Myasthenia gravis (MG) is associated with ectopic germinal centers in the thymus. Thymectomy and glucocorticoids are the main treatments but they induce operative risks and side effects, respectively. The aim of this study was to propose new therapies more efficient for MG. We hypothesized that molecules dysregulated in MG thymus and normalized by glucocorticoids may play a key role in thymic pathogenesis. Using gene chip analysis, we identified 88 genes complying with these criteria, the most remarkable being the B-cell chemoattractant (CXCL13). Its expression was increased in thymus and sera of glucocorticoid-untreated patients and decreased in response to treatment in correlation with clinical improvement. Normal B cells were actively chemoattracted by thymic extracts from glucocorticoid-untreated patients, an effect inhibited by anti-CXCL13 antibodies. In the thymus, CXCL13 was preferentially produced by epithelial cells and overproduced by epithelial cells from MG patients. Altogether, our results suggest that a high CXCL13 production by epithelial cells could be responsible for germinal center formation in MG thymus. Furthermore, they show that this gene is a main target of corticotherapy. Thus, new therapies targeting CXCL13 could be of interest for MG and other autoimmune diseases characterized by ectopic germinal center formation.
Introduction
Myasthenia gravis (MG) is a neuromuscular disease with an autoimmune or a congenital etiology.1 It is characterized by a defect in neuromuscular transmission, causing muscle weakness.2 In 85% of cases, autoimmune MG is caused by autoantibodies directed against the nicotinic acetylcholine receptors (AChRs) at the neuromuscular junction.3 Thymic hyperplasia occurs in 60% of MG patients with anti-AChR antibodies, is especially found in young women (< 40 years old), and consists in the formation of germinal centers (GCs) in the thymus.4 Although GCs are normally found in secondary lymphoid organs,5,6 ectopic GCs are described in many autoimmune diseases such as chronic arthritis, Sjogren syndrome, and Hashimoto thyroiditis.7-9 In MG disease, there is a clear association between the presence of GCs and anti-AChR antibody production, since B cells purified from MG thymic hyperplasia are able to spontaneously produce antibodies to AChR10 and thymectomy is an efficient therapy leading to a gradual decrease of anti-AChR antibody titer.11 In addition, thymic cells or fragments grafted in the SCID model produce anti-AChR antibodies accompanied by several signs of MG.12 However, the mechanism underlying the abnormal migration of B cells toward the thymus is so far unknown.
Although progress has been made in developing therapies for MG, this disease is still incapacitating.13,14 Among the treatments used, anticholinesterasic agents have a rapid effect based on the inhibition of acetylcholine degradation,14 but their action is only symptomatic. When applied appropriately, glucocorticoids with their anti-inflammatory properties appear to be effective in most severe cases. However, their therapeutic use is limited by their severe side effects, particularly during long-term treatment.15 Thymectomy is often indicated when the thymus is hyperplastic and is effective in most cases.14 However, it involves risks associated with the surgery,16 and the clinical status of the patients can remain unstable not only during the postoperative period but also for several months after thymectomy.17 Therefore, the existing treatments are far from being satisfactory in MG. Development of better therapies depends on a greater understanding of the mechanisms underlying thymic abnormalities, and namely the GC formation in the thymus.
Thus, the aim of this study was to identify novel molecules involved in the pathogenic mechanisms of autoimmune MG that could serve as potential targets for a selective therapy. Molecules dysregulated in MG patients and normalized by corticosteroids could serve such a function. Using DNA microarray technology, we identified a high proportion of genes associated with B-cell functions that fulfill these criteria. We focused on CXCL13, since this molecule is highly attractive for B cells and is important in GC formation and maintenance under physiological conditions.18-20 Our results showed an elevated CXCL13 production by thymic epithelial cells (TECs) during MG, probably responsible for B-cell attraction and thus for GC formation in the thymus.
Materials and methods
Biologic material
Thymic tissues and sera were taken from MG patients during thymectomy and from controls with healthy thymus during cardiac surgery at the Centre Chirurgical Marie-Lannelongue (Le Plessis-Robinson, France). Fresh blood was taken from healthy donors. Informed consent was provided according to the Declaration of Helsinki. These investigations were approved by the local Ethics Committee, CCPRB (Comité Consultative de Protection des Personnes dans la Recherche Biomédicale, Kremlin-Bicêtre, France).
All MG patients were females, younger than 40 years, receiving anticholinesterasic drugs, and seropositive for anti-AChR antibodies. The severity of MG symptoms was between 3A and 2B according to the MGFA classification.21 All patients in the study underwent thymectomy in the first 3 years following the onset of the disease and were chosen randomly. Patients with thymoma were excluded.
MG thymuses were taken from 24 MG patients with thymic hyperplasia not treated with corticosteroids (called untreated MG patients) and 21 MG patients treated with corticosteroids (called treated MG patients). Control thymuses were obtained from 21 age- and sex-matched adults and from 33 newborn girls all undergoing cardiac surgery.
Sera from 9 untreated MG patients and 9 treated ones were obtained before thymectomy. For 15 other MG patients, 5 of them having undergone corticotherapy, sera were obtained after thymectomy during clinical follow-up. Sera obtained from 10 sex- and age-matched adults were used as controls.
Cell isolation and culture conditions
Thymocytes were extracted mechanically by mincing freshly harvested thymuses in HBBS (Hanks balanced salts; Gibco-invitrogen, Cergypontoise, France). TECs were established as previously described.22 To obtain thymic fibroblasts, thymic explants were cultured as described,22 and after 7 days of culture, cells were submitted to a brief trypsin treatment to collect the fibroblasts that detach faster than TECs. The isolated cells were then cultured for an additional 7 days. The MITC (myoid immortalized thymic cell) cell line was established as previously described.23 Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood via Ficoll gradient (CMSMSL01-01; Eurobio, les-Ulis, France).
