spleens from mice vaccinated with DCs encoding the GFP-OVA fusion protein contained up to 4.7% OVA-specific CD8 T cells, compared with none in mice vaccinated with DCs encoding for GFP alone (Figure 4A). Induction of OVA-specific CTL responses was also measured 7 days after vaccination. DCs expressing the GFP-OVA induced CTL in recipient mice, whereas DCs expressing GFP alone did not (Figure 4B). Thus, our results indicate that nucleofected DCs can be used as a vaccine to induce strong antigen-specific immunity in naive wild-type mice.
Nucleofected DCs induce strong antigen-specific immune responses in vivo. (A) Percentage of OVA-reactive T cells was analyzed from naive mice and from mice vaccinated with DCs nucleofeted with pG or pGO. OVA-tetramer-positive CD8 T cells can be found in the spleens of C57BL/6 mice 5 days after the vaccination, only if the DCs used encoded OVA. (B) Spleen cells also exhibit OVA-specific CTL activity when OVA-encoding DCs were used in the vaccination. Results from 3 independent mice per group are shown.
Nucleofected DCs induce strong antigen-specific immune responses in vivo. (A) Percentage of OVA-reactive T cells was analyzed from naive mice and from mice vaccinated with DCs nucleofeted with pG or pGO. OVA-tetramer-positive CD8 T cells can be found in the spleens of C57BL/6 mice 5 days after the vaccination, only if the DCs used encoded OVA. (B) Spleen cells also exhibit OVA-specific CTL activity when OVA-encoding DCs were used in the vaccination. Results from 3 independent mice per group are shown.
DC vaccination at the time of tumor cell inoculation prevents establishment of solid tumors and is independent of CD4 T cells
The induction of immune responses against tumor cell epitopes is one of the most promising applications of DC vaccinations. In this study, we evaluated the requirements of protective immune responses induced by DC vaccination against a growing tumor. When EG.7-OVA cells (mouse thymoma cell line expressing OVA as a model antigen) are given subcutaneously to recipient mice, a single tumor develops around the site of inoculation within 2 weeks. If the tumor cells and control DC vaccine (pG) were given at the same time, many mice developed large tumors, whereas mice vaccinated with OVA-expressing DCs (pGO) developed significantly smaller tumors or none at all (Figure 5A). This primary response against tumor cells was independent of CD4 T-cell help, as mice depleted of CD4 T cells prior to DC vaccination were equally protected against the tumor cells (Figure 5A). These results show that the primary response against tumor cells is CD8 T-cell mediated (antigen-dependent) but independent of CD4 T-cell help.
DC vaccination induces long-term CTL memory that requires CD4 T cells
The dependence of memory CD8 T-cell response on CD4 T-cell help was analyzed by vaccinating mice 3 weeks before the tumor challenge. Although DCs nucleofected with pGO did not induce detectable CD4 T-cell proliferation either in vivo or in vitro, mice vaccinated with DCs nucleofected with the unmodified antigen plasmid (pGO) showed efficient CD8 T-cell responses and also were able to eradicate tumor cells 3 weeks after vaccination (Figure 5B). This memory CD8 T-cell response against tumor cells was significantly impaired when mice were depleted of CD4 T cells before vaccination (Figure 5B). These results show that if the priming takes place in the absence of CD4 T-cell help, protective memory response is abrogated.
Enhanced antigen presentation via MHC class II molecules in DCs induces CD25+ Tregs, which reduce protection against tumor cells
We next studied the antitumor immune response when DCs were nucleofected using psigGOL1tail. This plasmid induced significant CD4 and CD8 T-cell activation both in vitro and in vivo (Figure 4), and was therefore expected to activate the strongest primary (involving both CD4 and CD8 effector mechanisms) and secondary (CD4 help for CTL memory) antitumor responses. Surprisingly, C57BL/6 mice vaccinated with DCs nucleofected with psigGOL1tail and inoculated with tumor cells at the same time developed large tumors. This failure to protect was amended by depletion of CD25+ cells prior to vaccination and tumor inoculation (Figure 6A). We then tested whether DCs nucleofected with psigGOL1tail in fact induced CD25+ Treg cells by measuring numbers of Foxp3-expressing T cells (Figure 6B). The numbers of Foxp3+CD25+ cells were equal in mice immunized with DCs expressing either plasmid. To test for the possible induction of antigen-specific regulatory activity, purified CD4+CD25+ T cells from mice vaccinated with pGO or psigGOL1tail-nucleofected DCs were coincubated with OVA- and PPD-specific effector T cells in an in vitro restimulation assay (Figure 6C). CD4+CD25+ T cells from mice vaccinated with DCs nucleofected with either construct allowed comparable proliferation of PPD-specific effector T cells. In contrast, OVA-specific proliferation of effector T cells was strongly suppressed by Treg cells from mice vaccinated with DCs nucleofected with psigGOL1tail only. Vaccination with psigGOL1tail-nucleofected DCs did not impair tumor immunity against other tumor antigens, because mice immunized with a lysate of melanoma cells were equally well protected from melanoma challenge, when vaccinated with DCs nucleofected with psigGOL1tail or pGO, as they were when left without vaccination with these DCs (Figure 6D).
