Abstract
Severe combined immunodeficiency (SCID) is characterized by a complete block in T-lymphocyte differentiation. Most SCID also affects B-cell development or function, although a normal pool of pro–B cells is detectable. Treatment of SCID consists of allogeneic hematopoietic stem cell transplantation (HSCT), but in the absence of a myeloablative conditioning regimen, only T cells, and in some cases, natural killer (NK) cells, are of donor origin, while all other leukocytes subsets are of host origin. We hypothesized that donor B-cell development success could be conditioned by the competitive ability of recipient B-cell precursors in the bone marrow. We therefore compared the outcome of unconditioned HSCT in mice that differed with respect to their pro–B-cell compartments. B-cell reconstitution was limited in recipient mice containing a normal pro–B-cell pool, whereas immature and mature B-cell numbers reached wild-type levels in mice with compromised early B-cell precursors. Interestingly, host NK cells did not modify the outcome of unconditioned HSCT as long as the early B-cell compartment was compromised. These observations suggest that recipient B-cell precursors condition the reconstitution of the donor B-cell pool and, if extrapolative to humans, suggest that conditioning regimens targeting host pro–B cells may help improve B-cell reconstitution after allogeneic HSCT.
Introduction
Human severe combined immunodeficiency (SCID) is a heterogeneous group of inherited disorders impairing both cellular and humoral immunity due to the absence of T cells. Twelve different SCID phenotypes involving mutations in 11 genes have been identified to date.1,26 The most frequent form of human SCID results from defective cytokine-dependent survival and/or differentiation of lymphocyte precursors, secondary to mutations in one of the 3 genes: the common gamma chain of cytokine receptors (γc), the Janus kinase 3 (JAK-3), specifically associated with the cytoplasmic region of γc, or the interleukin-7 (IL-7) receptor α chain.2,3 These SCID forms are characterized by normal to increased numbers of circulating, although poorly functional, mature B cells (B+ SCID subtype).4,5 It is not clear whether early B-cell stages in the bone marrow also are affected in B+ SCID.5 A defect in the V(D)J rearrangement process of T-cell receptor (TCR) and B-cell receptor (immunoglobulin) genes caused by deficiencies in the recombinase activating gene (Rag)1 or Rag2, or in Artemis, a protein involved in the nonhomologous end joining repair pathway, leads to the B– SCID subtype and accounts for 30% of SCID cases.6 While lacking mature B cells, these patients exhibit normal numbers of precursor B cells.7,8
Allogeneic hematopoietic stem cell transplantation (HSCT) is the therapy of choice for SCID patients, who, if untreated, die within the first year of life. In the absence of a myeloablative conditioning regimen, there is a split chimerism in most SCID patients (> 80%), with T cells and natural killer (NK) cells being of donor origin in patients with a NK–B+ type of SCID.9,10 All other leukocyte subsets as well as hematopoietic lineages are of host origin in most cases. This chimerism pattern is unique compared to classic settings of HSCT and indicate that hematopoiesis remains of host origin,11 despite HSCT. A consequence of this split chimerism is the usual absence of donor B cells. In the survey realized by Haddad et al10 on 116 European patients, none of the B– and half of the B+ SCID patients, who did not receive a conditioning regimen, developed some B-cell function. Similarly, in a single-center study conducted by Buckley et al12 that involved 89 SCID patients, 45 of 72 surviving patients who received an HSCT without conditioning regimen were still receiving immunoglobulin replacement therapy at the last evaluation, despite the presence of donor T cells, indicating insufficient restoration of the B-cell compartment. Improving B-cell reconstitution after HSCT would significantly reduce the morbidity rate of B– SCID patients.13 The poor B-cell reconstitution in B– SCID (relative to B+ SCID subtypes) could relate to differences in the biology of host B-cell precursors.
To address this hypothesis, we have evaluated the efficacy of B-cell reconstitution after unconditioned HSCT in a series of mutant mouse strains differing in the “competitive fitness” of their early B-cell compartments. Our results point to a critical role for early pro–B cells, but not NK cells, in conditioning B-cell reconstitution after nonmyeloablative HSCT.
Materials and methods
Mice
Four- to six-week-old C57Bl/6Ly5.1 (CD45.1) mice were used as donors for HSCT and were obtained from Charles River Laboratories (l'Arbresle, France). Recipients included recombinase activating gene (Rag)–2–deficient (Rag2°), Rag-2, and common γ chain (γc)–deficient (Rag2°γc°) Rag-2, and IL-2Rβ–deficient (Rag2°IL2Rβ°), and fetal liver kinase-3 and IL-7Rα–deficient (Flt3IL7Rα°) mice.14 All recipient mice were of the CD45.2 background and were bred under pathogen-free conditions in our animal facilities (Institut Pasteur, Paris, and Necker Medical School, Paris). Where indicated, Rag2° mice have been irradiated with 3Gy using a 137Cs irradiator. All animal protocols were performed in compliance with the French Ministry of Agriculture regulations for Animal Experimentations (Act no. 87847, October 19, 1987; modified May 2001).