Different dexamethasone concentrations (0, 0.1, 0.2, 0.3, 0.4 mg/mL) were added for 24 and 48 hours to subcultured TECs.22 Supernatants were collected and stored at –80°C for enzyme-linked immunosorbent assay (ELISA). For flow cytometry analysis, TECs were treated for 4 hours with 10 μg/mL brefeldin-A before collection. For real-time reverse-transcriptase–polymerase chain reaction (RT-PCR), TECs were stored in TRIzol (Gibco-BRL, Cergy-pontoise, France) at –80°C until RNA isolation.
RNA isolation
RNAs were extracted from thymic tissues or cells in TRIzol according to the manufacturer's instructions. The FastPrep apparatus (QBiogen, Illkirch, France) was used for thymus fragments. RNA concentration and purity were determined spectrophotometrically.
DNA microarray analysis
Sample preparation. RNAs were purified on Qiagen columns (Courtaboeuf, France), and the quality was assessed using the bioanalyser 2001 from Agilent (Massy, France). Pools were made from equal amounts of total RNAs from thymuses of the following individuals: 4 adult controls, 5 untreated MG patients, and 5 treated MG patients. These samples from MG patients were from females aged 18 to 25 years with anti-AChR antibodies. The adult controls were age and sex matched. The thymuses of untreated patients were highly hyperplastic, while those of corticosteroid-treated patients were partially atrophic and included fewer germinal centers. The 3 RNA pools were tested against the same RNA reference: a pool of RNAs from 10 thymuses of newborn girls.
Labeling and hybridization. Samples were analyzed on the human 1 cDNA arrays from Agilent containing 16 200 probe sets (12 814 unique clones). Total RNA (20 μg) was labeled with cyanine 3 or 5 using the manufacturer's direct labeling protocol (Agilent). For each array, RNA reference pool was crossed with one of the other described RNA pools. Each comparison was repeated 4 or 5 times (14 arrays in total). Labeled cDNAs were hybridized overnight at 65°C and the slides washed following the manufacturer's instructions.
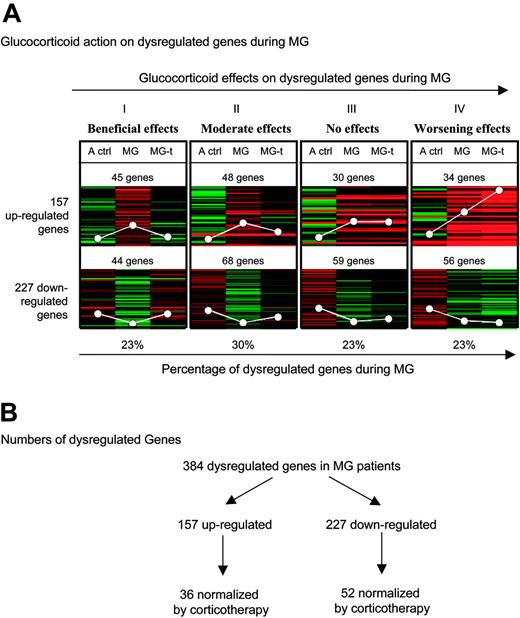
General effects of glucocorticoids on dysregulated genes during MG. (A) Eight K-median clustering analysis was applied to the median of ratios of the 3 comparisons: adult controls–reference (A ctrl), untreated MG patients–reference (MG), and treated MG patients–reference (MG-t) for the 384 dysregulated genes in MG extracted with SAM with an FDR less than 5% and an FC more than 1.8. The white circles represent average expression levels. (B) Numbers of dysregulated genes obtained in the microarray analysis data.
General effects of glucocorticoids on dysregulated genes during MG. (A) Eight K-median clustering analysis was applied to the median of ratios of the 3 comparisons: adult controls–reference (A ctrl), untreated MG patients–reference (MG), and treated MG patients–reference (MG-t) for the 384 dysregulated genes in MG extracted with SAM with an FDR less than 5% and an FC more than 1.8. The white circles represent average expression levels. (B) Numbers of dysregulated genes obtained in the microarray analysis data.
Data acquisition. Slides were scanned using the 428 Affimetrix scanner (MWG, Courtaboeuf, France). The images were analyzed with GenePix proV4.0 (Axon Instruments, Dipsi industrie, Chatillon, France). For each array, raw data were corrected by a nonlinear transformation (Lowess algorithm) using the TIGR Microarray Data Analysis System (http://www.tigr.org). To compare the arrays, each condition was centered on the median calculated from the repetitions and was then normalized by array. For each gene, a log 2 ratio of study sample over reference sample was calculated and the distribution by array was centered on zero.
Statistical analysis. To identify differentially expressed genes in thymuses from untreated MG patients compared with adult controls, the ratios were analyzed with the 2-class unpaired algorithm of the Significance Analysis of Microarrays software (SAM-version 1.21; Stanford University, Stanford, CA).24 The dysregulated genes were selected based on a false discovery rate (FDR) under 5% and an average fold change (FC) over 1.8. Among these genes, very few exhibited a low fluorescence intensity defined by a threshold corresponding to 1.5 background.
To determine the general effects of corticotherapy during MG, 8 K-median clustering analysis (Euclidean squared, log) using Acuity software, version 3.1 (Axon Instruments) was applied on the median of ratios of the 3 comparisons (adult controls–reference, untreated MG patients–reference, and treated MG patients–reference) for the genes identified by SAM. To assign a statistical value to these genes, we compared, using a nonparametric Mann-Whitney U test, the relative gene expression ratio between untreated and treated MG patients. A gene was considered significantly dysregulated if the P value was less than .05.
