Abstract
The determinants of sickle red blood cell (RBC) life span have not been well-defined but may include both intrinsic factors (eg, the tendency to sickle) and extrinsic factors (eg, the capacity of the reticuloendothelial system to remove defective RBCs). Fetal hemoglobin (HbF) is heterogeneously distributed among sickle RBCs; F cells contain 20% to 25% HbF, whereas the remainder have no detectable HbF (non-F cells). Autologous sickle RBCs were labeled with biotin and reinfused to determine overall survival, non–F- and F-cell survival, and time-dependent changes in HbF content (%HbF) for the surviving F cells. A total of 10 patients were enrolled, including 2 who were studied before and after the percentage of F cells was increased by treatment with hydroxyurea. As expected, F cells survived longer in all subjects. Non–F-cell survival correlated inversely with the percentage of F cells, with the time for 30% cell survival ranging from 6 days in patients with more than 88% F cells to 16 days in patients with less than 16% F cells. As the biotin-labeled RBCs aged in the circulation, the HbF content of the surviving F-cell population increased by 0.28%/d ± 0.21%/d, indicating that within the F-cell population those with higher HbF content survived longer.
Introduction
Patients with homozygous sickle cell disease (SCD) have 2 types of red blood cells (RBCs): longer-lived F cells, which contain both sickle hemoglobin (HbS) and fetal hemoglobin (HbF), and shorter-lived non-F cells, which contain HbS but no detectable HbF. The amount of HbF in each F cell undoubtedly varies to some extent,1 but there is a clear dichotomy between F and non-F cells. The percentage of F cells in blood depends on 2 factors: (1) a variable increase in the production of F cells in the bone marrow that is also seen in other states of increased erythropoietic stress2 and (2) the survival advantage of F cells that is unique to SCD and leads to an increase in their relative number as RBCs age in the circulation.3,4 Because of this difference in cell survival, the percent HbF in mature sickle RBCs is much higher than the corresponding value in newly released reticulocytes.1,2
Based on early studies showing that HbF inhibits sickling5 and has a beneficial clinical effect,6 therapeutic agents to increase HbF have been introduced. One of these, hydroxyurea (HU), has been approved by the Food and Drug Administration (FDA) based on a large multiinstitutional study7 that showed both increased HbF and a decreased number of painful episodes after treatment.
There are at least 2 important interactions between hemolysis and the other major consequence of SCD: vasoocclusion. The first is the presence of immature “stress” reticulocytes in the circulation due to the hemolysis-dependent increase in the rate of erythropoiesis. In ex vivo models, these are the first cells to adhere to postcapillary venules, thus narrowing the lumen and facilitating the entrapment of the poorly deformable dehydrated cells.8,9 The second connection between hemolysis and vasoocclusion is the role of free plasma hemoglobin (Hb) as a scavenger of NO.10-13 A significant portion of sickle RBC hemolysis appears to be intravascular, and free plasma Hb is markedly elevated. This extracellular Hb binds NO, resulting in a state of functional NO depletion and a shift in vascular tone toward vasoconstriction. It has been postulated that this contributes to vasoocclusion and the development of pulmonary hypertension in SCD. Therefore, by decreasing the number of stress reticulocytes and the level of free plasma Hb, high levels of F cells may have beneficial effects beyond dilution of the non-F sickle cells.
We have previously used the biotin label method to determine the life spans of F and non-F cells in patients with SCD for both unfractionated RBCs and the dense (dehydrated) fraction. The survival of non-F cells is about 2 weeks for most patients,3 whereas F cells survive much longer, typically about 6 weeks. Therefore, if most of the circulating RBCs are F cells, as occurs without treatment in some patients and after HU in others, hemolysis is less severe. However, there are still a number of questions concerning the factors that may influence this difference. These include whether F cells resulting from HU treatment and the remaining non-F cells have the same survival characteristics as pretreatment F and non-F cells, whether the survivals of F and non-F cells depend on the ratio of these cells in the circulation, and whether sickle RBC survival is influenced by the range in HbF content that is found in F cells.
We now examine in greater detail the survival characteristics of sickle RBCs and show that (1) the range of HbF content in F cells appears to be important, with cells on the high end of the range surviving longer than those on the low end, and (2) the survival of non-F cells correlates inversely with the fraction of F cells, with shorter non–F-cell life span in patients with a high percentage of F cells. This relationship appears to be independent of HU therapy.