Induction of tumor immunity against a self antigen requires depletion of CD25+ T cells
Tumor antigens are often cryptic self antigens that become expressed and visible to the immune system after malignant transformation of the cell. To model this situation, we used transgenic RIP-OVAlo mice, which express OVA in islet-β cells but remain ignorant of islet OVA despite having OVA-reactive CTL precursors.27 Unexpectedly, these mice appeared also to have an active tolerance mechanism toward OVA since all of the mice developed large tumors after tumor cell inoculation, and were completely resistant to the protective effect of DC vaccination (pGO plasmid). However, depletion of CD25+ cells prior to DC vaccination rendered these mice significantly less tolerant against OVA and in this case, the mice were well protected against the tumor cells (Figure 7A). We analyzed whether depletion of CD25+ cells would break tolerance toward pancreatic β-cells when RIP-OVAlo mice are immunized against OVA. Mice depleted of CD25+ cells did not show more lymphocyte infiltration in pancreatic islets after immunization when compared with similarly immunized controls (Figure 7B). Thus, DC vaccination against endogenously expressed tumor antigens can be enhanced by depletion of CD25+ regulatory cell population without induction of autoimmune response toward the self antigen.
Nucleofected DCs induce strong primary and memory antitumor immunity. (A) When tumor cells and DC vaccination are given at the same time, mice vaccinated with DCs encoding GFP-OVA fusion protein (pGO) are protected against tumor cells, whereas mice vaccinated with DCs encoding for GFP alone (pG) are not. The protective primary response was not affected even if the CD4 T cells were depleted before DC vaccination (pGO CD4 dep). (B) When memory response against tumor cells was measured by vaccinating the mice 3 weeks before tumor challenge, mice vaccinated using DCs nucleofected with pGO were almost completely protected against the tumor cells, whereas CD4 T-cell depletion abrogated the protection. P values were calculated using the Student t test.
Nucleofected DCs induce strong primary and memory antitumor immunity. (A) When tumor cells and DC vaccination are given at the same time, mice vaccinated with DCs encoding GFP-OVA fusion protein (pGO) are protected against tumor cells, whereas mice vaccinated with DCs encoding for GFP alone (pG) are not. The protective primary response was not affected even if the CD4 T cells were depleted before DC vaccination (pGO CD4 dep). (B) When memory response against tumor cells was measured by vaccinating the mice 3 weeks before tumor challenge, mice vaccinated using DCs nucleofected with pGO were almost completely protected against the tumor cells, whereas CD4 T-cell depletion abrogated the protection. P values were calculated using the Student t test.
CD4 T-cell activation by DC vaccination induces CD25+ cells capable of suppressing OVA-specific responses. (A) C57Bl/6 mice vaccinated with DCs nucleofected with psigGOL1tail developed large tumors within 2 weeks. If mice were depleted of CD25+ cells before vaccination (psigGOL1tail CD25dep), protection was significantly more efficient. P values were calculated using the Student t test. (B) Numbers of CD25+Foxp3+ cells were equal in mice vaccinated with DCs nucleofected with either construct. The percentages shown are the average of 6 mice per group. (C) OVA-specific suppression of T-cell proliferation by CD4+CD25+ T cells isolated from mice vaccinated with psigGOL1tail-, but not pGO-nucleofected DCs. CD4+CD25+ T cells were pooled from 3 mice per group and coincubated with splenocytes from PPD- or OVA-primed mice. PPD- or OVA-induced proliferation is shown as white or black bars, respectively, plus or minus the standard error of mean. (D) DCs nucleofected with either construct had no effect on the protective response against luciferase-expressing B16-F10-luc-G5 melanoma cells in vivo. White bars show the bioluminescence from unimmunized control mice (n = 2) plus or minus the standard error of mean, light gray bars show bioluminescence from mice immunized with tumor-cell lysate (n = 4), dark gray bars show bioluminescence from mice similarly immunized and then vaccinated with DCs nucleofected with pGO (n = 6), and black bars show bioluminescence from mice vaccinated with DCs nucleofected with psigGOL1tail (n = 6).