Bone marrow transplantation
Bone marrow (BM) cells were flushed from both tibias and femora of donor C57Bl/6Ly5.1 mice and treated with a 0.75% NH4Cl (Sigma-Aldrich, St Louis, MO). BM cells were stained with lineage (Lin) antibodies conjugated to phycoerythrin (PE) (all from Becton Dickinson Biosciences Pharmingen [BD], San Diego, CA): B220 (RA3-6B2), CD3 (17A2), CD4 (GK1.5), CD8 (53-5.8), NK1.1 (PK136), CD11b (M1/70), Gr-1 (RB6-8C5), and TER-119 (TER-119). Lin+ cells were depleted using an anti-PE magnetic selection kit (Miltenyi Biotech, Bergisch Gladbach, Germany). Lin– cells were then treated with anti–FcγII/III receptor antibody (2.4G2) (BD) to block nonspecific staining, before staining with fluorescein isothiocyanate–conjugated anti–Sca-1 (E13-161.7) antibody and allophycocyanin-conjugated anti-CD117 (c-kit, 2B8) antibody (both from BD). The Lin–Sca1+cKit+ (LSK) population was isolated to more than 95% purity using a MOFLO high-speed cell sorter (Dako, Glostrup, Denmark.). Ten thousand LSK cells were injected via the retro-orbital vein into nonirradiated recipients, which were analyzed 10 weeks later. Irradiated (3 Gy) Rag 2° mice were analyzed by serial monthly bleedings up to 6 months after transplantation.
Flow cytometry analysis
Cells were isolated from BM, spleen, and peritoneal cavity. Cells were treated with 2.4G2 to minimize nonspecific staining. Monoclonal antibodies to CD45.1 (A20), CD45.2 (104), CD19 (1D3), CD43 (S7), CD24 (30-F1), BP-1 (BP-1), CD25 (3C7), CD5 (53-7.3), CD11b (M1/70), CD21 (7G6), CD23 (B3B4), Gr-1 (Ly6-G) conjugated to fluorescein isothiocyanate, phycoerythrin, allophycocyanin, or biotin were obtained from BD Biosciences Pharmingen. Biotinylated antibodies were detected with streptavidin-phycoerythrin-cyanine-7. Goat anti–mouse IgM antibodies conjugated to fluorescein isothiocyanate were purchased from Jackson ImmunoResearch Laboratory (West Grove, PA). Rat anti–mouse IgD antibody (clone 11-26) conjugated to phycoerythrin was obtained from Southern Biotechnology Associates (Birmingham, AL). Propidium iodide (Sigma) was used to exclude dead cells, and live cells were found in the propidium iodide–negative population. Stained cells were analyzed using a FACSCalibur cytometer and CellQuest software (BD).
Results
Analysis of early B-cell precursors in alymphoid mice
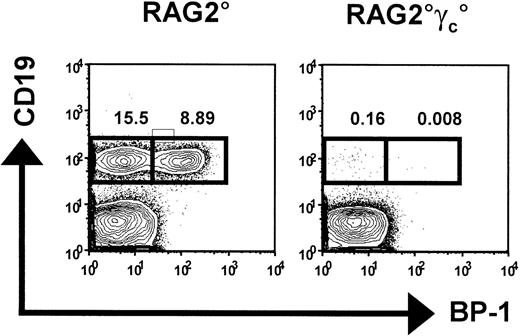
We tested the hypothesis that competition in the early B-cell precursor compartment might condition HSC engraftment and subsequent B-cell reconstitution in mice after unconditioned HSCT. We first analyzed early B-cell development in 2 mouse strains–Rag2°15 and Rag2°γc° mice16 –that have the T–B–NK+ and T–B–NK– phenotypes, respectively. As previously described, Rag2° are devoid of mature B and T cells, although pro–B cells are present in normal numbers (Figure 1). In contrast, all stages of B-cell development (including pro–B cells) are massively reduced in Rag2°γc° mice (Figure 1 and Hardy and Hayakawa17 ). Occupancy of the bone marrow B-cell niche by the recipient B-cell precursors in Rag2° mice might thus impede the development of donor B cells after HSCT. We therefore compared B-cell reconstitution following HSCT in unconditioned Rag2°γc° versus Rag2° mice.