Materials and methods
Biotin labeling of sickle RBCs
Approval was obtained from the University of Cincinnati College of Medicine Institutional Review Board for these studies. Informed consent was provided in accordance with the Declaration of Helsinki. Up to 10 mL autologous sickle cells were labeled with biotin and reinfused as previously described.3,4 The labeled RBCs were identified in postinfusion blood samples by flow cytometry after addition of streptavidin-phycoerythrin (SA-PE) and isolated with the aid of SA-coated magnetic beads.3,4
Determination of percent F cells and the survival of F cells
Selected postinfusion blood samples were first reacted with SA-PE to identify biotin-labeled RBCs3,4 and then with anti-HbF–FITC using a commercially available kit (Caltag, Burlingame, CA). The percentage of F cells in both the biotin-labeled and unlabeled populations was determined as a function of time after reinfusion using a 2-color flow cytometric analysis.
Percent survival. Survival is shown for F cells (♦) and non-F cells (□). The survival of each cell type was derived from the overall survival of biotin-labeled cells and the percentage of labeled F cells at each time point as described in “Materials and methods.” Each experiment is identified in accordance with Table 1.
Percent survival. Survival is shown for F cells (♦) and non-F cells (□). The survival of each cell type was derived from the overall survival of biotin-labeled cells and the percentage of labeled F cells at each time point as described in “Materials and methods.” Each experiment is identified in accordance with Table 1.
The survival curves for F and non-F cells were determined as follows:
(1) An arbitrary number of labeled RBCs (1000) was selected as the basis for the analysis. Immediately after reinfusion, the starting number of F- and non–F-labeled cells was calculated. For example, if there were 20% F cells, the starting values would be 200 F cells and 800 non-F cells.
(2) At selected times after reinfusion, the overall fractional RBC survival was calculated by dividing the percentage of total RBCs that were biotin labeled at that time by the percentage immediately after reinfusion.
(3) At the same postinfusion times, the percent F cells for the surviving labeled RBCs was determined. These data, together with the number of biotin-labeled RBCs at that time, yield the number of surviving F and non-F cells. Continuing the example from earlier in this section, if overall survival at 3 days is 0.8 and the surviving RBCs are 24% F cells, there would be 1000 × 0.8 × 0.24 (192) F cells and 800 – 192 (608) non-F cells. The fractional survival for the 2 cell types at 3 days would then be 192 of 200 (0.96) for F cells and 608 of 800 (0.76) for non-F cells. This analysis is continued for additional time points to yield the individual survival curves for F and non-F cells.
Magnetic isolation of biotin-labeled RBCs and HPLC
At the same times as flow cytometric F-cell analysis, the surviving biotin-labeled RBCs were isolated using SA-coated magnetic beads and the Hb composition of the isolated cells analyzed by high-performance liquid chromatography (HPLC) as previously described.3,4 The percentage of F cells from the flow cytometric assay and the percent HbF in the isolated labeled RBCs give related but not identical information. If the percent HbF in the surviving F cells remains constant with time, the values from both assays will increase at the same rate and their ratio will be constant. However, if the percent HbF in the surviving F cells increases with time, the HbF in the isolated labeled RBCs will increase faster than the percent F cells. In fact, the percent HbF in the surviving F cells can be calculated for each time point by dividing the percent HbF in the isolated cells by the fraction of F cells. For example, if at a given time the isolated labeled cells contain 20% HbF and at the same time 50% of the labeled cells are F cells (fraction 0.5), the F cells contain 40% HbF. These data provide information concerning the importance of the relatively small variability of HbF content in F cells. If the percent HbF in the surviving F cells increases with time after reinfusion, it can be concluded that F cells with a higher percentage of HbF survive longer than F cells with lower values.
Results
Patient data are shown in Table 1. Ten patients with SCD were studied. Two of these patients were studied before and after treatment with HU, giving a total of 12 experiments. These 2 patients showed a good response to HU, with increases in both percent HbF and percent F cells (Table 1). Of the remaining 8 patients, 2 were taking HU at the time of study. Therefore, 4 of the 12 experiments were performed during HU treatment.