Expression profiling by real-time PCR
Transcription of RNA and real-time PCR were performed as previously described.25 Primer sequences were as follows: human CXCL13 (NM_006419): sense, 5′-ctctgcttctcatgctgctg-3′, antisense, 5′-tgagggtccacacacacaat-3′; human CXCR5 (NM_001716): sense, 5′-cctcccagaacacactccat-3′, antisense, 5′-tgcttggtcaagatgactgc-3′; and human CD21 (M26004): sense, 5′-tggaaccacggtcacttaca-3′, antisense, 5′-ctccaggtgcctctttcttg-3′. Hybridization was performed at 62°C for 12 seconds for all the primer pairs used. Successful preparation of cDNA was independently checked by amplification of the 28S gene.
Thymic protein extraction
For ELISA measurements, total thymic proteins were extracted in solution containing 5% Tris HCl 20 mM (pH 7.4), 0.1% Triton X100, and one tablet of protease inhibitor cocktail (complete-mini; Roche-Diagnostics, Meylan, France) using the fast prep apparatus.
CXCL13 ELISA
Capture (MAB801) and detection (BAF801) CXCL13 antibodies (R&D, Lille, France) were used at 2.5 μg/mL and 0.25 μg/mL, respectively. Recombinant human CXCL13 (801-CX-025; R&D) was used as standard. Thymic proteins were normalized to 1500 μg/mL and sera were diluted at 1:50 in 0.1% BSA in PBS. Tetramethylbenzedine was used for color development and plates were read at 450 nm on an MRX reader (DYNEX-technologies commercialized by ThermoLab-systems, Cergy-pontoise, France). Several samples were tested several times in the same assay, or in different assays, and the variance was less than 20%.
Chemotaxis assay
To eliminate monocytes and adherent cells, PBMCs were cultured 24 hours prior to the assay in RPMI containing 0.5% fetal calf serum (FCS). To perform the assay, 24-well plates were used. Peripheral blood leukocytes (PBLs) were seeded in RPMI containing 0.5% fetal calf serum at 2 × 106 cells/insert (PI8P01250; Millipore, Saint-Quentin, France). Thymic extracts were prepared by mechanically lysing frozen thymic fragments in PBS using a Teflon-glass homogenizer. The suspension was then centrifuged and the concentration of proteins was analyzed in the supernatant. Proteins were adjusted to 3 mg/mL in RPMI, 0.5% fetal calf serum to obtain a final concentration of at least 240 ng CXCL13/mL (80 ng CXCL13/mg total proteins) in untreated MG patients; this is the optimal concentration for cell migration. For CXCL13 or CCL21 neutralization, MAB801 and MAB366 antibodies, respectively, were used (both from R&D). After 4 hours, cells were collected from both upper and lower compartments, stained with anti-CD19 (Dakocytomation, Trappes, France), and counted by flow cytometry analysis using a known concentration of unlabeled beads (340486; Becton Dickinson, Lyon, France). These analyses gave the total number of B cells in the upper and lower compartments of the transwell chambers. The percentage of migrating B cells was calculated as follows: B-cell number in the lower chamber/(B-cell number in the lower chamber + B-cell number in the upper chamber) × 100. The results are expressed as the percentage of migrating B cells after subtraction of spontaneous migration obtained by counting B cells in the medium without thymus extracts.
Flow cytometry analysis
Flow cytometry was carried out on permeabilized TECs as previously described26 using anti-CXCL13 MAB801 antibody (R&D) revealed by goat antimouse coupled to phycoerythrin (R0480; Dakocytomation).
Laser microdissection
Cryostat sections (7 μm) of frozen human thymic tissues were affixed to glass foil slides for membrane-based laser microdissection (Leica, Rueil-Malmaison, France), dried overnight, and stained by hematoxylin-eosin. GCs and mantle zones were isolated by laser capture microdissection using a Leica laser microdissection microscope. The microdissected regions were collected in RLT buffer (Qiagen). RNAs were extracted using the Qiagen RNeasy microkit following the manufacturer's instructions. Reverse transcription and real-time PCR were performed on microdissected GCs as described in “Expression profiling by real-time PCR.” The mantle zones were too small to provide enough material to perform RT-PCR.
Determination of total GC areas using the microarray scanner
Thymic sections were stained with anti-CD21 antibody (1/20, 555 421; Becton Dickinson) and antikeratin antibodies (1/50, M0717 and M0821; Dakocytomation), and then revealed with Alexa Fluor 594 (Molecular probes, Cergy-Pontoise, France)– and PE (R0480; Dakocytomation)–coupled antibodies, respectively. Slides were scanned with the 428 Affimetrix scanner using Jaguar software (MWG, Roissy, France). Total GC areas (CD21+) were determined by the number of Alexa-positive pixels out of the total number of pixels evaluated on the whole sections, using free Image J software version 1.33 (Research Services Branch, National Institute of Mental Health, Bethesda, MD).