Hematopoietic stem cell transplantation and donor B-cell engraftment
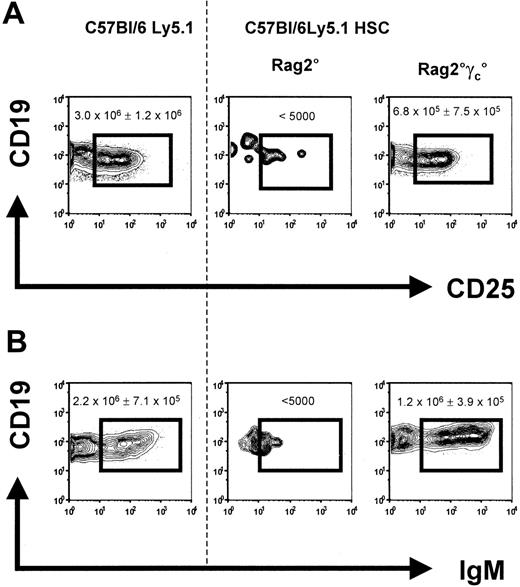
HSCT was performed by adoptively transferring 1 × 104 purified Lin–c-Kit+Sca-1+ cells from CD45.1 C57Bl/6 mice into nonirradiated recipient Rag2° and Rag2°γc° mice. Bone marrow, spleen, and lymph nodes were analyzed for B-cell reconstitution 10 weeks later. While donor pro–B and pre–B cells were not detectable in Rag2° mice that received transplants, they reached almost wild-type numbers in Rag2°γc° recipients (Figure 2A). Similarly, immature B cells (CD19+IgM+) were absent from the bone marrow in Rag2° recipients but were detected in about normal numbers in Rag2°γc° hosts (Figure 2B).
HSC engraftment was determined on the basis of the degree of CD45.1 chimerism within the myeloid lineage of recipient mice. Chimerism in this lineage reflects the level of HSC engraftment. As expected, myeloid chimerism in bone marrow was low in both types of recipients (Table 1), reflecting the nonconditioned status of the mice at the time of transplantation. Thus, the difference in B-cell reconstitution in Rag2° and Rag2°γc° recipients cannot be explained by a difference in HSC engraftment.
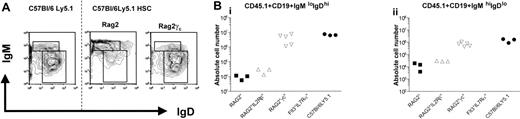
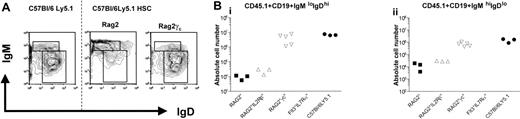
The generation of a steady-state peripheral B-cell pool was assessed in the spleen and peritoneal cavity. Splenic CD19+ B cells can be subdivided on basis of the differential expression of IgM and IgD, and 3 populations can be distinguished: (1) follicular B cells represent the largest fraction of splenic B cells and are identified as CD19+IgMloIgDhi cells; (2) CD19+IgMhiIgDlo cells contain marginal zone B cells, B1 cells, as well as transitional B cells, which have recently emigrated from the bone marrow18 ; and (3) CD19+IgM-IgD– cells represent cells that have undergone Ig isotype switching. All these B-cell subpopulations could be detected in both Rag2° as well as Rag2°γc° recipient mice (Figure 3A). However, while B-cell numbers reached almost wild-type levels in Rag2°γc° recipients, these numbers were, on average, 300-fold reduced in Rag2° mice (Figure 3B) that received transplants.
Bone marrow B cells in C57Bl/6Ly5.1, Rag2°, and Rag2°γc° mice. Bone marrow cells from these mice were stained for CD19 and BP-1. Shown are propidium iodide (PI)–negative cells within the lymphocyte gate. The boxed populations represent 2 subpopulations of the pro–B cells: CD19+BP-1– cells, which correspond to fraction B cells according to the Hardy scheme,17 and CD19+BP-1+ cells, which correspond to fraction C cells according to the Hardy scheme.17 Numbers indicate the percentage of cells in the respective box among lymphocytes.
Bone marrow B cells in C57Bl/6Ly5.1, Rag2°, and Rag2°γc° mice. Bone marrow cells from these mice were stained for CD19 and BP-1. Shown are propidium iodide (PI)–negative cells within the lymphocyte gate. The boxed populations represent 2 subpopulations of the pro–B cells: CD19+BP-1– cells, which correspond to fraction B cells according to the Hardy scheme,17 and CD19+BP-1+ cells, which correspond to fraction C cells according to the Hardy scheme.17 Numbers indicate the percentage of cells in the respective box among lymphocytes.