The survival of F cells and non-F cells
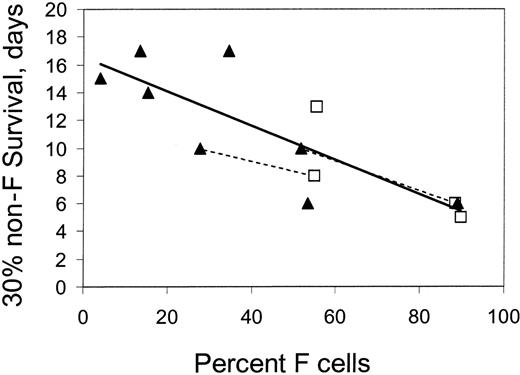
The separate survivals of F cells and non-F cells, calculated as described in “Materials and methods,” are shown in Figure 1 for the 12 experiments. As expected, the survival of F cells is invariably longer when compared with the survival of non-F cells in the same patient. There was only minor variation in the survival of F cells among patients. However, non-F cells exhibited a wide range of survival. Figure 2 shows non–F-cell survival from selected experiments in which the patient had either a very low (less than 16%) or very high (more than 88%) percentage of circulating F cells. The patients with low levels of F cells (and thus HbF) had a longer survival of non-F cells. Figure 3 plots the 30% survival (time when 30% of the labeled RBCs remain) of non-F cells as a function of percent F cells for all experiments. There is a strong inverse correlation between non-F survival and the percent F cells, with a slope that is significantly different than 0 (P < .001) and an intercept (corresponding to the 30% survival in a patient with no F cells) of 16.5 days. Extrapolation of this line to approach 100% F cells gives a 30% survival of 4 days for non-F cells. HU therapy did not appear to affect this relationship.
Survival of non-F cells in subjects with either a very low (less than 16%) or a very high (more than 88%) percentage of F cells.
Survival of non-F cells in subjects with either a very low (less than 16%) or a very high (more than 88%) percentage of F cells.
The 30% survival (time at which 30% of labeled cells remain) of non-F cells as a function of percent F cells in the circulation. Patients were either taking (□) or not taking (▴) HU. The dotted lines connect the points for 2 patients studied before and after treatment with hydroxyurea.
The 30% survival (time at which 30% of labeled cells remain) of non-F cells as a function of percent F cells in the circulation. Patients were either taking (□) or not taking (▴) HU. The dotted lines connect the points for 2 patients studied before and after treatment with hydroxyurea.
The average value for 30% F-cell survival was 24.3 ± 4.6 (1 SD) days. There was no correlation between F-cell survival and percent F cells.
The influence of HbF content on F-cell survival
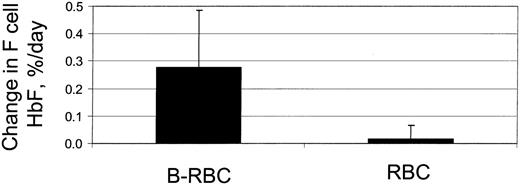
F cells are normally considered as a uniform population with each F-cell containing the same amount of HbF. However, there is undoubtedly a range of HbF content for each patient's F cells. The question is whether these relatively modest differences in HbF have a significant impact on in vivo sickling and cell survival. If so, the F cells with a higher content of HbF would have a longer in vivo survival, and thus older F cells would have a higher HbF content than younger F cells. As the labeled RBCs aged in vivo, the percent HbF in the remaining F cells would increase. Figure 4 shows the time-dependent changes in percent HbF for the labeled and unlabeled F cells in a typical subject. The unlabeled RBCs, which are at steady state and function as an internal control for the measurements, remain constant. The biotin-labeled RBCs, which are aging in the circulation, show a linear increase, implying that within the F-cell population survival is influenced by the amount of HbF within an F cell. The slope of this curve is the daily change in HbF content for the remaining F-cell population, expressed as percent per day. Figure 5 shows a comparison of these slopes for the labeled and unlabeled RBCs in 12 experiments, with a slope for the labeled cells of 0.28%/d ± 0.21%/d. These data demonstrate that, within an individual patient, F cells with higher HbF content survive longer than F cells with lower HbF.
Change in percent HbF in the rermaining F-cell population as a function of time after reinfusion for a representative patient. The open circles represent biotin-labeled RBCs, and the open squares represent unlabeled RBCs. The slope for labeled RBCs is the rate of increase in F-cell HbF (percent per day).
Change in percent HbF in the rermaining F-cell population as a function of time after reinfusion for a representative patient. The open circles represent biotin-labeled RBCs, and the open squares represent unlabeled RBCs. The slope for labeled RBCs is the rate of increase in F-cell HbF (percent per day).
HbF in F cells. The rate of increase of percent HbF in F cells for the surviving population of labeled cells (B-RBC) and unlabeled cells (RBC). n = 12, P < .005.