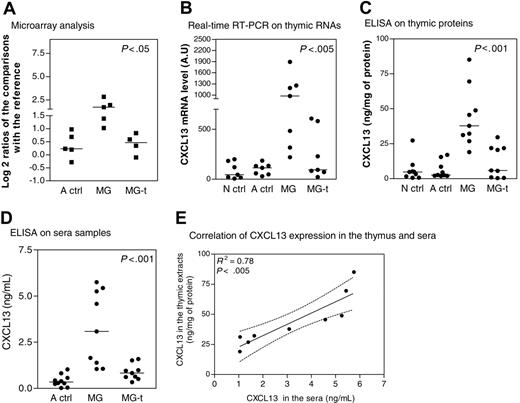
CXCL13 expression in thymus and sera during MG. (A) Microarray corresponding to CXCL13 gene expression level in thymuses of adult controls (A ctrl), untreated MG patients (MG), and MG patients treated by corticotherapy (MG-t) compared with the RNA reference obtained for each array. The y-axis shows the log 2 ratio centered on zero. Each dot corresponds to one comparison to the RNA reference. (B) Amplification by real-time PCR of CXCL13 gene in thymic samples from newborn girl controls (N ctrl), A ctrl, MG, and MG-t patients. Each dot represents the mean value of 3 different determinations. (C) Determination by ELISA of CXCL13 level expression in thymic samples from N ctrl, A ctrl, MG, and MG-t patients. Each dot represents the mean value of duplicates. (D) Determination by ELISA of CXCL13 level expression in sera samples from A ctrl, MG, and MG-t patients. Each dot represents the mean value of duplicates. In panels A-D, the bar represents the median value and the P values were obtained by the nonparametric one-way analysis of variance (Kruskal-Wallis test). (E) Correlation of CXCL13 expression determined by ELISA in thymic extracts and sera from MG untreated patients. The R2 and P value were obtained by the nonparametric correlation test (Spearman test).
CXCL13 expression in thymus and sera during MG. (A) Microarray corresponding to CXCL13 gene expression level in thymuses of adult controls (A ctrl), untreated MG patients (MG), and MG patients treated by corticotherapy (MG-t) compared with the RNA reference obtained for each array. The y-axis shows the log 2 ratio centered on zero. Each dot corresponds to one comparison to the RNA reference. (B) Amplification by real-time PCR of CXCL13 gene in thymic samples from newborn girl controls (N ctrl), A ctrl, MG, and MG-t patients. Each dot represents the mean value of 3 different determinations. (C) Determination by ELISA of CXCL13 level expression in thymic samples from N ctrl, A ctrl, MG, and MG-t patients. Each dot represents the mean value of duplicates. (D) Determination by ELISA of CXCL13 level expression in sera samples from A ctrl, MG, and MG-t patients. Each dot represents the mean value of duplicates. In panels A-D, the bar represents the median value and the P values were obtained by the nonparametric one-way analysis of variance (Kruskal-Wallis test). (E) Correlation of CXCL13 expression determined by ELISA in thymic extracts and sera from MG untreated patients. The R2 and P value were obtained by the nonparametric correlation test (Spearman test).
Results
DNA microarray experiment analysis
To identify genes mediating the effects of glucocorticoid treatments in MG, we first compiled a list of dysregulated genes in thymuses from untreated MG patients versus adult controls, and then analyzed the behavior of these genes in the thymus of glucocorticoid-treated MG patients.
Statistical analysis. Using SAM analysis with an FDR under 5% and an FC over 1.8, we identified 157 genes up-regulated and 227 genes down-regulated in thymuses from untreated MG patients compared with adult controls. Using K-median clustering analysis on these 384 dysregulated genes, we found that glucocorticoid effects on the dysregulated genes during MG could be divided into 4 patterns (Figure 1A). Glucocorticoids totally normalized the expression of 23% (pattern I) of these genes, and partially normalized 30% (pattern II) of them. In contrast, they exerted no effect on the expression of 23% of the dysregulated genes (pattern III) and they accentuated the dysregulated expression of 23% of them (pattern IV). The lists of these genes are given in Table S1, available on the Blood website (see the Supplemental Tables link at the top of the online article).
The beneficial effects of glucocorticoids may be due to their action on the genes of patterns I and II (53% of the dysregulated genes during MG). Their partial effects on some of the genes belonging to pattern II and their lack of effects on genes of pattern III may explain some of their limits in MG treatment. Finally, the effect of glucocorticoids on the genes of pattern IV may explain their important side effects, especially during long-term treatment.
Altogether, these analyses indicate that about only half of the dysregulated genes during MG are normalized by corticotherapy. On the contrary, many genes are even more dysregulated with corticosteroids, evidencing the side effects of corticosteroids. However, and since MG patients are improved after corticosteroid treatment, we hypothesize that the improvement is due to genes dysregulated during the pathology and normalized by the treatment.
Gene-by-gene analysis. To focus more precisely on the genes normalized by glucocorticoids, we carried out a gene-by-gene analysis on the genes belonging to patterns I and II (shown in Figure 1A) using a Mann-Whitney test. Figure 1B indicates that among the 227 down-regulated genes during MG, 52 were normalized by glucocorticoids (the list of these genes is given in Table S2), and among the 157 up-regulated genes during MG, 36 were normalized by glucocorticoids (Table 1). Altogether, 88 (36 + 52) genes were dysregulated in MG thymus and normalized by corticotherapy. Fifteen (42%) of the 36 genes were involved in the immune response and linked to B-cell biology. It is noteworthy that CXCL13 is the gene on which glucocorticoids exert the most significant effect (Table 1; Figure 2A). Indeed, CXCL13 expression was 2.82-fold higher in MG thymuses and was completely normalized in thymus of glucocorticoid-treated patients. We therefore decided to focus our research on the expression and function of CXCL13 in MG.
CXCL13 is overexpressed in MG thymuses and sera and normalized by corticotherapy
To confirm results obtained by DNA microarray (Figure 2A), we analyzed CXCL13 RNA and protein expression levels in the thymus. We found that CXCL13 was overexpressed in MG thymuses when compared with adult and newborn controls and normally expressed in MG patients treated by corticotherapy (Figure 2B-C). CXCL13 was also overexpressed in sera of untreated MG patients, while corticosteroid-treated patients exhibited normal levels (Figure 2D). A significant correlation was found between thymic and serum CXCL13 expression of the same MG patients (Figure 2E). Thus, CXCL13 expression was higher in MG patients untreated by corticosteroids in both the thymus and the serum and reduced to normal values in patients treated by corticotherapy.