Bone marrow B cells in reconstituted Rag2° and Rag2°γc°. Mice were analyzed 10 weeks after receiving transplants with 1 × 104 sorted Lin-cKit+Sca1+ cells from wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used for control. (A) Data represent profiles of CD19 versus CD25 expression on bone marrow cells from engrafted mice. (B) Profiles of CD19 versus IgM expression. Data represent profiles of gated CD45.1+ propidium iodide (PI)–negative cells. One representative analysis of at least 3 experiments is shown. Numbers indicate the absolute cell number in the respective quadrant. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice.
Bone marrow B cells in reconstituted Rag2° and Rag2°γc°. Mice were analyzed 10 weeks after receiving transplants with 1 × 104 sorted Lin-cKit+Sca1+ cells from wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used for control. (A) Data represent profiles of CD19 versus CD25 expression on bone marrow cells from engrafted mice. (B) Profiles of CD19 versus IgM expression. Data represent profiles of gated CD45.1+ propidium iodide (PI)–negative cells. One representative analysis of at least 3 experiments is shown. Numbers indicate the absolute cell number in the respective quadrant. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice.
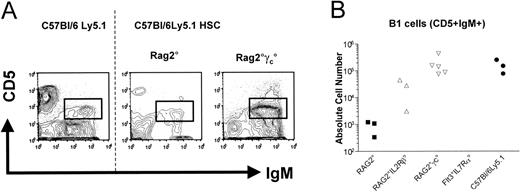
In the peritoneal cavity, B-1 cells can be distinguished by their characteristic surface phenotype, CD19+IgMhiCD5+. We detected a normal-sized population of B-1 cells in reconstituted Rag2°γc° mice, while the 3 Rag2° mice tested harbored only a small population (200-fold reduced as compared to Rag2°γc° mice) of B-1 cells in the peritoneal cavity (Figure 4).
B-cell development is conditioned by early B cells but independent of NK cells
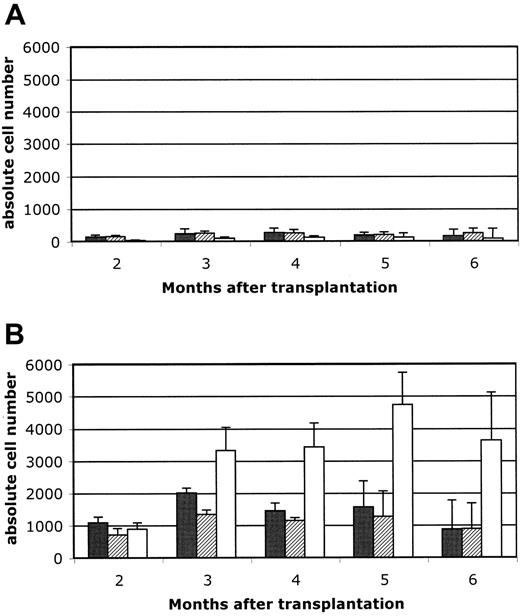
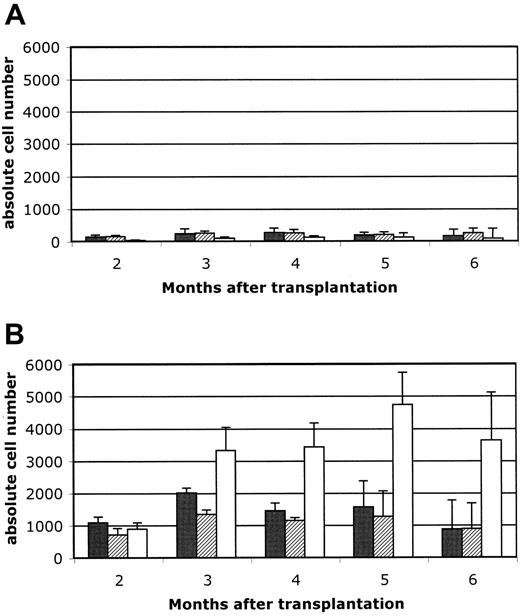
These results indicate that B-cell reconstitution after HSCT into unconditioned recipients is more efficient in Rag2°γc° than in Rag2° recipients, which supports the idea that endogenous B-cell precursors could condition the generation of donor-derived B cells. To validate this hypothesis, we determined the efficiency of B-cell reconstitution after transfer of hematopoietic precursors into unconditioned Rag2° hosts compared to Rag2° hosts conditioned with low-dose irradiation (3 Gy). We found that the low-dose conditioning regimen allowed for a 40-fold increase in peripheral B-cell numbers, while the effect on T-cell reconstitution was clearly less pronounced (Figure 5A,B).