CD4 T-cell activation by DC vaccination induces CD25+ cells capable of suppressing OVA-specific responses. (A) C57Bl/6 mice vaccinated with DCs nucleofected with psigGOL1tail developed large tumors within 2 weeks. If mice were depleted of CD25+ cells before vaccination (psigGOL1tail CD25dep), protection was significantly more efficient. P values were calculated using the Student t test. (B) Numbers of CD25+Foxp3+ cells were equal in mice vaccinated with DCs nucleofected with either construct. The percentages shown are the average of 6 mice per group. (C) OVA-specific suppression of T-cell proliferation by CD4+CD25+ T cells isolated from mice vaccinated with psigGOL1tail-, but not pGO-nucleofected DCs. CD4+CD25+ T cells were pooled from 3 mice per group and coincubated with splenocytes from PPD- or OVA-primed mice. PPD- or OVA-induced proliferation is shown as white or black bars, respectively, plus or minus the standard error of mean. (D) DCs nucleofected with either construct had no effect on the protective response against luciferase-expressing B16-F10-luc-G5 melanoma cells in vivo. White bars show the bioluminescence from unimmunized control mice (n = 2) plus or minus the standard error of mean, light gray bars show bioluminescence from mice immunized with tumor-cell lysate (n = 4), dark gray bars show bioluminescence from mice similarly immunized and then vaccinated with DCs nucleofected with pGO (n = 6), and black bars show bioluminescence from mice vaccinated with DCs nucleofected with psigGOL1tail (n = 6).
Discussion
We studied the efficacy of DC vaccination against lymphoma in a mouse model where the antigen used for immunization was also expressed in pancreatic islets and the animals' immune system was therefore shaped to recognize this antigen as a part of immunologic self. We investigated not only the consequences of using a self antigen in the DC vaccine but also the consequences of enhanced antigen presentation via MHC class II pathway to CTL induction, long-term CTL memory, and antitumor immunity.
DC vaccination against endogenously expressed tumor antigen is enhanced by depletion of CD25+ cells before vaccination. RIP-OVAlo mice on C57Bl/6 background expressing OVA under the control of rat insulin promoter were vaccinated using DCs nucleofected with pGO plasmid (pGO) (A). A group of mice were depleted of CD25+ cells 5 days before vaccination (pGO CD25dep). (B) Depletion of CD25+ cells 5 days before immunization with subcutaneous OVA in IFA does not increase lymphocyte infiltration into pancreatic islets within 2 weeks. Pancreases from 4 RIP-OVAlo mice in each group were analyzed in a blinded fashion for the level of lymphocytic infiltration (insulitis) in individual islets (each graded from 0 to 3 to count a score for each pancreas) using hematoxylin and eosin staining and light microscopy. At least 50 islets per pancreas were analyzed. P values were calculated using the Student t test. Black bars show insulitis score in untreated mice plus or minus the standard error of mean, white bars indicate OVA immunized mice, and gray bars indicate OVA-immunized and CD25-depleted mice.
DC vaccination against endogenously expressed tumor antigen is enhanced by depletion of CD25+ cells before vaccination. RIP-OVAlo mice on C57Bl/6 background expressing OVA under the control of rat insulin promoter were vaccinated using DCs nucleofected with pGO plasmid (pGO) (A). A group of mice were depleted of CD25+ cells 5 days before vaccination (pGO CD25dep). (B) Depletion of CD25+ cells 5 days before immunization with subcutaneous OVA in IFA does not increase lymphocyte infiltration into pancreatic islets within 2 weeks. Pancreases from 4 RIP-OVAlo mice in each group were analyzed in a blinded fashion for the level of lymphocytic infiltration (insulitis) in individual islets (each graded from 0 to 3 to count a score for each pancreas) using hematoxylin and eosin staining and light microscopy. At least 50 islets per pancreas were analyzed. P values were calculated using the Student t test. Black bars show insulitis score in untreated mice plus or minus the standard error of mean, white bars indicate OVA immunized mice, and gray bars indicate OVA-immunized and CD25-depleted mice.
Recent work in models of viral8 and bacterial9 infections as well as in a model using peptide immunization7 indicates that a primary CTL response does not, in fact, require CD4 T-cell help, but that CD4 T-cell help is indispensable for the generation of memory CD8 T cells. Because some controversy exists in whether this can be generalized to all CTL responses,28,29 it was important to test whether the absence of CD4 T cells at the time of DC vaccination impaired protection from subsequent injection of lymphoma cells. We found that if the vaccination was given 3 weeks before lymphoma cells, prior depletion of CD4 T cells abolished the protective effect of DC vaccination. This corroborated studies showing that CD4 T-cell help is required for CTL memory but importantly, also showed that CD4 help is needed for CTL memory against TAAs induced by activated DCs.