The overexpression of CXCL13 in MG thymus contributes to high number of B cells in MG thymic hyperplasia
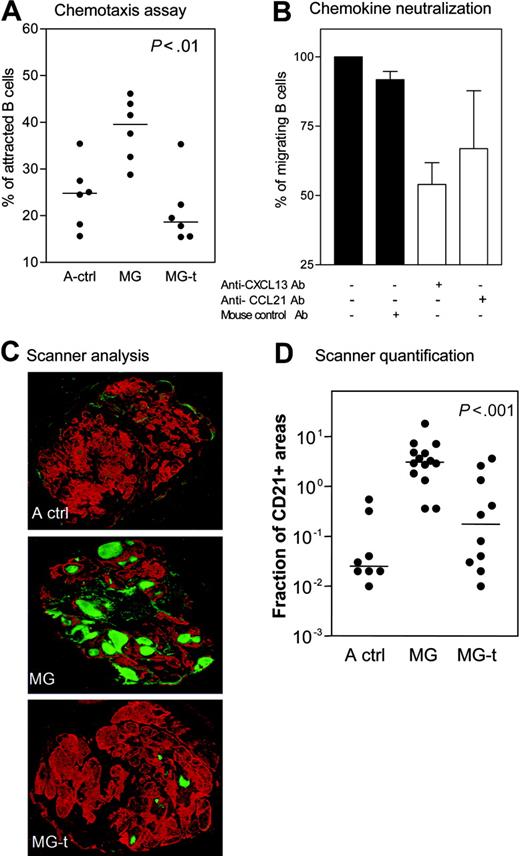
We compared the chemoattractive effect of thymic extracts from MG patients and adult controls on normal B cells. As expected, we found that the thymic extracts from untreated MG patients exhibited the strongest chemoattractant effect on B cells (Figure 3A). The chemoattraction was strikingly reduced in thymic extracts from corticosteroid-treated patients (Figure 3A). CXCL13 or CCL21 neutralization using specific antibodies resulted in a greater inhibition of B-cell attraction in the first case than in the latter (Figure 3B). The use of anti-CXCR5 antibody also resulted in a partial inhibition of B-cell migration (data not shown). Thus, it is likely that the overexpression of CXCL13 in MG thymus contributes to high number of B cells in MG thymic hyperplasia.
GC numbers are reduced in MG patients treated with corticosteroids
CD21 is a GC marker because of its high expression by germinal B cells.27 We analyzed the number of thymic GCs stained by anti-CD21 by means of an innovative use of the microarray scanner. As expected, the number of GCs was high in untreated MG patients. For the first time, we demonstrated that the number of GCs was dramatically reduced in corticosteroid-treated MG patients (Figure 3C). Image analysis of the CD21+ areas indicates a high significant difference between thymuses from treated and untreated MG patients (Figure 3D). These results demonstrate that glucocorticoids induce a striking reduction in the thymic GC formation.
Effect of glucocorticoids on GCs. (A) Specific chemoattraction of normal B cells by thymus extracts from adult controls (A ctrl), MG untreated (MG), and MG glucocorticoid-treated patients (MG-t). Each dot represents one sample after subtracting the spontaneous migration. (B) Four thymic extracts from MG patients used in panel A were incubated alone or with addition of anti-CXCL13, anti-CCL21, or mouse control antibody. The results are expressed as the mean and SEM. (C) Immunohistochemical analysis on thymic sections from A ctrl, MG, and MG-t patients (magnification, 5×). In red, epithelial network stained with antikeratin antibody; in green, FDC and B cells stained with anti-CD21 antibody. The whole sections were scanned using a microarray scanner. (D) Quantification of CD21 expression in the thymus of A ctrl, MG, and MG-t patients using Image J software. The P value was obtained by the nonparametric one-way analysis of variance (Kruskal-Wallis test). The horizontal lines in panels A and D represent the median value.
Effect of glucocorticoids on GCs. (A) Specific chemoattraction of normal B cells by thymus extracts from adult controls (A ctrl), MG untreated (MG), and MG glucocorticoid-treated patients (MG-t). Each dot represents one sample after subtracting the spontaneous migration. (B) Four thymic extracts from MG patients used in panel A were incubated alone or with addition of anti-CXCL13, anti-CCL21, or mouse control antibody. The results are expressed as the mean and SEM. (C) Immunohistochemical analysis on thymic sections from A ctrl, MG, and MG-t patients (magnification, 5×). In red, epithelial network stained with antikeratin antibody; in green, FDC and B cells stained with anti-CD21 antibody. The whole sections were scanned using a microarray scanner. (D) Quantification of CD21 expression in the thymus of A ctrl, MG, and MG-t patients using Image J software. The P value was obtained by the nonparametric one-way analysis of variance (Kruskal-Wallis test). The horizontal lines in panels A and D represent the median value.
All these results show that CXCL13 expression and function is increased in the thymus of untreated MG patients in correlation with a high number of GCs, while CXCL13 production is dramatically reduced in the thymus of MG patients treated with glucocorticoids and correlates with a low number of GCs.