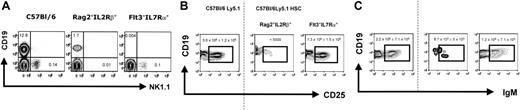
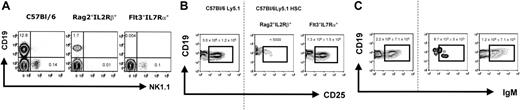
We next considered if factors other than the differences in the endogenous B-cell precursor pool might have influenced the B-cell engraftment potential. For example, Rag2° mice contain a normal compartment of NK cells, while Rag2°γc° double-deficient mice exhibit only the earliest NK precursors, while all later stages are virtually absent.19 Thus, it was possible that NK cells present in the unconditioned Rag2°, but not Rag2°γc°, recipient mice inhibit engraftment of the transplanted cells or inhibit donor B-cell differentiation. To study this possibility, we took advantage of other immunodeficient mouse strains that dissociate the development of early B-cell precursors from that of NK cells. For example, Rag2°IL2Rβ° double-deficient mice display a normal pro–B-cell compartment in the bone marrow, although they completely lack NK cells. In contrast, Flt3°IL7Rα° mice lack all early B-cell precursors but develop normal numbers of NK cells (Figure 6A and data not shown). We therefore assessed B-cell reconstitution after HSCT in unconditioned Rag2°IL2Rβ° and Flt3°IL7Rα° mice. Donor-derived B cells reached wild-type numbers in Flt3°IL7Rα° mice at early (Figure 6B) and later (Figure 6C) stages of bone marrow B-cell development as well as in the spleen and peritoneal cavity (Figure 7A,B). In contrast, early and later stages of B-cell development were absent or strongly (around 1000-fold) reduced in the bone marrow of reconstituted Rag2°IL2Rβ° mice. In these mice, donor-derived B cells in the spleen and peritoneal cavity were 200-fold (Figure 3B) and 10-fold (Figure 4B) reduced, respectively, compared to Flt3°IL7Rα° transplant recipients. The small difference in the number of peritoneal cavity B cells observed in the different mice is likely due to the fact that B-1a cells (as opposed to B-2 B cells) have self-renewal capacity and thus can accumulate with time in all mice. This would explain the reduced but detectable B-1a cells in the Rag2°IL2Rβ° recipients (Figure 4B), which result from expansion of a small number of B cells that can develop in these mice.
HSC chimerism was very low in Rag2°IL2Rβ° mice (at levels similar to that observed in Rag2° and Rag2°γc° mice), while HSC chimerism was somewhat higher in Flt3°IL7Rα° recipients (Table 1). Nevertheless, the differences in donor HSC chimerism cannot explain the striking differences in B-cell restoration in the 4 types of recipient mice, which is clearly correlated with a deficiency in the development of the B-cell precursor compartment (Table 1).
Discussion
Our results indicate that the efficiency of B-cell reconstitution in mice after unconditioned HSCT depends on the availability of niches/resources in the bone marrow environment. This is based on comparing HSC mice that received transplants that are severely deficient in B-cell precursors in the bone marrow (Rag2°γc° or Flt3°IL7Rα°) with mice that contain normal numbers of pro–B cells (Rag2° or Rag2°IL2Rβ°). The results indicate that presence of early pro–B-cell precursors conditions the overall efficiency of B-cell reconstitution. Since the different mouse mutants tested in this study lack pre-B, immature, and mature B cells, we conclude that a major determinant of B-cell reconstitution in the context of this transplantation protocol is competition at the earliest B-cell stages (pro–B cells). As such, the few transplanted HSCs and their progeny appear to have to compete with these endogenous pro–B cells for resources during development. Once donor-derived B cells leave the bone marrow, they enter the pool of long-lived, mature B cells, where they may accumulate to a steady-state level that is determined by different homeostatic controls.
Splenic B cells from reconstituted Rag2° and Rag2°γc° mice. Mice were analyzed 10 weeks after bone marrow transplantation with 104 sorted Lin–cKit+Sca1+ cells of wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used as control. (A) Splenic B cells from these mice were stained for CD45.1, CD19, IgM, and IgD. Data represent profiles of IgM and IgD expression on gated CD45.1+CD19+ PI– cells. The dotted line separates the flow cytometric results from unmanipulated C57Bl/6Ly5.1 mice and those from the reconstituted recipient mice. Absolute cell numbers are given in panel 3B. (B) Absolute cell numbers of CD45.1+CD19+IgMloIgDhi cells (i) and CD45.1+CD19+IgMhiIgDlo (ii) cells found in the respective recipient mice.