As CD4 T-cell help was clearly needed for memory CD8 T-cell–mediated eradication of lymphoma cells, we investigated whether this could further be enhanced by vaccinating mice using DCs that were made to present antigen efficiently also via MHC class II pathway. However, this was not the case and paradoxically, all mice developed large tumors. Although the traditional view holds that activated, mature DCs only induce effector CD4 T cells, recent findings have shown that activated DCs can also expand CD25+CD4+ Tregs.30,31 Therefore, we investigated whether the paradoxical loss of protection after targeting antigen to MHC II presentation in DCs (MHC-II DCs) would be due to induction of CD25+ Tregs. We found that depletion of CD25+ T cells prior to vaccination restored the ability of MHC-II DCs to induce protective immunity. Our further in vitro and in vivo experiments revealed that MHC-II DCs induced CD25+ Treg activity that was antigen-specific, as such Tregs suppressed OVA-specific T-cell proliferation in vitro and did not impair protection against melanoma provided by immunization with melanoma cell lysate. This indicated that rather than improving CD4 T-cell help for CTLs, deliberate antigen targeting to MHC II processing pathway, in fact, activates CD25+ Tregs. Thus, our results suggest that there is an optimal level of CD4 T-cell activation provided by the DC vaccine that must not exceed a critical threshold. This is an important caveat and suggests that artificial antigen targeting may in some instances dampen the antitumor response.
When TAAs belonging to immunologic self are used for vaccination, 2 important questions must always be addressed. First, is it possible to induce protective CTL immunity at all, and will this inevitably lead to destruction of healthy cells expressing the same antigen? We were able to model this situation using the RIP-OVAlo mice, which express OVA constitutively in islet β-cells. Although RIP-OVAlo mice retain OVA-reactive CTL precursors in their T-cell repertoire, they remain healthy under normal situations, because they are ignorant to OVA-expressing islet β-cells.27 We tested whether vaccination with activated DCs presenting OVA would break this ignorance and lead to autoimmunity. We found, however, that it was impossible to induce tumor protection using DC vaccination. This suggested that in addition to ignorance, these mice have a component of active regulation that controls CTL induction. It has been previously shown that depletion of CD25+ cells can break immunologic unresponsiveness to autologous tumors in vivo and lead to development of tumor-specific and nonspecific effector cells.32,33 Our depletion experiments proved this to be true in our model, as protection against lymphoma was restored when the mice were depleted of CD25+ T cells. However, although CD25+ Tregs maintain immunologic tolerance toward self antigens,34,35 autoimmunity against islet β cells did not develop in our mice. Technically, this could be due to a lower level of antigen expression in islet β cells, rendering these cells insensitive to immune attack compared with tumor cells. Alternatively, distinction between the tumor cells and islet β cells was maintained due to compartmentalization of the immune response to subcutaneous regions,36 or due to some other factors related to the DCs. However, the presumed lower level of antigen expression on islet β cells also models a situation where there is a quantitative difference in T-cell avidity to tumor cells and healthy cells, either due to the number of TCR ligands per cell (as in our model situation) or due to slight differences in the antigenic epitope (as between self- and neoantigens). Such differences may exist when using tumor neoantigens that are slightly modified self antigens.
While corroborating earlier studies showing the efficacy of DC vaccination in achieving tumor immunity37-40 and the requirement of CD4 T cells for long-term CTL memory,7-9 our results suggest that intentionally enhanced endosomal antigen processing may shift the balance from beneficial CD4 T-cell help toward stimulation of immunosuppressive Tregs. Our findings also demonstrate that lymphoma cells may be eradicated using DC vaccination but this requires simultaneous elimination of CD25+ Tregs when the lymphoma antigen is endogenously expressed. These findings indicate that CD4+CD25+ Tregs are important in the control of not only natural but also vaccination-induced immune responses, and warrants their monitoring in the further development of DC vaccination for the treatment of cancer.
Prepublished online as Blood First Edition Paper, April 18, 2006; DOI 10.1182/blood-2005-11-008615.
Supported by the Finnish Academy, Sigrid Juselius Foundation (Finland), Juvenile Diabetes Foundation International, Finnish Diabetes Research Foundation and Turku Graduate School of Biomedical Sciences.
M. Maksimow designed research, performed research, collected data, analyzed data, and wrote the paper; M. Miiluniemi performed research and collected data; F.M.-I. performed research, collected data, and analyzed data; S.J. designed research; and A.H. designed research, analyzed data, and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mrs Anne Sovikoski-Georgieva for excellent secretarial assistance, Mrs Anitta Niittymäki for taking care of the animals, and Ms Minna Santanen for excellent cell culture work.