The increase of CXCL13 in MG thymus is not only due to cells within GCs
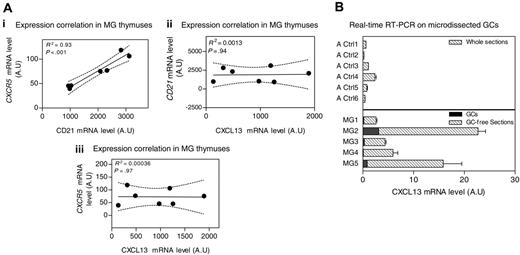
CXCL13 is known to be produced by cells within GCs.18,28-30 Therefore, the increased CXCL13 expression in MG patients could be due to the high GC number. We analyzed the correlation between CXCL13 and CD21 or CXCR5, both normally expressed on B cells27,31 and thus in GCs. While expression of CD21 and CXCR5 was clearly correlated, no correlation could be found between CXCL13 and CD21 or CXCR5 mRNA levels in MG patient thymuses (Figure 4A). This suggests that the high levels of CXCL13 found in MG thymuses are not supplied only from the GCs.
Source of CXCL13 in the thymus of MG patients. (A) Correlation between CXCR5 and CD21 mRNA levels (i), CXCL13 and CD21 mRNA levels (ii), and CXCL13 and CXCR5 mRNA levels (iii) in samples from MG untreated patient thymuses. The R2 and P value were obtained by the nonparametric correlation test (Spearman test). (B) Amplification of CXCL13 gene by real-time RT-PCR on total sections from 6 adult controls (Ctl1-6), 5 thymic GC-free sections from untreated MG patients (MG1-5), and their corresponding microdissected GCs. RT-PCR was performed in duplicate. Error bars represent the mean value of the 2 duplicates ± SEM.
Source of CXCL13 in the thymus of MG patients. (A) Correlation between CXCR5 and CD21 mRNA levels (i), CXCL13 and CD21 mRNA levels (ii), and CXCL13 and CXCR5 mRNA levels (iii) in samples from MG untreated patient thymuses. The R2 and P value were obtained by the nonparametric correlation test (Spearman test). (B) Amplification of CXCL13 gene by real-time RT-PCR on total sections from 6 adult controls (Ctl1-6), 5 thymic GC-free sections from untreated MG patients (MG1-5), and their corresponding microdissected GCs. RT-PCR was performed in duplicate. Error bars represent the mean value of the 2 duplicates ± SEM.
To strengthen these results, CXCL13 mRNA expression was determined on thymic GC-free sections and the corresponding microdissected GC from untreated MG patient thymuses. We found that MG GC-free sections exhibited a higher CXCL13 mRNA level than total thymic sections from adult controls. Furthermore, the CXCL13 levels provided by microdissected GCs represented 1% to 12% of the total CXCL13 produced by the whole section (Figure 4B). Thus, GC cells could not explain the higher production of CXCL13 observed in thymus from untreated MG patients. We next examined which cells are able to produce CXCL13 in the thymus.
CXCL13 is normally produced by TECs in the thymus
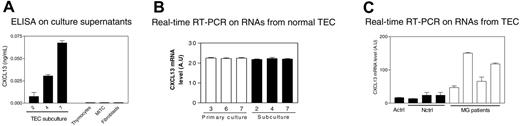
We tested the main thymic populations isolated from normal thymus for CXCL13 production: TECs, thymocytes, thymic fibroblasts, and MITCs. TECs were the only cells producing CXCL13 (Figure 5A).
CXCL13 is produced by TECs and is overproduced by TECs from MG patients. (A) Determination by ELISA of CXCL13 concentration in the culture supernatants of subcultured TECs (at days 2, 4, and 7), thymocytes, MITCs, and thymic fibroblasts. Each histogram represents the mean value of 3 different experiments and SEM. (B) CXCL13 gene amplification by real-time RT-PCR on RNAs isolated from TECs obtained at days 3, 6, and 7 of primary cultures and days 2, 4, and 7 of subcultures. Each histogram represents the mean value and SEM of 2 different experiments. (C) Amplification by real-time PCR of CXCL13 gene on RNAs isolated from TECs prepared from 4 patient thymuses (MG1-4), 1 adult control (A ctrl), and 3 newborn controls (N ctrl1-3). Each histogram represents the mean and SEM of 2 different experiments.
CXCL13 is produced by TECs and is overproduced by TECs from MG patients. (A) Determination by ELISA of CXCL13 concentration in the culture supernatants of subcultured TECs (at days 2, 4, and 7), thymocytes, MITCs, and thymic fibroblasts. Each histogram represents the mean value of 3 different experiments and SEM. (B) CXCL13 gene amplification by real-time RT-PCR on RNAs isolated from TECs obtained at days 3, 6, and 7 of primary cultures and days 2, 4, and 7 of subcultures. Each histogram represents the mean value and SEM of 2 different experiments. (C) Amplification by real-time PCR of CXCL13 gene on RNAs isolated from TECs prepared from 4 patient thymuses (MG1-4), 1 adult control (A ctrl), and 3 newborn controls (N ctrl1-3). Each histogram represents the mean and SEM of 2 different experiments.
CXCL13 concentration in TEC culture supernatants increased from days 2 to 7 of subculture, suggesting an increase of CXCL13 production during the culture (Figure 5A). However, CXCL13 mRNA levels remained unchanged throughout the culture period (Figure 5B), suggesting that the increase of CXCL13 concentration in the culture supernatant is due to an accumulation of CXCL13 rather than to an increase of its production rate by TECs.
These results show that TECs are the primary source of CXCL13 in the thymus and suggest that the high levels of CXCL13 in MG may be due to a dysregulated expression in this cell type.
We then compared the CXCL13 mRNA level in TECs established from MG and control thymuses. The 4 TEC samples from MG patients overproduced CXCL13 (Figure 5C), suggesting a defect in the regulation of CXCL13 production by TECs during MG, leading to overexpression of this chemokine in the thymus.