Splenic B cells from reconstituted Rag2° and Rag2°γc° mice. Mice were analyzed 10 weeks after bone marrow transplantation with 104 sorted Lin–cKit+Sca1+ cells of wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used as control. (A) Splenic B cells from these mice were stained for CD45.1, CD19, IgM, and IgD. Data represent profiles of IgM and IgD expression on gated CD45.1+CD19+ PI– cells. The dotted line separates the flow cytometric results from unmanipulated C57Bl/6Ly5.1 mice and those from the reconstituted recipient mice. Absolute cell numbers are given in panel 3B. (B) Absolute cell numbers of CD45.1+CD19+IgMloIgDhi cells (i) and CD45.1+CD19+IgMhiIgDlo (ii) cells found in the respective recipient mice.
An interesting comparison can be made between the reconstitution characteristics of the 2 “permissive” mutants used in this study, namely Flt3°IL7Rα° mice and Rag2°γc° mice. Deficiency in Flt3 affects the very early stages of hematopoietic development, including multipotent hematopoietic progenitors.20,21 Thus, Flt3° mice may enable more efficient engraftment of hematopoietic precursors, a notion that is supported by the higher degree of donor-recipient HSC chimerism in these mice compared to all other recipients. Multipotent hematopoietic progenitors are not affected in Rag2°γc° mice (nor in Rag2° or Rag2°IL2Rβ° mice) and, thus, overall HSC engraftment in these strains is low using a nonconditioning HSCT scheme. Nevertheless, this increased HSC chimerism in Flt3°IL7Rα° mice does not impact significantly on overall B-cell reconstitution as compared with Rag2°γc° recipients. These observations suggest that the degree of HSC engraftment might not be the only critical factor in determining the efficiency of B-cell reconstitution after unconditioned HSCT. The ability to compete out recipient early pro–B-cell precursors also appears to be a limiting factor. In this respect, early B-cell precursors from Flt3°IL7Rα° as well as Rag2°γc° are clearly at a competitive disadvantage compared to wild-type precursors, due to their inability to proliferate in response to IL-7.
Can we extrapolate the results from our mouse studies to propose new therapeutic HSCT options to SCID patients? Clinical transplantation studies have shown a mild difference in B-cell function after HSCT in NK–B+ and NK+B– SCID patients.9,10,13 This difference can be partially explained by the 15% of B+SCID forms with normally functional B cells (ie, IL-7R and CD45 deficiencies) and by the fact that a donor B-cell microchimerism accounting for the antibody production cannot be excluded in γc-deficient patients. Three sets of data point to a role of γc-dependent cytokines on multiple levels of human B-cell development. First, B cells from mothers of X-SCID patients, who are obligate carriers of a defective γc allele on one X chromosome, display a nonrandom pattern of X-chromosome inactivation in their developing bone marrow B precursors and mature B cells.22 Second, Johnson et al5 recently have documented the capacity of γc-dependent cytokines in promoting the proliferative expansion of human CD19+ B-lineage cells. Finally, mature B cells from NK–B+SCID patients are poorly functional even in the presence of a restored T-cell pool following HSCT.13 As consequence, the B-cell precursor stages could be reduced in the γc-deficient patients due to a decreased proliferative capacity of these γc-deficient cells compared to their wild-type counterparts, facilitating the engraftment of some donor precursor B cells.
Peritoneal cavity B1 cells from engrafted mice. Cells were stained for CD45.1, IgM, and CD5. (A) Profiles of CD5 and IgM expression on gated CD45.1+IgM+PI– cells are presented. One representative analysis of at least 3 experiments is shown. Absolute cell numbers are given in panel B. (B) The absolute cell numbers of the indicated cells found in the respective recipient mice are presented. The dotted line separates flow cytometric results from unmanipulated C57Bl/6 Ly5.1 mice and those from reconstituted recipient mice.
Peritoneal cavity B1 cells from engrafted mice. Cells were stained for CD45.1, IgM, and CD5. (A) Profiles of CD5 and IgM expression on gated CD45.1+IgM+PI– cells are presented. One representative analysis of at least 3 experiments is shown. Absolute cell numbers are given in panel B. (B) The absolute cell numbers of the indicated cells found in the respective recipient mice are presented. The dotted line separates flow cytometric results from unmanipulated C57Bl/6 Ly5.1 mice and those from reconstituted recipient mice.