HbF in F cells. The rate of increase of percent HbF in F cells for the surviving population of labeled cells (B-RBC) and unlabeled cells (RBC). n = 12, P < .005.
Discussion
The studies presented here show that the life span of non-F cells correlates inversely with the percentage of F cells in the circulation. The patients with a greater percentage of F cells have lower non–F-cell survival. In 2 patients studied before and after effective administration of HU, the same trend was observed. There are 2 potential explanations for this result. The first is that the intrinsic properties of non-F cells determine their survival, which in turn influences F-cell enrichment and the percentage of F cells. For example, lower intracellular HbS concentration due to the presence of α thalassemia could result in better survival of non-F cells and therefore less enrichment of F cells, leading to a lower percentage of F cells.14 In the current study, 3 subjects (Table 1: patients 1, 4, and 6) had low MCV and presumptive α thalassemia. All 3 of these subjects had longer non–F-cell survival. However, several factors in the current study make it less likely that α thalassemia was the predominant factor in determining the observed variability in non–F-cell life span: (1) While on average patients with α thalassemia have decreased MCHC, which is thought to lead to longer cell survival, this was not the case for these 3 subjects with low MCV (Table 1). (2) The 2 patients who were studied before and after HU, neither of whom had decreased MCV prior to treatment, showed a change in non–F-cell survival consistent with the change in percent F cells that occurred with treatment. (3) After elimination of the 3 patients with presumptive α thalassemia, analysis of the remaining data show that the slope of the line analogous to that shown in Figure 3 is still different from 0 at the P = .025 level. Furthermore, if faster removal of non-F cells contributed significantly to higher percent HbF levels in the absence of α thalassemia, higher HbF would be associated with lower blood Hb levels. However, patients with high blood HbF tend to have higher Hb levels
A second possible explanation for the observed correlation between non–F-cell survival and percent F cells is that a higher percentage of F cells in the circulation shortens the survival of non-F cells. The best evidence for this is that the increased HbF levels in 2 patients after hydroxyurea therapy was associated with reduction in non–F-cell survival, as predicted by this relationship in other patients. It is unlikely that this is a direct effect on the non-F cells.
A factor that could influence the life span of non-F cells, and is potentially dependent on the percentage of F cells, is the sensitivity of surveillance for damaged RBCs. Patients with high HbF may have preservation of spleen function, and this could contribute to the faster removal of non-F cells in these subjects. It is possible that some steps in the identification and removal of damaged RBCs may be influenced by the rate of RBC turnover, so that under conditions in which there is a greater load upon the cell-removal process there is also a decreased sensitivity of surveillance. This possibility is supported by the studies of Loegering and colleages,15-17 in which the infusion of either neuraminidase-treated RBCs or RBC ghosts led to the suppression of reticuloendothelial system (RES) function in rats. A similar mechanism, with RES blockade by antibody-coated RBCs, may be involved in the treatment of idiopathic thrombocytopenic purpura (ITP) with anti-D.18,19
The data presented in Figures 4 and 5 show that F cells are not a uniform population and that relatively small differences in HbF content modify in vivo cell survival. The percent HbF value for unlabeled F cells was 22.6% (mean for all experiments). This is the average value for all the circulating cells, which have ages ranging from 0 days up to the age of the oldest F cells. Because the percent HbF of labeled F cells increases with time, the value for younger F cells is less than the average value. The 30% survival of F cells was 24.3 days (mean for all experiments), giving a total survival (24.3/0.7) of 34.7 days and an average age of 17.4 days. At the mean measured change in HbF per day of 28%, the youngest cells would have 22.6 – (0.28 × 17.4), or 17.7%. Conversely, the oldest cells would have a value of 27.5%. Thus the estimated range of percent HbF in F cells is substantial, from 17.7% to 27.5%. Dover et al1 used a single-cell immunodiffusion assay to quantitate HbF in F erythrocytes and F reticulocytes and found that reticulocytes contained 7.7 pg (weighted average of values in Table 1 from Dover et al1 ) and F erythrocytes contained 10.9 pg. While this method measures the absolute amount of HbF in the cell rather than percent HbF, age-dependent HbF increases in F cells were of the same magnitude as the current study.
Prepublished online as Blood First Edition Paper, March 30, 2006; DOI 10.1182/blood-2005-09-008318.
Supported by National Institutes of Health (NIH) grant HL70871 to the Cincinnati Comprehensive Sickle Cell Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.