Glucocorticoids inhibit CXCL13 production by TECs
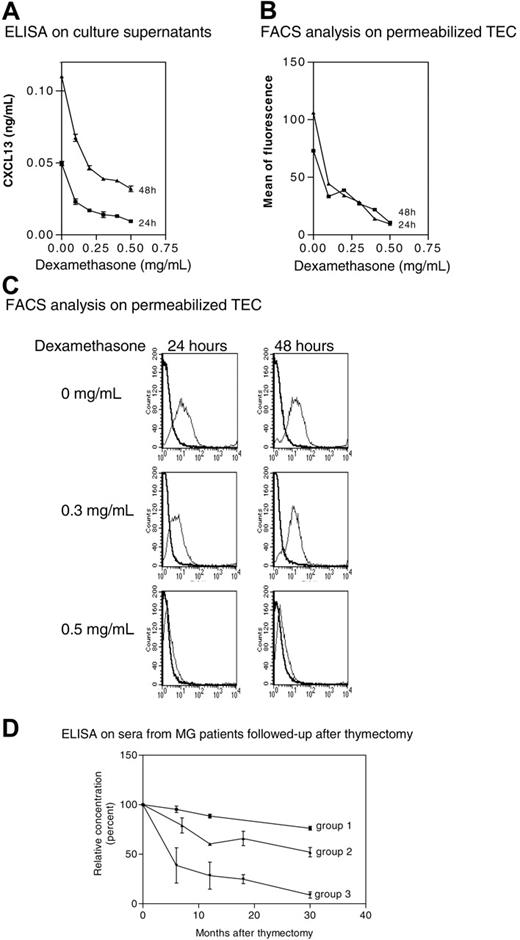
We tested the effects of corticosteroids on CXCL13 production in vitro by normal TECs after permeabilization. We found that dexamethasone inhibited CXCL13 expression in a dose-dependent manner (Figure 6A-C). It is noteworthy that under our culture conditions, the cellular viability reached 88% (data not shown), excluding an apoptotic or necrotic effect. No staining was observed on nonpermeabilized TECs, indicating the absence of CXCL13 at TEC surface. These observations show that CXCL13 expression in normal TECs is down-regulated by corticosteroids in vitro.
Effects of treatments on CXCL13 expression. (A) Subcultures of normal TECs were treated with dexamethasone for 24 and 48 hours, and CXCL13 levels were measured by ELISAin the culture supernatant. Results are expressed as the mean ± SEM of 3 different experiments. (B) Fluorescence intensities were determined on TECs treated with dexamethasone by flow cytometry analysis. A representative experiment out of 2 is shown. (C) Representative experiment (1 of 3) of flow cytometry analysis. As dexamethasone concentration increases, the positive peak (thin line) is shifted to negative values (large line). (D) CXCL13 concentrations were determined by ELISA in sera obtained at the time of thymectomy (time 0) and at different times after thymectomy in 15 MG patients followed up after surgery. Results are expressed as mean and SEM of percentage for each group. The CXCL13 serum level at the time of thymectomy is defined as 100%. Group 1: 3 MG patients who never underwent corticotherapy and did not improve after thymectomy; group 2: 7 MG patients who never underwent corticotherapy and improved after thymectomy; and group 3: 5 MG patients who underwent corticotherapy and improved after thymectomy.
Effects of treatments on CXCL13 expression. (A) Subcultures of normal TECs were treated with dexamethasone for 24 and 48 hours, and CXCL13 levels were measured by ELISAin the culture supernatant. Results are expressed as the mean ± SEM of 3 different experiments. (B) Fluorescence intensities were determined on TECs treated with dexamethasone by flow cytometry analysis. A representative experiment out of 2 is shown. (C) Representative experiment (1 of 3) of flow cytometry analysis. As dexamethasone concentration increases, the positive peak (thin line) is shifted to negative values (large line). (D) CXCL13 concentrations were determined by ELISA in sera obtained at the time of thymectomy (time 0) and at different times after thymectomy in 15 MG patients followed up after surgery. Results are expressed as mean and SEM of percentage for each group. The CXCL13 serum level at the time of thymectomy is defined as 100%. Group 1: 3 MG patients who never underwent corticotherapy and did not improve after thymectomy; group 2: 7 MG patients who never underwent corticotherapy and improved after thymectomy; and group 3: 5 MG patients who underwent corticotherapy and improved after thymectomy.
Thymectomy induces a decrease in CXCL13 concentration in patient sera
Since TECs are a major source of CXCL13 production, we should expect a decrease in CXCL13 concentration in the sera of MG patients after thymectomy. Indeed, the serum level of CXCL13 decreased in all the patients during the first months following thymectomy (Figure 6D). Of interest, we found a correlation between the decrease of CXCL13 and a clinical improvement; most patients (groups 2 and 3) improved in the months following thymectomy and they showed a striking decrease in their CXCL13 serum concentration, while patients not improved after thymectomy (group 1) exhibited the lowest reduction in CXCL13 serum concentration. Moreover, patients treated with corticoids at the time of thymectomy (group 3) showed the most rapid decrease in CXCL13 concentration. These results confirm that the thymus is a significant source of CXCL13 in MG and that CXCL13 is both implicated in MG pathophysiology and serves as a glucocorticoid target.