In contrast, NK+B– SCID patients, caused by Rag1, Rag2, and Artemis deficiencies, lack all mature B cells due to their inability to rearrange the gene segments encoding the Ig and TCR genes.23 Nevertheless, the earlier B-cell developmental stages that are independent of signals derived from rearranged Ig genes, including the pro–B-cell stage, are unaffected in these patients as in mice.7,8 Pro–B cells from B– SCID patients are expected to be indistinguishable from normal pro–B cells in their response to extracellular stimuli. Thus, the frequent absence of the B-cell reconstitution in B– SCID following a nonconditioned HSCT might be explained by the competitive fitness of the recipient B-cell precursors that likely occupy critical niches in the recipient bone marrow. This explanation is strongly supported by our results following transplantation of Sca-1+ wild-type bone marrow cells into 3 Gy–irradiated Rag2° mice. Despite this low irradiation dose, the number of circulating B cells was significantly increased in comparison to the nonirradiated recipients (at all time points up to 6 months after transplantation).
Reconstitution of peripheral blood lymphocytes. Reconstitution of peripheral blood lymphocytes in unconditioned (A) versus 3Gy-conditioned (B) Rag2° recipients. Mice were reconstituted with 104 Sca1+ BM cells and peripheral blood cells analyzed at 8 weeks after transfer and then monthly up to 6 months. Five mice per group were analyzed. In A and B, ▪ indicates CD3/CD4; ▨, CD3/CD8; and □, B220/IgM. Data are expressed as means ± standard deviation.
Reconstitution of peripheral blood lymphocytes. Reconstitution of peripheral blood lymphocytes in unconditioned (A) versus 3Gy-conditioned (B) Rag2° recipients. Mice were reconstituted with 104 Sca1+ BM cells and peripheral blood cells analyzed at 8 weeks after transfer and then monthly up to 6 months. Five mice per group were analyzed. In A and B, ▪ indicates CD3/CD4; ▨, CD3/CD8; and □, B220/IgM. Data are expressed as means ± standard deviation.
Nevertheless, the role of the competition for early B-cell niches may not be sufficient to explain all of the observed differences between T- and B-cell restoration following HSCT in SCID patients. In NK+B–SCID, some residual early T-cell precursors also may be located within thymic stromal niches. Nevertheless, the putative presence of these early thymocytes does not appear to impair the generation of donor T cells, even in patients who received nonconditioned HSCT.9 Consequently, to fully explain the absence of B-cell reconstitution in these HSCT settings, other associated factors might play a role. For example, stromal cell niches could be submitted to different control mechanisms in the thymus and in the bone marrow. In this way, B-cell precursors would have a limited expansion capacity in comparison with their early T-cell counterparts.
Endogenous BM B cells from Rag2°IL2Rβ° and Flt3IL7Rα° mice and B cells generated after reconstitution of these recipient mice. (A) CD19 versus NK1.1 profile of the bone marrow cells of the different recipient mice. Shown are CD3-negative bone marrow lymphocytes. Note that the CD19+ in the C57Bl/6 mouse contain Ig– precursor (pro– and pre–B cells) as well as Ig+ immature and mature B cells, while in the Rag2°IL2Rβ° mouse CD19+ cells only comprise pro–B cells, as B-cell development is arrested at the transition of the pro– to pre–B-cell stage (according to Hardy et al17 ) due to the Rag2-mutation. Absolute cell numbers for CD19+ and NK1.1+CD3– cells are given. (B) CD19 versus CD25 profiles. Phenotype of the bone marrow B cells from reconstituted Rag°IL2Rβ° and Flt3IL7Rα° mice, then weeks after transplantation with 1 × 104 sorted Lin–cKit+Sca1+ cells from wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used for control. (C) Profiles of CD19 versus IgM. Shown are CD45.1+PI– cells falling in a lymphocyte gate. One representative analysis of at least 3 experiments is shown. Numbers indicate the absolute cell number in the respective quadrant. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice.
Endogenous BM B cells from Rag2°IL2Rβ° and Flt3IL7Rα° mice and B cells generated after reconstitution of these recipient mice. (A) CD19 versus NK1.1 profile of the bone marrow cells of the different recipient mice. Shown are CD3-negative bone marrow lymphocytes. Note that the CD19+ in the C57Bl/6 mouse contain Ig– precursor (pro– and pre–B cells) as well as Ig+ immature and mature B cells, while in the Rag2°IL2Rβ° mouse CD19+ cells only comprise pro–B cells, as B-cell development is arrested at the transition of the pro– to pre–B-cell stage (according to Hardy et al17 ) due to the Rag2-mutation. Absolute cell numbers for CD19+ and NK1.1+CD3– cells are given. (B) CD19 versus CD25 profiles. Phenotype of the bone marrow B cells from reconstituted Rag°IL2Rβ° and Flt3IL7Rα° mice, then weeks after transplantation with 1 × 104 sorted Lin–cKit+Sca1+ cells from wild-type C57Bl/6Ly5.1 mice. Age-matched C57Bl/6Ly5.1 mice were used for control. (C) Profiles of CD19 versus IgM. Shown are CD45.1+PI– cells falling in a lymphocyte gate. One representative analysis of at least 3 experiments is shown. Numbers indicate the absolute cell number in the respective quadrant. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice.