Discussion
In the majority of chronic inflammatory diseases such as multiple sclerosis, asthma, and psoriasis, multiple chemokines/receptors are present within lesions.32,33 For example, synovial tissue from patients with rheumatoid arthritis expresses chemokines including MCP-1, MIP-1α, IL-8, and RANTES, and infiltrating lymphocytes within synovial fluid express the chemokine receptors CXCR3 and CCR5.34-37 To date, no clear pathogenic role for a particular chemokine in the pathogenesis of MG has been shown, except IP10 and its receptor (CXCR3), which are increased in MG and experimental autoimmune MG (EAMG).38 Our study demonstrates for the first time the role of the CXCL13 chemokine in the pathophysiology of this disease and its overexpression in the thymus, the effector organ during MG. A recent work on another autoimmune disease, Sjögren syndrome, reports similar results, indicating that CXCL13 is expressed in the target organ of almost all patients.39 Thymic extracts from MG patients displayed a high chemoattractant effect toward B cells, an effect inhibited by anti-CXCL13 antibody. It is noteworthy that CCL21 neutralization resulted in a lower B-cell migration inhibition than CXCL13 neutralization. Based on our data, we propose the following hypothesis: a high number of B cells are chemoattracted by the MG thymus overproducing CXCL13. In contact with the autoantigen present in the thymus,40 and in the inflammatory environment described for MG hyperplasia,25 B cells would be activated, leading in turn to the formation of GCs typical of thymus from untreated MG patients. When patients are treated with glucocorticoids, CXCL13 production is strikingly reduced, leading to a reduced number of chemoattracted B cells in the thymus, and in turn a reduced number of GCs.
In MG patients, the higher production of CXCL13 both in thymus and serum, the correlation between the thymic and peripheral CXCL13 levels, and the decrease after thymectomy strongly suggest that the thymus is a major producer of CXCL13. However, CXCL13 concentrations in patient serum remained sometimes higher than the level in controls, even many years after thymectomy (data not shown), suggesting the possible existence of another source of CXCL13 in the periphery. The question of the nature of the thymic cells producing CXCL13 could be raised. From previous reports, it is known that CXCL13 is produced by follicular dendritic cells (FDCs)18,28 and GC-specific CD4+ CD57+ T cells.29,30 However, other data show that mature macrophages produce CXCL13, indicating that cellular types other than GC cells can be a source of this chemokine.41 In this study, we demonstrate that TECs are significant producers of this chemoattractant molecule.
The causes of the overproduction of CXCL13 by TECs from MG patients are not known. Several hypotheses could be raised: (1) CXCL13 production could be induced due to the influence of the thymic environment. The triggering event(s) in MG disease is not known, but thymic hyperplasia is accompanied by signs of inflammatory activity.25 However, since TECs are obtained after several days of culture out of the thymic environment, this hypothesis is unlikely. Furthermore, treatment of TEC cultures with cytokines involved in GC formation (TNF-α or LTα1β2) did not result in an increase in CXCL13 concentration in the supernatants (data not shown). (2) Agenetic polymorphism of CXCL13 could explain these results. Although there are no data describing such a polymorphism for CXCL13, several high-producer alleles of proinflammatory cytokines, such as TNF-α and IL-1, have been associated with MG.42-44 Whether a CXCL13 high-producer allele is associated with MG disease needs to be explored. (3) Another possibility is that the natural T-regulatory CD4+CD25+ cells that are defective in MG thymic hyperplasia45 could influence the production of chemokines by stromal cells. This hypothesis deserves further investigation.
The very effective control of inflammation exerted by glucocorticoids is largely mediated by the inhibition of the transcriptional activity of several genes encoding proinflammatory cytokines such as IL-1β, lymphotoxin-β, IL-1α, IL-8, IFN-α, and INF-β46 ; chemokines such as CCL5, CCL3, CCL2, and CCL11; adhesion molecules such as ICAM-1, VCAM-1, and E-selectin; and mediator-synthesizing enzymes such as i-NOS, COX-2, and cytoplasmic PLA2.47 However, glucocorticoids induce also many undesirable side effects. Common side effects widely described in the literature include weight gain, hypertension, diabetes, anxiety/depression/insomnia (“steroid psychosis”), glaucoma, osteoporosis, cataracts, opportunistic infections, muscle weakness, personality changes, and others.48 Early attempts to circumvent these side effects were largely unsuccessful, probably because of the large panel of genes on which glucocorticoids exert an effect. In this study, we found that glucocorticoids dysregulate 23% of genes whose expression is normal in MG thymuses. These findings further highlight the lack of specificity of corticosteroid treatments and support the need to identify more specific therapeutic targets among the glucocorticoid-regulated genes. Our results demonstrate for the first time that CXCL13 gene is a major target of glucocorticoids. Chemokines and their receptors might be good therapeutic targets in the treatment of inflammatory diseases. Strategies that have already been used to reduce chemokine/receptor activity include neutralizing antibodies, peptide antagonists, nonpeptide antagonists, and virally derived peptides.49-51 The strategy of blocking the chemokine system to combat diseases is under investigation for the treatment of several diseases, and several chemokine receptors have been already targeted in the search for antagonists.51 Therefore, the targeting of CXCL13 in early steps of MG could inhibit B-cell chemotaxis, and therefore limit thymic inflammation. This approach appears realistic, and is expected to be more specific and to have fewer side effects than corticosteroids.
In conclusion, this study demonstrates the key role of CXCL13 gene in MG pathophysiology and its inhibition by glucocorticoids. Based on our data, CXCL13 could represent a future therapeutic target for this disease.
Prepublished online as Blood First Edition Paper, March 16, 2006; DOI 10.1182/blood-2005-06-2383.
Supported by grants from the National Institutes of Health (NS39869), the European Commission (QLG1-CT-2001-01918 and QLRT-2001-00225), and the Association Française contre les Myopathies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Patrice Nancy for providing RNA from TECs established from MG patients. We thank Dr Revital Aricha, Dr Neli Boneva, Dr Sylvia Cohen-Kaminsky, Dr Tali Feferman, Prof Sara Fuchs, Dr Isabelle Petit, and Prof Idit Shahar for helpful discussions and critical reading of the paper. We thank Shelley Schwartzbaum for editing the paper.