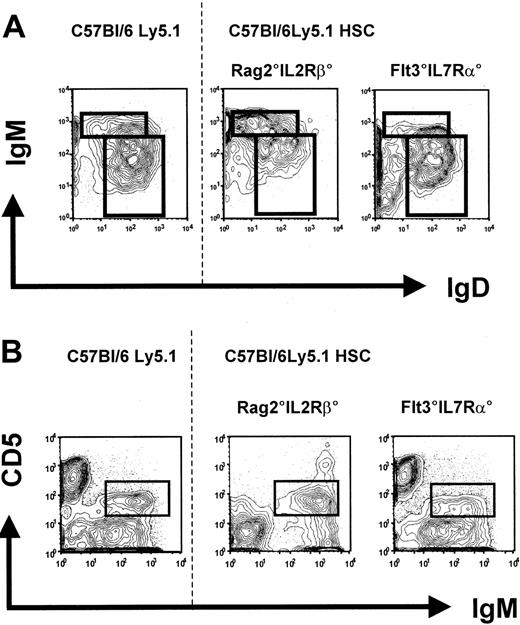
Peripheral B cells from reconstituted Rag2°IL2Rβ° and Flt3IL7Rα° mice 10 weeks after transplantation with Lin–cKit+Sca1+CD45.1+ wild-type cells. Age-matched C57Bl/6Ly5.1 mice were used as controls. (A) IgM and IgD profiles on gated CD45.1+CD19+PI– splenic cells of the indicated mice. Absolute cell numbers are given in Figure 3B. (B) Peritoneal cavity B1 cells from engrafted mice. Cells were stained for CD45.1, IgM, and CD5. Data represent profiles of CD5 and IgM expression on gated CD45.1+IgM+PI– cells. One representative analysis of at least 3 experiments is shown. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice. Absolute cell numbers are given in Figure 4B.
Peripheral B cells from reconstituted Rag2°IL2Rβ° and Flt3IL7Rα° mice 10 weeks after transplantation with Lin–cKit+Sca1+CD45.1+ wild-type cells. Age-matched C57Bl/6Ly5.1 mice were used as controls. (A) IgM and IgD profiles on gated CD45.1+CD19+PI– splenic cells of the indicated mice. Absolute cell numbers are given in Figure 3B. (B) Peritoneal cavity B1 cells from engrafted mice. Cells were stained for CD45.1, IgM, and CD5. Data represent profiles of CD5 and IgM expression on gated CD45.1+IgM+PI– cells. One representative analysis of at least 3 experiments is shown. The dotted line separates the flow cytometric results from the unmanipulated C57Bl/6Ly5.1 from that of the reconstituted recipient mice. Absolute cell numbers are given in Figure 4B.
Nevertheless, the data demonstrated herein and the clinical observations lead us to propose that recipient B-cell precursors represent an obstacle toward the development of donor B-cell precursors following nonconditioned HSCT. From a clinical standpoint, our results suggest that specific therapy targeting B-cell precursors might improve B-cell reconstitution after HSCT in selected SCID patients. A fully myeloablative regimen can lead to full B-cell immunity development but is associated with a higher risk of toxicity, particularly in infected patients.24 A selective in vivo depletion of pro–B cells by using monoclonal antibodies could represent an interesting alternative strategy. The increasing use of monoclonal antibodies to B cells in various human conditions has proved its clinical efficacy, even in the context of HSCT.25 However, clinical grade antibodies capable of selectively depleting pro–B cells are not available presently. This proposed strategy could be tested in the experimental models we have described before any clinical application. As such, these mutant mouse strains provide valuable models to test new approaches to improve the current results of HSCT for inherited primary immunodeficiencies such as SCID.
Prepublished online as Blood First Edition Paper, April 13, 2006; DOI 10.1182/blood-2006-01-0061.
Supported by grants from INSERM, the Institut Pasteur, Ligue National Contre le Cancer, Ministère de la Recherche, and the Contrat Européen Consert “Concerted Safety and Efficiency Evaluation of Retroviral Transgenesis in Gene Therapy of Inherited Diseases” (contract no. LSHB-CT-2004-005242; coordinator, G. Wagemaker). A.L. received a fellowship from Harvard University.
A.L. and C.A.J.V. contributed equally to this work.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Odile Richard-LeGoff and Erwan Corcuff for their technical assistance, and Isabelle Andre-Schmutz for fruitful discussions.